Abstract
Background
The potential drug-drug interaction (pDDI) increases as the number of concomitant medications increases. Patients with cardiovascular disorders are at higher risk for drug- drug interactions because of the types and number of drugs they receive. While drug interactions are reported to be common, there is no published report of the prevalence of such interactions among Indian cardiac patients. The aim of the present study was to identify the pattern of pDDI and document any observed interaction. It was also planned to evaluate the demography of patients and correlate it with the drug-drug interactions.
Method
A prospective observational study from Oct 2007 to Apr 2008 was carried out in 'cardiology department' of a hospital in South India. Those patients who were taking at least two drugs and had a hospital stay of at least 48 hours were included in the study. The medications of the patients were analyzed for possible interactions. Factors associated with pDDI were studied. The actual interactions that were observed during the hospital stay in the study subjects were documented.
Results
A total of 812 patients were included in the study. 388 pDDIs were identified among 249 patients. The incidence of pDDI was 30.67%. The most common potential interactions were between aspirin & heparin (29.38%), and clopidogrel & heparin (7.21%). Drug classes most commonly involved were antiplatelets, anticoagulants and diuretics. Majority of interactions were of moderate severity, delayed onset, and pharmacodynamic in nature. Total 68 actual interactions were observed in the observed cases.
Conclusion
The present study identified pDDIs and also documented interactions in cardiovascular patients. Factors which had correlation with adverse drug interactions were identified. This study highlights the need for screening prescriptions of cardiovascular patients for pDDIs and proactive monitoring of patients who have identified risk factors; this helps in detection and prevention of possible adverse drug interactions.
Keywords: Potential Drug-Drug interactions, Cardiology, Severity, Bleeding, Antiplatelets, Anticoagulants, Diuretics
Background
Drug therapy is growing more complex, thus appropriate drug prescription becomes increasingly challenging. Adverse drug reactions (ADRs) include adverse effects, extension effects, drug interactions, idiosyncratic reactions, and hypersensitivity reactions.1 Adverse drug-drug interactions are a major cause of morbidity and mortality. In the Harvard Medical Practice Study of adverse events, 20% of events in an acute hospital in-patient setting were drug related. Of these, 8% were considered to be due to Drug- Drug Interactions (DDIs).2
In a study on the admissions of two hospitals in Britain, it was reported that adverse drug reactions were responsible for a significant proportion of admissions and drug interactions accounted for around 16% of adverse drug reactions resulting in hospital admissions.3 In a recent review, it has been reported that approximately 0.05% of the emergency department visits, 0.6% of the hospital admissions and 0.1% of the re-hospitalizations are caused by ADRs due to DDIs.4 The clinical outcome of a potential Drug-Drug Interaction (pDDI) is often unknown. However, exposure to pDDIs is associated with a significantly increased risk for hospitalization.5
A prospective study reported that 30.3% of patients admitted to an emergency department in USA were at the risk of pDDIs and this increased to 48% after being treated at the emergency department.6 The prevalence of pDDIs in the medication of ambulatory patients at hospital admission during hospitalization and at discharge was reported by various studies.7-14 In a study which assessed pDDIs for each patient at hospital admission, at discharge, and 3 months after discharge reported that pDDIs dramatically increased during the hospital admission period compared to the prehospitalization period and fell after discharge but not to the level of pre hospitalization level.15
According to WHO the number of cases of cardiovascular cases will increase from 29 million in the year 2000 to about 69 million cases in the year 2015.16 The potential drug-drug interaction increases as the number of concomitant drug increases.17 The incidence of drug interactions among the cardiac patients is more common than patients of other departments.18 A study reported by Cruciol-Souza showed that overall frequency of pDDIs was 49.7% in cardiology.19
Although drug interactions are reported to be common in cardiology17-19, there is no published report of the prevalence of such interactions among South Asian cardiac patients. The aim of this study was to assess for the prevalence of pDDIs during hospitalization among inpatients admitted to a cardiology unit of a tertiary care hospital.
Methods
A prospective observational study was carried out for a period of seven months (between October 2007 and April 2008) in a south Indian teaching hospital. Ethical approval was obtained from the relevant Institutional ethics committee prior to study initiation. Patients admitted consecutively to cardiology wards were included in the study. Prescriptions with two or more drugs prescribed were selected for the study. Prescriptions from each patient during his/her hospitalization in the ward during the study were included. Demographic information (age and sex), length of hospital stay, main diagnosis (ICD-10) and details of comorbidities were obtained from the clinical records.
Data collection
Drug interactions were identified using a computerized DDI database system (DrugReax-Micromedex, 2008). This computer program describes all potential interactions and states whether information is available on specific drugs within a class of drugs. It also briefly indicates the clinical relevance of the interaction, whether the interaction has been well established in the literature and gives literature citations.20 In determining pDDIs within prescriptions, current, new and discontinued medications were considered. All drugs were classified with Anatomical Therapeutic Chemical Classification (ATC code, level one) (WHO, 2008).
Patients diagnosed with cardiac problems either as main diagnosis or as additional diagnosis were identified using the International Classification of Diseases-10th Edition (ICD-10, WHO, 1992). Certain demographic characteristics were studied to establish factors that predict the presence of pDDIs. Factors studied were: (a) patient characteristics [gender, age (more than 18 years old), social history, concurrent morbidities and length of stay], (b) drug characteristic [number of drugs, number of therapeutic drug classes (number of ATC codes)]. The number of drug pairs was calculated according to the number of drugs per prescription.
Classification of potential drug-drug interaction
Based on the profile of medications prescribed, the drug- drug interactions were identified and classified according to Drug-Reax database. According to severity, pDDIs were classified as: 1) Major –The effects are potentially life threatening or capable of causing permanent damage (2) Moderate- The effects may cause deterioration in patients' clinical status and additional treatment or extension of hospital stay (3) Minor- The effects are usually mild. Consequences may be bothersome or unnoticeable but should not significantly affect the therapeutic outcome.
Documentation of adverse drug interactions
Patients identified with pDDIs were followed. Whenever patients with severe pDDIs were noted and if there is sufficient evidence for the interaction, it is intimated to treating clinician and the medicines were changed. If the pDDIs were mild or moderate or there is no significant evidence for such interaction, the cases are followed as such and documented if there are any actual interactions.
Statistical analysis
Frequencies expressed as percentages were used to summarize sex, diagnosis, number of drugs dispensed frequency of pDDIs, drugs involved in the pDDIs and severity of pDDIs. Mean with 95% confidence interval was used to summarize age, length of stay. Chi-square test was used to find the association between sex, number of drugs and pDDIs. Logistic regression analysis was used to determine associations of patient's age, sex, medications given, length of stay and risk factors with pDDIs adjusting for effect of other variables. Pearson correlation was used to find the correlation between numbers of drugs, length of stay with pDDIs. p values of < 0.05 were considered statistically significant. All analysis was performed using SPSS for window 15 (SPSS Inc., South Asian Ed, Bangalore).
Results
A total of 982 patients were admitted in the department of cardiology during the study period. Among them 812 patients were studied. The remaining 170 patients failed to meet the inclusion criteria. These patients were either on single drug or the duration of stay was less than 48 hours. Of the 812 studied patients, 249 patients had at least one pDDI. Overall the incidence rate of pDDI was 30.67%. The patient characteristics are represented in table 1. Among the 812 studied patients, a significant proportion of these patients were in the age group of 51-60 years [232 (28.57%)]. The mean age of the study population was 57.27±14.6 (95% CI 54.17-57.16) years. On average, each patient had 3 coded diagnoses. Anterior wall myocardial infarction was the most common diagnosis [184 (22.66%)] followed by inferior wall myocardial infarction [87 (10.71%)] and other diagnoses. The mean duration of hospital stay of the patients was 5.56±3.77 (range 2-35) days. The mean number of drugs prescribed per patient in the study population was 10.23±4.76 (range 2-24).
Table 1. The characteristics of the study population.
| Characteristics (n=812) | Number (%) |
|---|---|
| Age – years Mean±SD (Range) | 57.27±14.0(3-92) |
| Sex | |
| Male | 555 (68.3) |
| Female | 257 (31.7) |
| Length of stay – days | |
| < 5 | 500 (61.58) |
| 6-10 | 234 (28.82) |
| 11-15 | 55 (6.77) |
| 16-20 | 20 (2.46) |
| > 21 | 3 (0.37) |
| Main Diagnosis | |
| Anterior wall MI | 184 (22.66) |
| Inferior wall MI | 87 (10.71) |
| Hypertension | 78 (9.60 |
| Unstable Angina | 68 (8.37) |
| Stable Angina | 51 (6.28) |
| Rheumatic Heart Disease | 45 (5.54) |
| Cardiomyopathy | 33 (4.06) |
| Infer posterior wall MI | 32 (3.94) |
| Non Cardiac Chest Pain | 25 (3.08) |
| Non ST segment elevated MI | 24 (2.96) |
| Atrial Fibrillation | 22 (2.70) |
| Atypical Chest Pain | 13 (1.60) |
| Syndrome X | 12 (1.48) |
| Anteroseptal MI | 10 (1.23) |
| Congestive heart failure | 9 (1.11) |
| Others | 119 (14.66) |
| Number of coded diagnosis per patient |
3 (Range 2-4) |
Potential drug-drug Interactions
On evaluation of the number of drugs administered to the individual patients, the mode [87 (10.7% of patients)] was 6 drugs. The mean number of drug pairs identified was 58.42 (range 21-78). The frequency of pDDIs per patient ranged from 1 to 6. Out of 249 patients, 154 had at least one interaction. The results are represented in table 2.
Table 2. No of drugs, No of drugs pairs per patient's severity and Frequency of drug-drug interaction.
| Characteristics | Number (%) |
|---|---|
| No of drugs dispensed | |
| 2 | 7 (0.86) |
| 3-5 | 105 (12.93) |
| 6-10 | 362 (44.58) |
| 11-15 | 220 (27.09) |
| 16-20 | 95 (11.70) |
| >20 | 23 (2.83) |
| Severity of DDI | |
| Minor | 28 (7.20%) |
| Moderate | 234 (60.30%) |
| Severe | 126 (32.50%) |
| Frequency of drug-drug interaction | |
| 1 | 154 (61.85) |
| 2 | 67 (26.91) |
| 3 | 18 (7.23) |
| 4 | 5 (2.01) |
| 5 | 4 (1.61) |
| 6 | 1 (0.40) |
| No of drugs pairs Mean (95% CI) | 58.42(21-78) |
Classification of potential drug-drug interactions
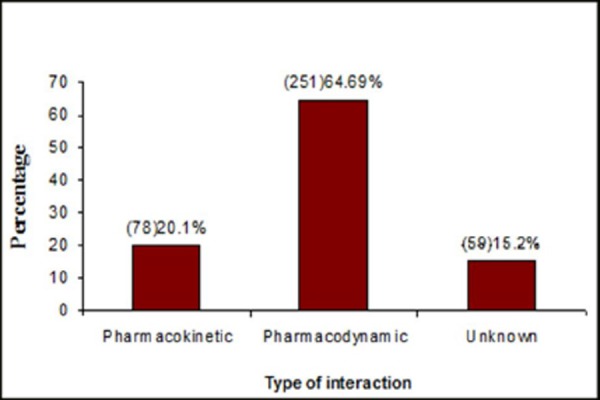
The classification of the potential drug-drug interactions were made based on their mechanism like pharmacodynamic, pharmacokinetic or unknown. Among 388 pDDIs, [251 (64.69%)] were pharmacodynamic drug interactions, [78 (20.1%)] were pharmacokinetic and [59 (15.2%)] were unknown. (Fig. 1)
The significance of pDDIs was classified according to a three level scale. Of the 388 pDDIs, the majority were of mild & moderate significance. Interactions with major severity accounted for 32.5% (126) of the total pDDIs while those with moderate and minor severity accounted for 60.3% (234) and 7.20% (28) respectively. (Fig. 2) Most interactions were classified as delayed interactions, accounting for 52% (203) of the total interactions. Whereas 165 (43%) were of a rapid onset type.
Figure 2. Significance level of the drug-drug interactions.
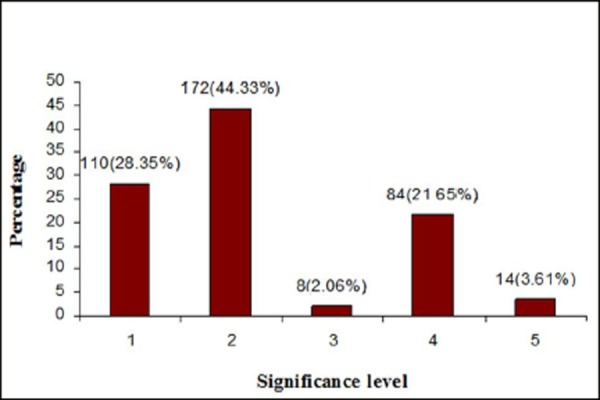
Table 3. List of common drugs involved in potential drug- drug interaction.
| Drug | ATC code | No. DDI (%) |
|---|---|---|
| Aspirin | B01AC06 | 174 (44.85) |
| Heparin | B01AB01 | 166 (42.78) |
| Clopidogrel | B01AC04 | 86 (22.16) |
| Warfarin | B01AA03 | 45 (11.59) |
| Atorvastatin | C10AA05 | 28 (7.22) |
| Ramipril | C09AA05 | 27 (6.95) |
| Torsemide | C03CA04 | 24 (6.19) |
| Digoxin | C01AA05 | 22 (5.67) |
| Furosemide | C03CA01 | 21 (5.41) |
| Spironolactone | C03DA01 | 21 (5.41) |
| Diltiazem | C08DB01 | 19 (4.89) |
| Enoxaparin | B01AB05 | 16 (4.12) |
| Amiodarone | C01BD01 | 14 (3.60) |
| Metoprolol | C07AB02 | 14 (3.60) |
| Captopril | C09AA01 | 12 (3.09) |
Risk factors for potential drug interactions
Statistical analysis, by Pearson correlation showed that there was an extremely significant linear relationship (r = 0.99, 95% CI = 0.9968-0.9978, p <0.0001) between the number of drugs prescribed for a patient and the occurrence of pDDIs. Similarly there was a significant linear relationship (r = 0.6962, 95% CI = 0.1652-0.9143, p < 0.0173) between length of hospital stay and occurrence of pDDIs.
Observed drug interactions
In total, 68 adverse drug interactions were observed among the 388 pDDIs identified. This gives an incidence of 17.53%. Bleeding was the most important interaction in 60 cases followed by Torsemide toxicity (8 events). The most common objective drug (drug which is originally prescribed) was Heparin followed by Warfarin. The common precipitant drug is Aspirin (Drug which is added to the already prescribed drugs). The results are presented in the Table 4.
Table 4. Observed adverse drug-drug interactions.
| Objective Drug | Precipitant Drug | No. of ADI (%), n=68 | Adverse outcome |
|---|---|---|---|
| Heparin | Aspirin | 26 (6.70) | Bleeding |
| Warfarin | 12 (3.09) | Bleeding | |
| Warfarin | Clopidogrel | 8 (2.06) | Bleeding |
| Heparin | 11 (2.84) | Bleeding | |
| Clopidogrel | Torsemide | 8 (2.06) | Torsemide Toxicity |
| Enoxaparin | 1 (0.26) | Bleeding | |
| Aspirin | 2 (0.52) | Bleeding |
Discussion
Drug-drug interactions (DDIs) are a concern for all the stake holders, especially patients and this risk increases as greater numbers of medications are commonly used to manage complex conditions. Drug- drug interactions can result in anything from minor morbidities up to fatal consequences.20 Studies have shown that up to 11% of outpatients' experience symptoms associated with DDIs and DDIs are responsible for up to 2.8% of hospital admissions.21
A total of 388 pDDIs were identified in 249 patients involving 51 different drugs with a total of 74 different drug combinations. pDDIs were identified in about 30.67% of the study subjects. Data on the incidence of pDDIs in cardiac patients was not available, but the data on Incidence of DDIs in medical wards was available. Cardiac patients have previously been found to have a higher chance of having drug interactions compared to other group of patients.19, 23, 24
Many of the commonly used cardiovascular drugs interact with one another. These drugs can be used together to treat cardiac conditions following a risk-benefit assessment. It is likely that many clinicians balance the risks of pDDIs against the benefits when prescribing patients with multidrug regimens. An example would be combined anticoagulant- anti-platelet therapy where an increase in the risk of haemorrhage with the combined therapy needs to be considered against the risks of thromboembolism without it. Benefits with multidrug regimens are unlikely to always outweigh their risks; therefore decisions regarding prescriptions must always be tailored to suit each patient.
Of the total pDDIs identified majority were of moderate severity. A study by Herrlinger and Vonbach reported interactions in cardiac failure patients and highlighted that more than 90% of interactions were of moderate or major in severity, a result that is very similar to the present study.25, 26
Of the pDDIs identified, 52% were of delayed onset in nature. This implies that even if there was an interaction occurring during the concomitant administration, it may not manifest itself immediately. If these combination of drugs were to be continued on an outpatient basis, this could potentially lead to decreased efficacy leading to therapeutic failures or potential for delayed adverse events. Hence the duration of concomitant drug use should also be taken into account when prescribing relevant interacting drugs.
Of the pDDIs observed, majority were of pharmacodynamic in nature followed by pharmacokinetic interactions. These findings are in contrast to the study reported by Vonbach and Aparasu who reported 76% of pharmacokinetic and 22% of pharmacodynamic interactions.21, 26
Patients in the age group of >80 years had higher percentage of pDDIs. Since this age group usually has many intercurrrent illnesses, they might be subject to polypharmacy which increases the chances for pDDIs. In many of the reported studies, age more than 60 was reported as an independent risk factor for pDDIs.19, 26, 27
The average number of drugs prescribed per patient for the study population was 10.23±4.76 (range 2-24). It was seen that there is a linear increase in the percentage incidence of drug interactions with an increase in the number of drugs prescribed to the patient. There was a statistically significant (r- 0.99, p<0.0001) correlation between number of drug prescribed to the occurrence of pDDIs in the patients. The present study substantiates the similar reports by other authors.19, 21, 25, 27
The average duration of hospital stay in the current study was 5.54±3.76 days (range 2-35) days. It was also seen that there is preponderance for increased incidence of pDDIs in the population as the duration of stay increases. A linear correlation was found (r= 0.69, and p<0.0173) between length of stay and percentage of drug interactions. Available studies also have shown that increased length of stay increases the probability of occurrences pDDIs.22 This might be because the chance of getting multiple drug increases with longer stays in the hospital which in turn increase the risk for pDDIs.
The class of drugs most commonly involved in pDDIs according to the anatomical therapeutic code (ATC Code) were antiplatelets, anticoagulants and diuretics. These three classes together accounted for 71% of the identified pDDIs. This might be due frequent use of this drug class among the cardiac patients in the present study. This study found an increased risk of bleeding associated with pDDIs among patients prescribed thrombolytic agents. The increased risk was observed when aspirin was combined with other thrombolytic agents. It was found that aspirin was involved in total of 44.84% (174) of pDDIs, which was slightly more than the reported incidence by other authors.26, 28, 29
Proper management of DDIs is based on recognition the pDDIs and consequently taking the suitable measures like therapeutic drug monitoring and dose adjustment, inclusion of protective agents like omeprazole for gastric protection to reduce the likelihood of an adverse outcome.26 Most of the interacting combinations in present study like aspirin/heparin, clopidogrel/heparin, heparin/warfarin, heparin/streptokinase might increase the risk of bleeding. There is a need for proper monitoring of PT, aPTT, and INR in patients with these combinations. The actual interactions observed also reflected this with more bleeding incidents involving anti-coagulants like heparin and warfarin.
Similarly when potassium supplements are given in combination with potassium-sparing diuretics, ACE inhibitors, and angiotensin receptor blockers, there is a need for regular monitoring of serum potassium level and renal function. Digoxin was also found to interact with number of drugs. When patients are on combinations involving digoxin, monitoring the level of this drug may help to avoid potential toxicity by suitable dosage adjustment. In this scenario of increased risk of interactions and potential adverse outcomes in cardiology patients, there is a need to enhance the monitoring of patients with pDDIs and take suitable preventive measures.
Conclusions
This study showed that the overall incidence of pDDIs was 30.67%. Out of the documented pDDIs adverse drug interaction was observed in 17% of pDDIs. It was observed that prevalence of pDDIs increased linearly with number of drugs and length of stay. Increased number of pDDIs was noted in patients older than 80 years of old. The majority of interactions were pharmacodynamic in nature, having moderate severity. Anti-platelets and anti-coagulants were commonly implicated in many PDDIs in this study and therefore require intensive monitoring during therapy. This study provided a reference data for the surveillance of pDDIs in cardiac patients. Development of similar data base in other Indian and South Asian centers might help to evaluate the economic, clinical, and humanistic outcomes of clinically important DDIs in the South Asian context.
ACKNOWLEDGEMENTS
The authors would like to acknowledge all health care professional of Kasturba Hospital, Manipal University and Staff of Department of Pharmacy Practice, Manipal College of Pharmaceutical Sciences, Manipal University for the support and encouragement extended for this work.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors have no competing interests.
FUNDING
None
Please cite this paper as: Patel V K, Acharya L D, Rajakannan T, Mallayasamy S, Guddttu V, Padmakumar R. Potential drug interactions in patients admitted to cardiology wards of a south Indian teaching hospital. AMJ 2011, 4, 1, 9-14 DOI:http//dx.doi.org/10.4066/AMJ.2011.450
References
- 1.Johnson JA, Bootman JL.. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155:1949–56. [PubMed] [Google Scholar]
- 2.Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA. et al. The nature of adverse events in hospitalized patients: results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 3.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH.. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641–651. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton RA, Briceland LL, Andritz MH.. Frequency of hospitalization after exposure to known drug-drug interactions in a Medicaid population. Pharmacotherapy. 1998;18:1112–20. [PubMed] [Google Scholar]
- 6.Delafuente JC.. Understanding and preventing drug interactions in elderly patients. Critical Reviews in Oncology/Hematology. 2003;48:133–43. doi: 10.1016/j.critrevonc.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Costa AJ.. Potential drug interactions in an ambulatory geriatric population. Fam Pract. 1991;8:234–236. doi: 10.1093/fampra/8.3.234. [DOI] [PubMed] [Google Scholar]
- 8.Ratz Bravo AE, Tchambaz L, Krahenbuhl-Melcher A, Hess L, Schlienger RG, Krahenbuhl S.. Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf. 2005;28:263–275. doi: 10.2165/00002018-200528030-00007. [DOI] [PubMed] [Google Scholar]
- 9.Gosney M, Tallis R.. Prescription of contraindicated and interacting drugs in elderly patients admitted to hospital. Lancet. 1984;2:564–567. doi: 10.1016/s0140-6736(84)90775-x. [DOI] [PubMed] [Google Scholar]
- 10.Manchon ND, Bercoff E, Lemarchand P, Chassagne P, Senant J, Bourreille J.. Incidence and severity of drug interactions in the elderly: a prospective study of 639 patients. Rev Med Interne. 1989;10:521–525. doi: 10.1016/s0248-8663(89)80069-4. [DOI] [PubMed] [Google Scholar]
- 11.Wiltink EH.. Medication control in hospitals: a practical approach to the problem of drug-drug interactions. Pharm World Sci. 1998;20:173–177. doi: 10.1023/a:1008652812023. [DOI] [PubMed] [Google Scholar]
- 12.Gronroos PE, Irjala KM, Huupponen RK, Scheinin H, Forsstrom J, Forsstrom JJ.. A medication database-a tool for detecting drug interactions in hospital. Eur J Clin Pharmacol. 1997;53:13–7. doi: 10.1007/s002280050330. [DOI] [PubMed] [Google Scholar]
- 13.Straubhaar B, Krahenbuhl S, Schlienger RG.. The prevalence of potential drug-drug interactions in patients with heart failure at hospital discharge. Drug Saf. 2006;29:79–90. doi: 10.2165/00002018-200629010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Egger SS, Drewe J, Schlienger RG.. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58:773–778. doi: 10.1007/s00228-002-0557-z. [DOI] [PubMed] [Google Scholar]
- 15.Kohler GI, Bode-Boger SM, Busse R, Hoopmann M, Welte T, Boger RH.. Drug-drug interactions in medical patients: effects of in hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13. doi: 10.5414/cpp38504. [DOI] [PubMed] [Google Scholar]
- 16.Disease Burden in India. Estimations and causal analysis. Available at: http://www.whoindia.org/LinkFiles/Commision_on_Ma croeconomic_and_Health_Bg_P2Burden_of_Disease_Es timations_and_Casual_analysis.pdf. Accessed on 21/11/09.
- 17.Frazier SC.. Health outcomes and polypharmacy in elderly individuals: An integrated literature review. J Gerontol Nurs. 2005;31:4–11. doi: 10.3928/0098-9134-20050901-04. [DOI] [PubMed] [Google Scholar]
- 18.Nolan PE Jr, Marcus FI.. Cardiovascular drug use in elderly. Am J Geriatr Cardiol. 2000;9:127–129. doi: 10.1111/j.1076-7460.2000.80021.x. [DOI] [PubMed] [Google Scholar]
- 19.Cruciol-Souza JM, Thomson JC.. Prevalence of potential drug-drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci. 2006;9:427–433. [PubMed] [Google Scholar]
- 20.DRUGDEX® System [database on CD-ROM]. Version 5.1. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc.; [Google Scholar]
- 21.Aparasu R, Baer R, Aparasu A.. Clinically important potential drug-drug interactions in outpatient settings. Research in Social and Administrative Pharmacy. 2007;3:426–437. doi: 10.1016/j.sapharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Mahmood M, Malone DC, Skrepnek GH, Abarca J, Armstrong EP, Murphy JE, Grizzle AJ, Ko Y. Woosley RL Potential drug-drug interactions within Veterans affairs medical centers. Am J Health Syst Pharm. 2007;64:1500–1505. doi: 10.2146/ajhp060548. [DOI] [PubMed] [Google Scholar]
- 23.Carter BL, Lund BC, Hayase N, Chrischilles E.. A Longitudinal Analysis of hypertensive Drug Interactions in a Medicaid Population. Am J Hyp. 2004;17:421–427. doi: 10.1016/j.amjhyper.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Bernhard S, Stephan K, Raymond S.. The prevalence of potential drug-drug interactions in patients with heart failure at hospital discharge. Drug saf. 2006;29:79–90. doi: 10.2165/00002018-200629010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Herrlinger C, Klotz U.. Drug metabolism and drug interaction in the elderly. Best Pract Res Clin Gastroentrol. 2001;15:897–918. doi: 10.1053/bega.2001.0249. [DOI] [PubMed] [Google Scholar]
- 26.Vonbach P, Dubied A, Krahenbuhl S, Beer JH.. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur J Int Med. 2008;19:413–20. doi: 10.1016/j.ejim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Weideman RA, McKinney WP, Bernstein IH.. Predictors of potential drug interactions. Hosp Pharm. 1998;33:835–40. doi: 10.1093/ajhp/56.15.1524. [DOI] [PubMed] [Google Scholar]
- 28.Pote S, Tiwari P, D'Cruz S.. Medication prescribing errors in a public teaching hospital in India: A prospective study. Pharmacy Practice. 2007;5:17–20. doi: 10.4321/s1886-36552007000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan FA, Taylor DM, Leow FH, Doolan G, Knott JC.. Potential interactions between drugs taken by emergency department patients of an Australian hospital. J Pharmacy Practice and Research. 2006;36:266–70. [Google Scholar]


