Abstract
Background
Alterations in brain density and signaling associated with monoamine receptors are believed to play a role in depressive disorders. This study evaluates the functional status of α2A-adrenoceptors in postmortem frontal cortex of depressed subjects.
Methods
G-protein activation and inhibition of adenylyl cyclase (AC) activity induced by the α2-adrenoceptor agonist UK14304 were measured in triplicate in samples from 15 suicide victims with an antemortem diagnosis of major depression and 15 matched control subjects.
Results
Basal [35S] guanosine γ thio-phosphate (GTPγS) binding and cyclic adenosine monophosphate accumulation did not differ between groups. In depressed victims, an increase in [35S] GTPγS binding potency (EC50 = .58 μmol/L vs. EC50 = 3.31 μmol/L; p < .01; depressed vs. control) and a significant reduction in the maximal inhibition of AC activity (Imax = 27 ± 4% vs. Imax = 47 ± 5%; p < .01) were observed after incubation with the α2-adrenoceptor agonist UK14304. No differences were found between antidepressant-free and antidepressant-treated subjects. A significant relationship between EC50 values for [35S] GTPγS and Imax values for AC assay was found (n = 30; r = −.43; p < .05).
Conclusions
The dual regulation of α2A-adrenoceptor signaling pathways raises the possibility that factors affecting the G-protein cycle and/or selective access of Gαi/o–protein to AC might be relevant to receptor abnormalities in depression, providing further support for the involvement of α2A-adrenoceptors in the pathogenesis of depression.
Keywords: adenylyl cyclase, α2-adrenoceptor, cAMP, depression, G-protein, human brain
The biochemical bases of depressive disorders have classically focused on the role of serotonin and norepinephrine and their specific receptors (1). The α2-adrenoceptors are of special interest, because they regulate the activity of monoaminergic neurons (2), acting via inhibitory G–proteins (Gi/o), resulting in—among others—the inhibition of the adenylyl cyclase (AC) activity. An upregulation of α2-adrenoceptors has been reported in brain of depressed subjects and/or suicide victims (3,4) as well as in platelets of depressed untreated patients (5). An increased α2A-adrenoceptor messenger RNA in prefrontal cortex of suicide victims has also been reported (6). Moreover, a higher functional sensitivity of α2-adrenoceptors on agonist-stimulation of [35S] guanosine 5′-[γ-thio]triphosphate (GTPγS) coupling in brain samples (7) and clonidine-dependent increase of cerebral blood flow (8) has been reported in depression. In agreement with these data, chronic administration of antidepressants results in a reduced functional activity of α2-adrenoceptors in rat brain (2) and human platelets (5,9). Under these premises, association of several polymorphisms of the genes encoding the different α2-adrenoceptor subtypes with depression and suicide are being evaluated (10,11).
Several studies have investigated the basal level of AC activity and G-protein subunit densities in brain tissue from depressed suicide victims (7,12,13). Some studies in platelets have suggested the lack of alterations (9) or, alternatively, subsensitivity in the α2-adrenoceptor–induced inhibition of cyclic adenosine monophosphate (cAMP) formation in major depression (14). However, no data are available on the response of the AC system to α2-adrenoceptors in brain of depressed subjects. The aim of this study was to examine, in the same group of cortical tissue samples from a population of suicide victims with antemortem diagnosis of major depression, the α2-adrenoceptor– dependent G-protein activation and AC activity process and the possible influence of the pharmacological treatment in the modulation of α2-adrenoceptor response.
Methods and Materials
Subject Selection
Brain samples were dissected at autopsy from 15 suicide victims and 15 control subjects in whom the medical examiner had reliably determined the cause of death (Table 1 and Supplement 1). Suicide and control subjects were individually matched by gender, age, postmortem delay, and freezing storage time.
Table 1.
Demographic Characteristics, Diagnoses, Prescribed Treatment, and Toxicological Analysis of Individual Cases of Suicide Victims with Depressive Disorders and Their Respective Control Subjects
| Case | Gender (F/M) | Age (yrs)a | PM (h) | Cause of Death | Treatment (prescription at death) | Drug Blood Levels (μg/mL) |
|---|---|---|---|---|---|---|
| Case 1b | F | 35 | 39 | Caustic intoxication | TMT, SMZ | negative |
| Control 1b | F | 33 | 44 | Motor vehicle accident | negative | |
| Case 2b | M | 73 | 60 | Gunshot wound | ATD, BDZ | CIT (.1) |
| Control 2b | M | 79 | 66 | Motor vehicle accident | negative | |
| Case 3b | F | 72 | 49 | Jumping from a height | Untreated | negative |
| Control 3 | F | 79 | 39 | Motor vehicle accident | negative | |
| Case 4b | M | 65 | 30 | Drug intoxication | APS, ATD, BDZ | IMI (.17); TIA (13); SUL(3.3) terbinafine (1.17); EtOH (2 g/L) |
| Control 4 | M | 65 | 50 | Motor vehicle accident | negative | |
| Case 5 | F | 58 | 27 | Hanging | ATD, BDZ | negative |
| Control 5b | F | 58 | 37 | Motor vehicle accident | BDZ, PC | negative |
| Case 6 | M | 42 | 20 | Jumping from a height | APS, ATD | Clotiapine; BDZ, Metamizol |
| Control 6 | M | 41 | 19 | Work accident | negative | |
| Case 7b | F | 88 | 9 | Jumping from a height | ATD, BDZ | SER (.03) |
| Control 7 | F | 81 | 19 | Cardiac arrest | negative | |
| Case 8b | F | 68 | 25 | Jumping from a height | ATD | SER (.4) |
| Control 8b | F | 68 | 38 | Motor vehicle accident | ASA | negative |
| Case 9 | F | 64 | 27 | Jumping from a height | ATD, BDZ | MIA; CIT |
| Control 9 | F | 66 | 16 | Motor vehicle accident | negative | |
| Case 10 | F | 64 | 25 | Jumping from a height | APS, ATD, BDZ | CIT |
| Control 10 | F | 67 | 35 | Cardiac arrest | negative | |
| Case 11b | F | 71 | 19 | Jumping from a height | TEO, ASA | TEO, AAS |
| Control 11 | F | 70 | 18 | Motor vehicle accident | negative | |
| Case 12 | F | 73 | 18 | Jumping from a height | LIT, BDZ | Not performed |
| Control 12b | F | 74 | 19 | Motor vehicle accident | Paracetamol | |
| Case 13 | M | 43 | 34 | Hanging | ATD, BDZ | CIT, BDZ |
| Control 13 | M | 44 | 21 | Motor vehicle accident | negative | |
| Case 14 | M | 43 | 15 | Train jumping | ATD, BDZ | BDZ |
| Control 14 | M | 43 | 10 | Motor vehicle accident | negative | |
| Case 15 | M | 73 | 17 | Drowning | APS, ATD | AMI; NOR; Trazodone; BDZ; Ibuprofen |
| Control 15 | M | 71 | 14 | Blast accident | Not performed |
Total depressed suicide subjects: nine women, six men, 62 ± 4 years old; 28 ± 4 hours postmortem delay (PM). Total control subjects: nine women, six men, 63 ± 4 years old; 30 ± 4 hours PM. Group values are means ± SEM. Blood levels when performed quantitatively are expressed in μg/mL. Blood levels of ethanol (EtOH) are expressed in g/L. Several benzodiazepines (BDZ) and metabolites were also detected in blood samples. Demographic parameters of depressed suicide and control subjects were compared by Student t test. Analysis of covariance was performed to control results for gender, age at death, postmortem delay, storage time, and the presence or absence of antidepressant drugs.
TMT, trimetroprim; SMZ, sulfametoxazol; ATD, antidepressants; CIT, citalopram; APS, antipsychotics; IMI, imipramine; TIA, tiapride; SUL, sulpiride; PC, piroxicam; SER, sertraline; ASA, acetylsalicylic acid; MIA, mianserin; TEO, theophylline; LIT, lithium; AMI, amitriptyline; NOR, nortriptyline.
Age at death in years.
Case also used in the study of González-Maeso et al., 2002.
Membrane Preparation
Samples were processed under code to blind the diagnostic group of the subjects. For [35S] GTPγS binding and AC assays, membrane preparation (P2 fraction) was obtained as described previously (7,15). For detailed protocols see Supplement 1.
[35S] GTPγS Binding and AC Assays
The α2-adrenoceptor–mediated functional G-protein activity was assessed by the [35S] GTPγS binding assay as described previously (7). The AC assay protocol was modified from one previously described (15) (Supplement 1).
Analysis of Data
Demographic parameters of depressed and control subjects were compared by Student t test. Differences in [35S] GTPγS binding assays or cAMP accumulation parameters between subjects with depression and control subjects were evaluated by unpaired Student t test. Pearson’s coefficient of correlation was calculated to evaluate the relationship between Imax cAMP inhibition and EC50 [35S] GTPγS binding values. One-way analysis of variance test, followed by Newman–Keuls post hoc test, was used to discriminate between the antidepressant-free and antidepressant-treated subgroups. The level of significance was chosen at p < .05. For more details see Supplement 1.
Results
α2-Adrenoceptor–Mediated [35S] GTPγS Binding and AC Activity Inhibition
Because subjects had been matched for gender, age at death, and postmortem delay, the analysis of covariance also confirmed the lack of influence of these parameters on [35S] GTPγS binding and AC activity.
The concentration–response curve for agonist UK14304-induced [35S] GTPγS binding in the depression group was shifted to the left (normalized −log EC50 = 6.2 ± .2) when compared with that obtained in the control group (normalized −log EC50 = 5.5 ± .2; t = 2.93, p < .01) (Figure 1A), whereas the Emax. value did not display significant changes (1338 ± 141 fmol/mg protein vs. 1311 ± 96 fmol/mg protein, in the suicide and control groups, respectively) (for basal [35S] GTPγS binding values, see Table S1 in Supplement 1). The simultaneous fitting of stimulatory concentration–response curves confirmed the presence of differences between depressed suicide subjects and control subjects [F (3,247) = 3.038, p < .05]. The differences were ascribed to a higher potency in the suicide group [F (1,247) = 3.962, p < .05] without changes in Emax. parameters [F (1,247) = .746, p > .05]. The tendency to a higher potency (lower EC50 values) was evident in both antidepressant-free (−logEC50 = 6.2 ± .2 vs. 5.4 ± .1 in matched control subjects) and antidepressant-treated (−logEC50 = 6.3 ± .2 vs. 5.5 ± .3) subgroups, although without reaching statistical significance [F (3,26) = 2.736; p = .06].
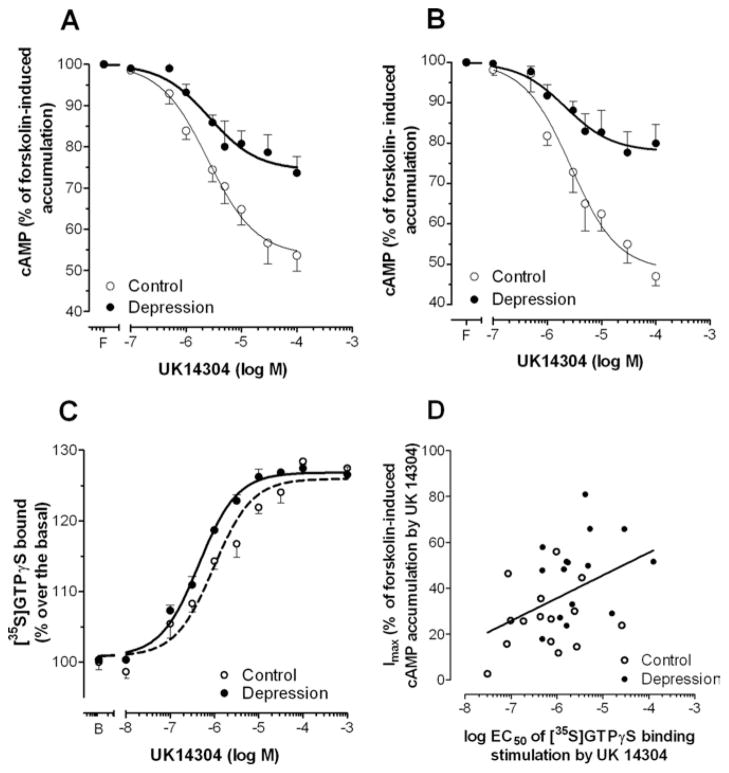
Figure 1.
The α2-adrenoceptor– dependent cyclic adenosine monophosphate (cAMP) accumulation and [35S] guanosine 5′-[γ-thio]triphosphate (GTPγS) binding in membranes of frontal cortex of depressed (closed circles) and matched control subjects (open circles). (A) Concentration–response curves of the inhibition of the forskolin-induced cAMP accumulation by the α2-adrenoceptor agonist UK14304 (10−7–10−4 mol/L). Points represent mean ± SEM of total group (15 depressed subjects and 15 control subjects). (B) Concentration–response curves of the inhibition of the forskolin-induced cAMP accumulation by the α2-adrenoceptor agonist UK14304 (10−7–10−4 mol/L) in the antidepressant-free group (seven depressed subjects and seven matched control subjects). F represents basal UK14304-free forskolin-stimulated cAMP values. (C) Concentration–response curves of the [35S] GTPγS binding stimulation by theα2-adrenoceptor agonist UK14304 (10−8–10−3 mol/L) in depressed suicide victims (solid line) and matched control subjects (broken line). Points represent mean ± SEM of 15 depressed suicide victims and 15 control subjects. B represents basal [35S] GTPγS binding values. (D) Linear correlation between normalized (logEC50) values for UK14304-induced [35S] GTPγS binding stimulation and the maximal inhibitory effect on forskolin-induced cAMP accumulation (% Imax.) promoted by UK14304 in the whole group of subjects analyzed (n = 30).
The incubation with increasing concentrations of the α2-adrenoceptor agonist UK14304 resulted in a concentration-dependent decrease of forskolin-induced cAMP accumulation in both control and depressed suicide groups (Figure 1B). The Imax value (maximal inhibition of forskolin-stimulated cAMP production) to UK14304 was significantly lower in the depressed suicide group (27 ± 4%) than in control subjects (47 ± 5%) (t = 3.40, p < .01) No differences in the potency of the α2-adrenoceptor agonist UK14304 to inhibit AC activity between control (normalized −logIC50 = 5.6 ± .1) and depressed suicide (normalized −logIC50 = 5.6 ± .2) groups were observed (Figure 1B) (for basal cAMP and forskolin-induced cAMP accumulation, see Table S1 in Supplement 1). The simultaneous analysis of inhibitory concentration–response curves indicated the existence of differences between depressed suicide and control subjects [F (3,246) = 23.80, p < .0001]. The differences were ascribed to a lower maximal inhibitory effect (Imax) in the depression group [F (1,246) = 33.23, p < .001] without changes in IC50 values [F (1,246) = 2.179, p > .05]. With regard to the existence of antidepressant treatment, the results from antidepressant-free (Imax = 22 ± 1% vs. Imax = 51 ± 3% in matched control group) and antidepressant-treated (Imax = 30 ± 3% vs. Imax = 47 ± 2% in matched control group) subjects showed a tendency to a decrease of forskolin-induced cAMP accumulation in both subgroups [F (3,26) = 3.83, p < .05], although it only reached statistical significance in the antidepressant-free group (Figure 1B, insert).
Relationship Between α2-Adrenoceptor–mediated [35S] GTPγS Binding Stimulation and AC Inhibition
A significant and positive correlation was found between the normalized EC50 values for UK14304-induced [35S] GTPγS binding stimulation and the maximal inhibitory effect on forskolin-stimulated AC (% Imax) induced by the agonist in the whole group of subjects analyzed (n = 30; r = .43, p < .05) (Figure 1C).
Discussion
The results of this study strongly support the role of α2-adrenoceptor–mediated signaling in the pathophysiology of depressive disorders. Our data demonstrate a dual modification in the frontal cortex of depressed suicide victims with an increase in the α2A-adrenoceptor–mediated G-protein coupling and desensitization in the AC inhibition induced by this receptor.
The higher potency of the α2-adrenoceptor agonist UK14304 to promote the exchange of guanosine 5′-diphosphate by [35S] GTPγS in brain of depressed subjects indicates a supersensitive receptor coupling to inhibitory Gαi/o-proteins (Figure 1A). The finding validates the results of a previous study (7) and demonstrates that the α2A-adrenoceptor is the concrete subtype involved in this response (Figure S1 in Supplement 1). Early works have repeatedly demonstrated by receptor radioligand binding assays a greater α2-adrenoceptor density in the brain of depressed patients (3,4). In the depressed group displaying a supersensitive α2A-adrenoceptor coupling to G proteins, the cAMP accumulation revealed a lower α2A-adrenoceptor efficacy, suggestive of a subsensitive α2A-adrenoceptor signaling at this level (Figure 1). Similar results have been observed in platelets by some but not all authors (9,14). The discrepancy between [35S] GTPγS binding stimulation and AC inhibition has also been found in other biological paradigms, such as the cannabinoid CB1 receptor modulation by antidepressant drugs (15).
The present results demonstrate the existence of a positive relationship between the potency values for UK14304-induced G-protein coupling (EC50) and the maximal efficacy of AC inhibition by the same agonist (Imax), suggesting that both findings could be the consequence of a single altered phenomenon affecting signaling pathways (Figure 1C). The existence of this relevant modification of the α2A-adrenoceptor– dependent transduction signaling in depression is compatible with several hypotheses, including the existence of overexpression and/or functional hypersensitivity of regulators of G-protein signaling in brain of depressed suicide victims: these proteins accelerate the endogenous GTPase activity of Gαi/o-proteins, reducing maximal agonist inhibition of AC (16) and other signaling cascades. Other explanations include the heterogeneity of brain AC isoforms showing different sensitivities to G protein subunits or the influence of PTX-insensitive proteins (i.e., Gαq/11, Gαz, and/or Gβ γ dimmers in the decreased response to AC), as [35S] GTPγS binding assays are restricted to guanine nucleotide exchange on Gαi/Gαo-protein subunits (17). Finally, the possibility exists of an increased Gαi/Gαo-protein localization in lipid raft membranes with increased access to α2-adrenoceptors and reduced availability to interact with AC, a mechanism already proposed to explain the altered Gαs-protein status in brain from depressed subjects (18).
Chronic treatment with antidepressants has been shown to modify α2-adrenoceptor–mediated activity in rat brain (2) and human platelets (5,9). In our experiments, no significant differences were observed between antidepressant-free and antidepressant-treated subjects either with respect to the enhancement of the agonist potency to induce G-protein coupling or with regard to the decreased maximal inhibition of forskolin-induced cAMP accumulation in both groups, probably due to the reduced number of cases.
The modification in the α2-adrenoceptor– dependent cellular transduction in the brain of subjects with depression is of particular interest, taking into account the increasing evidence of involvement of downstream targets of the cAMP cascade in affective disorders (i.e., cAMP response element-binding protein) (19). The recent finding of enhanced antidepressant effect on neurogenesis and neurotrophism by α2-adrenoceptor antagonist drug administration holds the critical role of this receptor in depression (20). In this regard, the full understanding of the nature of the complex regulation of α2-adrenoceptor–mediated noradrenergic functionality in depression will contribute to a better knowledge of both the biological basis and the pharmacological treatment of the disease.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia e Innovacion–Fondo Europeo de Desarrollo Regional (SAF 04/2784 and SAF 09/8460 to JJM; SAF 04/00941 and SAF07/61862 to AP, and SAF08/01311 and RETICS-Trastornos Adictivos to JAG-S), The Basque Government (IT199-07) and the Centro de Investigación Biomédica en Red de Salud Mental CIBERSAM, Instituto de Salud Carlos III. The cooperation of the staff members of the Basque Institute of Legal Medicine and the Romand University Center of Legal Medicine at Geneva is acknowledged.
The authors JAG-S and JJM have received support for research from Lilly Laboratories in the past 3 years. The author JJM has received compensation as a collaborator with the companies Lilly and Brainco Biopharma. The authors AP and EMV have received support for research from Faes Farma, S.A.
Footnotes
The authors RD-A, JG-M, and FP-C reported no biomedical financial interests or potential conflicts of interest. The present study is not related to any of these professional or collaborative relationships.
Supplementary material cited in this article is available online.
References
- 1.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:98S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 2.Mongeau R, de Montigny C, Blier P. Electrophysiologic evidence for desensitization of α2-adrenoceptors on serotonin terminals following long-term treatment with drugs increasing norepinephrine synaptic concentration. Neuropsychopharmacology. 1994;10:41–51. doi: 10.1038/npp.1994.6. [DOI] [PubMed] [Google Scholar]
- 3.Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, García-Sevilla JA. Selective increase of α2A-adrenoceptor agonist binding sites in brains of depressed suicide victims. J Neurochem. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- 4.De Paermentier F, Mauger JM, Lowther S, Crompton MR, Katona CLE, Horton RW. Brain α-adrenoceptors in depressed suicides. Brain Res. 1997;757:60– 68. doi: 10.1016/s0006-8993(97)00138-8. [DOI] [PubMed] [Google Scholar]
- 5.García-Sevilla JA, Ventayol P, Pérez V, Rubovszky G, Puigdemont D, Ferrer-Alcón M, et al. Regulation of platelet α2A-adrenoceptors, Gi proteins and receptor kinases in major depression: Effects of mirtazapine treatment. Neuropsychopharmacology. 2004;29:580–588. doi: 10.1038/sj.npp.1300356. [DOI] [PubMed] [Google Scholar]
- 6.Escribá PV, Ozaita A, García-Sevilla JA. Increased mRNA expression of α2A-adrenoceptors, serotonin receptors and μ-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 7.González-Maeso J, Rodríguez-Puertas R, Meana JJ, García-Sevilla JA, Guimón J. Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: Selective supersensitivity of α2A-adrenoceptors. Mol Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- 8.Fu CHY, Reed LJ, Meyer JH, Kennedy S, Houle S, Eisfeld BS, Brown GM. Noradrenergic dysfunction in the prefrontal cortex in depression: An [15O] H2O PET study of the neuromodulatory effects of clonidine. Biol Psychiatry. 2001;49:317–325. doi: 10.1016/s0006-3223(00)01050-7. [DOI] [PubMed] [Google Scholar]
- 9.García-Sevilla JA, Padró D, Giralt MT, Guimón J, Areso P. α2-adrenoceptor-mediated inhibition of platelet adenylate cyclase and induction of aggregation in major depression. Effect of long-term cyclic antidepressant drug treatment. Arch Gen Psychiatry. 1990;47:125–132. doi: 10.1001/archpsyc.1990.01810140025005. [DOI] [PubMed] [Google Scholar]
- 10.Martín-Guerrero I, Callado LF, Saitua K, Rivero G, García-Orad A, Meana JJ. The N251K functional polymorphism in the α2A-adrenoceptor gene is not associated with depression: A study in suicide completers. Psychopharmacology. 2006;184:82– 86. doi: 10.1007/s00213-005-0266-2. [DOI] [PubMed] [Google Scholar]
- 11.Perroud N, Aitchison KJ, Uher R, Smith R, Huezo-Diaz P, Marusic A, et al. Genetic predictors of increase in suicidal ideation during antidepressant treatment in the GENDEP project. Neuropsychopharmacology. 2009;34:2517–2528. doi: 10.1038/npp.2009.81. [DOI] [PubMed] [Google Scholar]
- 12.Lowther S, Crompton MR, Katona CLE, Horton RW. GTPγS and forskolin-stimulated adenylyl cyclase activity in post-mortem brain from depressed suicides and controls. Mol Psychiatry. 1996;1:470– 477. [PubMed] [Google Scholar]
- 13.Valdizán EM, Gutierrez O, Pazos A. Adenylate cyclase activity in postmortem brain of suicide subjects: Reduced response to β-adrenergic stimulation. Biol Psychiatry. 2003;54:1457–1464. doi: 10.1016/s0006-3223(03)00589-4. [DOI] [PubMed] [Google Scholar]
- 14.Roy A, Kafka MS. Platelet adrenoceptors and prostaglandin responses in depressed patients. Psychiatry Res. 1989;30:181–189. doi: 10.1016/0165-1781(89)90159-5. [DOI] [PubMed] [Google Scholar]
- 15.Mato S, Vidal R, Castro E, Diaz A, Pazos A, Valdizán EM. Long-term fluoxetine treatment modulates cannabinoid type 1 receptor-mediated inhibition of adenylyl cyclase in the rat prefrontal cortex through 5-hydroxytryptamine 1A receptor-dependent mechanisms. Mol Pharmacol. 2010;77:424– 434. doi: 10.1124/mol.109.060079. [DOI] [PubMed] [Google Scholar]
- 16.Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR. Endogenous RGS protein action modulates μ-opioid signaling through Gαo. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem. 2003;278:9418–9425. doi: 10.1074/jbc.M208885200. [DOI] [PubMed] [Google Scholar]
- 17.Milligan G. Principles: Extending the utility of [35S]GTPγS binding assays. Trends Pharmacol Sci. 2003;24:87–90. doi: 10.1016/s0165-6147(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 18.Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN, Rasenick MM. Postmortem brain tissue of depressed suicides reveals increased Gsα localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J Neurosci. 2008;28:3042–3050. doi: 10.1523/JNEUROSCI.5713-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, et al. α2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.