Abstract
Food intake and body weight are regulated by a complex system of neural and hormonal signals, of which the anorexigenic neurotransmitter serotonin (5-hydroxytryptamine or 5-HT) is central. In this study, rat models of obesity and weight loss intervention were compared with regard to several 5-HT markers. Using receptor autoradiography, brain regional-densities of the serotonin transporter (SERT) and the 5-HT2A and 5-HT4 receptors were measured in (i) selectively bred polygenic diet-induced obese (pgDIO) rats, (ii) outbred DIO rats, and (iii) Roux-en-Y gastric bypass (RYGB)-operated rats. pgDIO rats had higher 5-HT4 and 5-HT2A receptor binding and lower SERT binding when compared to polygenic diet-resistant (pgDR) rats. The most pronounced difference between pgDIO and pgDR rats was observed in the nucleus accumbens shell (NAcS), a brain region regulating reward aspects of feeding. No differences were found in the 5-HT markers between DIO rats, chow-fed control rats, and DIO rats experiencing a weight loss. The 5-HT markers were also similar in RYGB and sham-operated rats except for a downregulation of 5-HT2A receptors in the NAcS. The higher receptor and lower SERT binding in pgDIO as compared to pgDR rats corresponds to what is reported in overweight humans and suggests that the dysfunctions of the 5-HT system associated with overeating or propensity to become overweight are polygenically determined. Our results support that the obesity-prone rat model has high translational value and suggests that susceptibility to develop obesity is associated with changed 5-HT tone in the brain that may also regulate hedonic aspects of feeding.
INTRODUCTION
Appetite and body weight are regulated by a complex system of peripheral and central signals acting on the homeostatic and hedonic systems of the brain to trigger hunger or satiety. Perturbations in any of these systems can lead to obesity and associated diseases including type 2 diabetes mellitus and cardiovascular diseases. Gastrointestinal hormones, neuropeptides, and neurotransmitter systems are involved in homeostatic regulation of energy balance. However, there are also hedonic aspects of feeding behavior, such as reward; those studies have largely focused on the opioid and dopaminergic systems.
Serotonin (5-hydroxytryptamine or 5-HT) suppresses food intake and especially the 5-HT2C and 5-HT1B receptors have been implicated in appetite regulation (1). Other 5-HT receptors, including the 5-HT2A and 5-HT4 receptors, are, however, also involved in feeding. 5-HT2A receptor agonism suppresses feeding (2,3), and 5-HT2A receptor activation in the hypothalamus has also been shown to inhibit neuropeptide Y-induced feeding (4). In outbred diet-induced obese (DIO) rats (5), as well as in mice modeling the dietary-genetic aspect of obesity (6), 5-HT2A receptor upregulation takes place. The 5-HT4 receptors have also been implicated in appetite and body weight regulation. Injection of the 5-HT4 receptor agonist BIMU8 into the nucleus accumbens (NAc) decreases food intake in fed and food-deprived mice and administration of the 5-HT4 receptor antagonist RS39604 into the NAc increases food intake in fed, but not in food-deprived mice (7). Moreover, 5-HT4 receptor knockout mice exhibit reduced response to stress- or 3,4- Methylenedioxymethamphetamine (MDMA)-induced anorexia as compared to wild-type mice (7,8), and 5-HT4 receptor antagonism using RS39604 in the NAc reduces MDMA-induced food intake suppression (9).
In humans, we have previously reported a positive correlation between BMI and 5-HT2A receptor binding (10), 5-HT4 receptor binding (11) and an inverse correlation between BMI and serotonin transporter (SERT) binding (12) as measured by in vivo imaging with positron emission tomography. These associations were detected across multiple brain regions and are suggested to represent a compensatory mechanism in response to low 5-HT activity in the obese state, as the 5-HT2A and 5-HT4 receptor binding are upregulated in response to low extracellular 5-HT levels (13–16).
Different animal models are used to study aspects of ingestive behavior and obesity, including the outbred DIO rodents. In DIO rodents, dietary manipulations such as feeding high fat (HF) or cafeteria diet induce obesity (17). Whereas these models may be useful for elucidating certain aspects of obesity, they do not mirror human obesity that is caused by a combination of hereditary, environmental, and behavioral factors. In some outbred Sprague-Dawley rats, ~50% of the cohort becomes obese (obesity-prone) when exposed to a high energy (HE; 31% fat, 25% sucrose) diet, whereas the other half exhibit no significant weight gain (diet-resistant) as compared to chowfed controls (18). This has led to the selective rat breeding for polygenic high (pgDIO) and low (pgDR) weight gainers (19). The pgDIO rats share a number of traits with human obese subjects, including polygenic inheritance, susceptibility to glucose intolerance, insulin resistance and hyperleptinemia, increased carcass adiposity and, once established, persistence of obesity (19,20).
While different treatment schemes for obesity currently are used, bariatric surgery is the only treatment of obesity resulting in a substantial and sustained weight loss with a proven mortality benefit (21). The Roux-en-Y gastric bypass (RYGB) is one of the most common and effective of these procedures. RYGB-induced weight loss is caused by decreased food intake, increased energy expenditure, and altered endocrine signaling via the gut-brain axis (22). However, changes in brain circuitry and neurochemistry after RYGB surgery are rather unexplored.
The aim of this study was to perform a comparative analysis of three individual animal models of obesity and weight loss intervention with regard to brain regional SERT, 5-HT2A, and 5-HT4 receptor binding in order to determine the extent of 5-HT dysfunction in these animal models. In neuroimaging studies, we have recently demonstrated changes in cerebral 5-HT markers in relation to BMI (10–12) and therefore, the translational value of the animal models was also assessed.
METHODS AND PROCEDURES
Animals
Outbred DIO rats
Five-week-old male Sprague-Dawley rats (~150 g; n = 24) (Taconic, Ry, Denmark) were housed in pairs at 24–26 °C under a 12-h light/dark cycle (lights off at 15:00) and placed on an ad libitum HF diet (diet no. D12492; Research Diets, New Brunswick, NJ; containing 5.24 kcal/g with 20% of the energy as protein, 60% as fat, and 20% as carbohydrate) for 10 weeks (23). After 10 weeks, the 24 DIO rats were randomized into two groups: one was continued on HF diet and one was switched to chow (diet no. 1324; Altromin GmbH, Lage, Germany; 2.85 kcal/g with 26.7% of the energy as protein, 12.6% as fat, and 60.7% as carbohydrate). Together with an age-matched group (n = 12) that had previously been fed chow, they were assessed by real-time food intake monitoring for 34 days. For real-time food intake analysis, rats were rehoused and transferred with their partnering rat to fully automated food intake monitoring cages (HM-2; MBRose ApS, Faaborg, Denmark), with standard equipment settings as previously described (23). Briefly, the feeding behavior of each animal was constantly monitored during the habituation period (at least 5 days) to assure that the food intake of each animal returned to pre-rehousing level. On the day of rehousing, the rats were subcutaneously injected with a microchip (no. 402575; eVet, Haderslev, Denmark) allowing the HM-2 food intake monitoring system to simultaneously identify and in real-time mode track feeding behavior of the individual animal throughout the entire experiment. Body weight was determined every second day during the experiment. Body weight and micro-structural food intake analyses were performed using a data reporting software (HMView; MBRose, Faaborg, Denmark). All animal procedures were approved by the Danish Council for Animal Ethics (J. No.2007/561-1343).
Polygenic diet-induced obese and diet-resistant rats
Selectively bred male pgDIO (n = 16) and pgDR (n = 16) Sprague-Dawley rats from the in-house colonies in the New Jersey research laboratory of one of the authors (B.L.) were used. Rats were weaned at 21 days of age and placed ad libitum on a chow diet (no. 5008; Ralston Purina, St. Louis, Mo; 3.30 kcal/g with 23.4% of the energy as protein, 4.5% as fat, and 72.1% as carbohydrate). At 28 days of age, the rats were randomized into two groups per genotype (n = 8 per subgroup): one was continued on chow and one was switched to an ad libitum HE diet (Research Diets no. C11024F, New Brunswick, NJ; 4.47 kcal/g with 21% of the energy content as protein, 31% as fat, and 48% as carbohydrate). Food intake and body weight were monitored every 3–4 days for 60 days. All rats were singly housed at 23–24 °C on a 12-h light/dark cycle (lights off at 18:00). These studies were in compliance with the Animal Care Committee of the East Orange Veterans Affairs Medical Center and the “Guiding Principles for Research Involving Animals and Human beings” of the American Physiological Society.
RYGB-operated rats
Twenty-six male Wistar rats were acclimatized for one week before being operated at a body weight of 469 ± 3 g. The rats were housed individually under a 12-h light/dark cycle (lights off at 19:00) and at a temperature of 19–23 °C. The rats had ad libitum access to chow (Rat and Mouse No.1 Maintenance; Special Diets Services, Essex, UK; 3.52 kcal/g with 17.49% of the energy as protein, 7.42% as fat, and 75.09% as carbohydrate). The rats were randomized to gastric bypass surgery or sham surgery. The gastric bypass procedure was as previously described (22). Briefly, the jejunum was divided 15 cm distal to the pylorus creating a biliopancreatic limb. The cecum was identified and the ileum followed proximally creating a common channel of ~25 cm. At the proximal end of the common channel, a 7-mm-side-to-side jejuno-jejunostomy was made between the biliopancreatic limb and the common channel. The stomach was transected close to the gastrooesophageal junction with vagal sparring leaving the gastric pouch with no more than 3 mm of gastric mucosa. The gastric pouch was anastomosed end-to-side to the alimentary tract. Sham-operated animals had a 7 mm gastrotomy on the anterior wall of the stomach and a 7 mm jejunotomy performed with subsequent closure. All operations were performed by the same surgeon. Body weight and food intake were measured daily over the postoperative period of 60 days. Three RYGB and one sham-operated rat died postoperation, corresponding to the mortality rate associated with this type of operation (24). These rats were part of another project where the effect of fasting was examined. Thus, half the rats were fasted overnight prior to termination. Animal procedures were approved by the Home Office, UK (J. No. PL 70-6669).
Autoradiography
Upon completion of the behavioral experiments, brains were collected from all rats included in the study. All brains were removed rapidly after decapitation and frozen in powdered dry ice and stored at −80 °C. The brains were sectioned on a CM3050S cryostat (Leica, Ballerup, Denmark) in 12-μm-thick coronal sections at −20 °C. The sections were thaw-mounted on Superfrost Plus glass slides (Thermo Scientific, Braunschweig, Germany) and allowed to dry at room temperature (RT) before being stored at −80 °C until further processed. Sections were collected from three brain levels where the following regions were analyzed (i) bregma 1.60 mm: motor cortex, caudate-putamen, and NAc shell (NAcS), (ii) bregma −3.14 mm: hippocampus and hypothalamus, and (iii) bregma −7.8 mm: the dorsal raphe nucleus (25). Total binding and nonspecific binding (NSB) assessment was carried out on slides with representative adjacent brain sections. Sections were thawed for 1 h at RT prior to preincubation. All brain sections were processed together. The autoradiographic protocols were as follows:
5-HT2A receptor binding was measured with the radioligand [3H]MDL100907, kindly provided by Professor Christer Halldin, Karolinska Institute, Stockholm, Sweden. Sections were preincubated for 15 min in 50 mmol/l Tris-HCl buffer containing 0.01% ascorbic acid (pH 7.4) at RT. Following preincubation, sections were incubated for 1 h in 50 mmol/l Tris-HCl containing 0.01% ascorbic acid and 2 nmol/l [3H]MDL100907 (4 times Kd, data not shown) at RT. NSB was determined in the presence of 10 μmol/l ketanserin tartrate (Tocris Biosciences, Bristol, UK). Following incubation, slides were washed in ice-cold 50 mmol/l Tris-HCl (pH 7.4) for 2 × 5 min followed by a 20-s dip in ice-cold distilled water. Slides were dried for 1 h before being fixed in a paraformaldehyde vapor desiccator jar overnight at 4 °C. Hereafter, slides were exposed to a pre-erased BAS TR2040 TR-IP (Science Imaging Scandinavia AB, Nacka, Sweden) for 7 days at 4 °C along with two sets of high and low activity [3H]microscales (GE Healthcare, Hillerød, Denmark). The TR-IP was scanned with a BAS-2500IPR (Fujifilm Europe GmbH, Düsseldorf, Germany).
5-HT4 receptor binding was investigated with [3H]SB207145 (kindly donated by GlaxoSmithKline, Brentford, UK) as previously described (13). Briefly, sections were preincubated in 50 mmol/l Tris-HCl, containing 0.01% ascorbic acid and 10 μmol/l pargyline (pH 7.4) for 15 min at RT. After preincubation, sections were incubated for 1 h at RT in assay buffer with 1 nmol/l [3H]SB207145 added (1–7 times Kd, (26)). Preincubation and incubation buffer for NSB contained 10 μmol/l RS39604 (Tocris). Slides were washed for 2 × 20 s in ice-cold 50 mmol/l Tris-HCl (pH 7.4) followed by a 20-s dip in ice-cold distilled water. The remaining protocol was as described above except for a longer exposure time of 14 days.
SERT binding was assessed with (S)-[N-methyl-3H]citalopram (kindly donated by H. Lundbeck A/S, Copenhagen, Denmark) as previously described (27). Briefly, slides were preincubated for 20 min at RT in 50 mmol/l Tris-HCl, 120 mmol/l NaCl, and 5 mmol/l KCl (pH 7.4). Slides were then incubated for 1 h at RT in buffer with 2 nmol/l (S)-[N-methyl-3H]citalopram (2–3 times kd, (28)). NSB was determined in the presence of 10 μmol/l paroxetine HCl (donated by GlaxoSmithKline). Slides were washed for 3 × 2 min in ice-cold assay buffer followed by a 10-s dip in ice-cold distilled water. The remaining protocol was as described for 5-HT2A receptor autoradiography.
Image analysis
The digital images obtained from scanning the imaging plates were analyzed using ImageJ (Image processing and analysis in Java, http://rsb.info.nih.gov/ij/ version 1.42). The receptor binding was quantified as densitometric measurements of the autoradiograms and the co-exposed [3H]microscale standards were used to calibrate pixel density to radioactivity expressed as nCi/mg tissue equivalents, which was subsequently converted to radioligand binding expressed as fmol/mg tissue equivalents using the decay-corrected specific activity of the ligand. SB was calculated by subtracting NSB from total binding. Regions of interest were free-hand drawn except in the NAcS where a line drawing of the respective section level from a digital version of the rat brain in stereotaxic coordinates (25) was overlaid a representative autora diogram in Adobe Illustrator version CS5 (Adobe, San Jose, CA). The atlas-overlayed autoradiogram was then opened in imageJ and regions of interest's drawn as previously described (13). Slide processing and analyses of autoradiograms were blinded to the investigator.
Statistics
All statistical analyses were done with Prism version 5.02 (GraphPad software, San Diego, CA) with the exception of three-way ANOVAs, which were carried out in SYSTAT version 13 (Systat Software, Chicago, IL).
Food intake and body weight data from the DIO rat experiment were analyzed using a one-way ANOVA, and subgroups were tested against each other using Tukey post hoc tests. Autoradiography data from DIO rats were tested using two-way ANOVA (experimental group × brain region). Thereafter, Bonferroni-corrected t-tests were conducted to test for differences between subgroups.
Food intake and body weight data for pgDIO and pgDR rats were tested using a two-way ANOVA (genotype × diet) with subsequent Bonferroni-corrected t-tests to test for differences between subgroups. A three-way ANOVA was used to analyze autoradiography data for pgDIO and pgDR rats (genotype × diet × brain region). The effects of genotype and region on receptor and SERT binding were tested with a two-way ANOVA with subsequent Bonferroni-corrected t-tests for detection of subgroup differences; only subgroups sharing genotype or diet were compared. Thus, pgDR chow was not tested against pgDIO HE, and pgDR HE was not tested against pgDIO chow.
Two-tailed student's t-tests were used to analyze food intake and body weight data from the RYGB and sham-operated rats. Autoradiography data were tested with two-way ANOVA (operation type × brain region) with subsequent Bonferroni-corrected t-tests for subgroup differences. P values < 0.05 were considered significant. Data are expressed as mean ± s.e.m. unless otherwise stated.
RESULTS
Food intake and body weight analyses
Outbred DIO rats
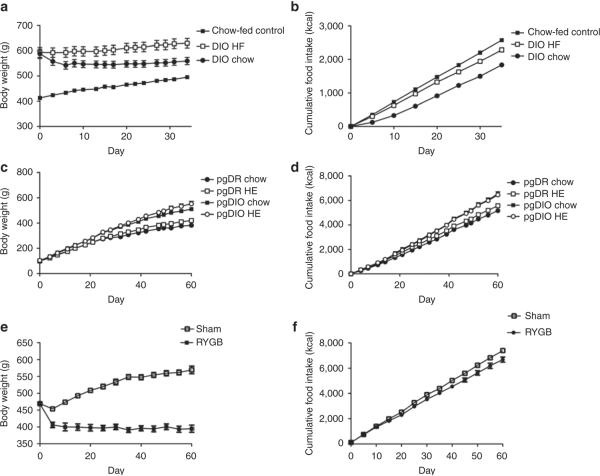
During the period of food intake monitoring, the body weight of DIO HF rats increased over the entire period, while DIO rats switched from HF diet to chow lost weight over the first 10 days without any further changes in body weight throughout the remainder of the experiment (Figure 1a). Chow-fed control rats progressively gained weight according to the normal weight gain curve for rats at this age (Figure 1a). Chow-fed control rats exhibited the highest cumulative body weight gain over the monitoring period. However, DIO HF and DIO chow-fed rats had undergone 10 weeks of HF diet exposure and were thus heavier prior to the feeding regulation period and also showed a significantly higher body weight at termination of the experiment (Table 1). The three groups of rats significantly differed in their cumulative food intakes in parallel with their changes in their body weight (Figure 1b and Table 1).
Figure 1.
Body weight and cumulative food intake for (a,b) outbred DIO rats (n = 12 per group), (c,d) pgDIO and pgDR rats (n = 8 per group), and (e,f) RYGB rats (RYGB: n = 10, sham-operated: n = 12 per group). DIO, diet-induced obese; HE, high energy; HF, high fat; pgDIO, polygenic diet-induced obese; pgDR, polygenic diet-resistant; RYGB, Roux-en-Y gastric bypass.
Table 1.
In vivo data for DIO rats, DIO rats after weight loss, and chow-fed control rats
| Chow-fed control | DIO HF | DIO chow | One-way ANOVA P value | |
|---|---|---|---|---|
| Average daily body weight gain (g) | 2.4 ± 0.087***,### | 1.1 ± 0.079§§§ | −0.85 ± 0.18 | P < 0.0001 |
| Body weight at termination (g) | 495.2 ± 4.0***,## | 629.5 ± 18.9§§ | 559.1 ± 14.5 | P < 0.0001 |
| Average daily food intake (kcal) | 73.8 ± 1.1**,### | 66.3 ± 1.9§§§ | 52.3 ±1.5 | P < 0.0001 |
Data analyzed using one-way ANOVA with Tukey post-test. n = 12 per group.
DIO, diet-induced obese; HF, high fat.
P < 0.01.
P < 0.001 between chow-fed control and DIO HF.
P < 0.01.
P < 0.001 between chow-fed control and DIO chow.
P < 0.01
P < 0.001 between DIO HF and DIO chow.
Polygenic DIO and diet-resistant rats
Prior to the introduction of the HE diet, pgDIO and pgDR rats had similar body weights (pgDR: 100 ± 2 g, pgDIO: 104 ± 2 g). However, during the period where food intake was monitored, pgDIO rats gained more weight than pgDR rats. No apparent body weight differences were observed within genotype groups during the first 25 days of body weight monitoring. Thereafter, small body weight differences were observed between pgDIO chow and pgDIO HE and between pgDR chow and pgDR HE (Figure 1c). Both genotype and diet had significant main effects on body weight at termination and body weight gain. Post hoc analyses indicated significant differences between the pgDIO and pgDR genotype groups. However, no differences were present within the genotype groups irrespective of diet (Table 2).
Table 2.
In vivo data for pgDIO and pgDR rats
| pgDR chow | pgDR HE | pgDIO chow | pgDIO HE | Interaction | Genotype | Diet | |
|---|---|---|---|---|---|---|---|
| Average daily body weight gain (g) | 4.7 ± 0.14*** | 5.3 ± 0.14### | 6.8 ± 0.16 | 7.5 ± 0.32 | P = 0.90 | P < 0.0001 | P = 0.004 |
| Body weight at termination (g) | 382.3 ± 10.1*** | 420.3 ± 9.7### | 510.1 ± 11.4 | 552.4 ± 19.7 | P = 0.87 | P < 0.0001 | P = 0.005 |
| Average daily food intake (kcal) | 86.1 ± 2.3*** | 92.8 ± 2.7## | 109.0 ± 2.9 | 107.7 ± 4.1 | P = 0.20 | P < 0.0001 | P = 0.37 |
| Fat pad weight/body weight (%) | 3.95 ± 0.3* | 4.8 ± 0.6### | 6.6 ± 0.7§§§ | 10.5 ± 0.8 | P = 0.02 | P < 0.0001 | P = 0.0009 |
Data tested with two-way ANOVA with Bonferroni-corrected post-tests, n = 8 per group.
HE, high energy; pgDIO, polygenic diet-induced obese; pgDR, polygenic diet-resistant.
P < 0.05.
P < 0.001 between pgDR chow and pgDIO chow.
P < 0.01.
P < 0.001 between pgDR HE and pgDIO HE.
P < 0.001 between pgDIO chow and pgDIO HE.
Genotype, but not diet, had significant main effect on food intake of pgDIO and pgDR rats (Table 2). pgDIO rats consumed more calories than pgDR rats on both diets (Figure 1d). Post hoc analyses indicated that significant differences in cumulative food intake only were present between genotype groups and not within genotype groups (Table 2). Both diet and genotype affected the fat pad/body weight ratio, and pgDIO rats on chow and HE diet differed significantly in this parameter (Table 2). Thus, although genotype was the determinant of body weight and food intake, intake of the HE diet had its main effect in pgDIO rats, which became fatter than all other groups.
RYGB rats
RYGB rats lost 72 ± 11 g during the 60-day post-operation period. Weight loss was mainly seen in the first 10 days postoperation hereafter their weight stabilized (Figure 1e). Sham-operated control rats continued to gain weight postoperation (Figure 1e). Cumulative food intake increased in a linear fashion for both groups. However, RYGB rats consumed significantly less calories than sham-operated control rats (Figure 1f and Table 3). At termination, RYGB rats weighed an average of 169 g less (31%, P < 0.001) than sham-operated control rats.
Table 3.
In vivo data for RYGB and sham-operated rats
| RYGB rats | Sham-operated rats | |
|---|---|---|
| Average daily body weight gain (g) | −1.3 ± 0.20*** | 1.7 ± 0.094 |
| Body weight at termination (g) | 395.2 ± 9*** | 564.6 ± 7.8 |
| Average daily food intake (kcal) | 97.9 ± 3.0** | 108.5 ± 1.8 |
Data tested with student's t-tests. RYGB: n = 10, Sham-operated: n = 12 per group.
RYGB, Roux-en-Y gastric bypass.
P < 0.01.
P < 0.001.
Autoradiography
Outbred DIO rats
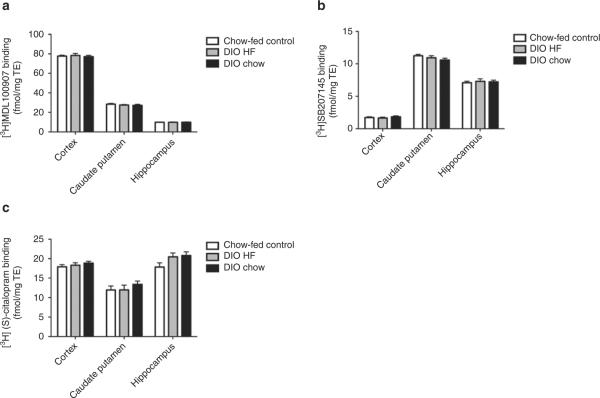
Experimental group had no main effect on 5-HT2A receptor binding (P = 0.32), 5-HT4 receptor binding (P = 0.79), or SERT binding (P = 0.058) in the DIO HF, DIO chow, and chow-fed control rats. Likewise, no differences between groups were found when testing within brain regions (Figure 2). Thus, no overall or region-specific variations were found in the outbred DIO rats.
Figure 2.
Brain regional 5-HT receptor and transporter binding in outbred diet-induced obese rats. (a) 5-HT2A receptor-specific binding, (b) 5-HT4 receptor-specific binding, and (c) SERT-specific binding in chow-fed control, DIO HF, and DIO chow rats. Data tested with two-way ANOVA with Bonferroni-corrected post-tests. n = 11–12 per group. DIO, diet-induced obese; HF, high fat; SERT, serotonin transporter.
Polygenic DIO and diet-resistant rats
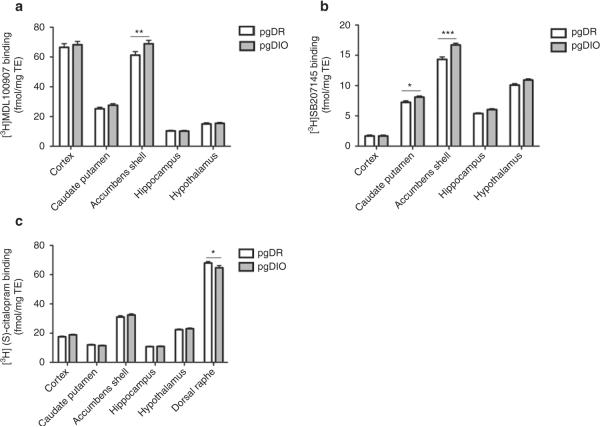
For pgDIO and pgDR rats, a main effect of genotype was found on 5-HT2A receptor binding (P = 0.018) and 5-HT4 receptor binding (P < 0.001) but not SERT binding (P = 0.93). Diet had no effect on SERT binding (P = 0.93), 5-HT2A receptor binding (P = 0.36), or 5-HT4 receptor binding (P = 0.69). Therefore, the data obtained from the pgDR rats on chow and HE diet were pooled, and the data obtained from pgDIO rats on chow and HE diet were pooled. Post hoc analyses showed that pgDIO rats had higher 5-HT2A receptor binding in the NAcS (P < 0.01) (Figure 3a), higher 5-HT4 receptor binding in the NAcS (P < 0.001) and in the caudate-putamen (P < 0.05) (Figure 3b) and lower SERT binding in the dorsal raphe nucleus (P < 0.05) (Figure 3c) as compared to pgDR rats. Accordingly, genotype was the main determinant of receptor binding levels while diet did not affect receptor and SERT binding.
Figure 3.
Brain regional 5-HT receptor and transporter binding in selectively bred polygenic diet-induced obese and diet-resistant rats. (a) 5-HT2A receptor-specific binding, (b) 5-HT4 receptor-specific binding, and (c) SERT-specific binding for pgDIO and pgDR rats. Data tested with three-way ANOVA and subsequent two-way ANOVA with Bonferroni-corrected post-tests, *P < 0.05, **P < 0.01, and ***P < 0.001. For two-way ANOVA analyses and Bonferroni-corrected post-tests, pgDR chow and pgDR HE rats were pooled and pgDIO chow and pgDIO HE rats were pooled. n = 14–16 per group. HE, high energy; pgDIO, polygenic diet-induced obese; pgDR, polygenic diet-resistant; SERT, serotonin transporter.
RYGB rats
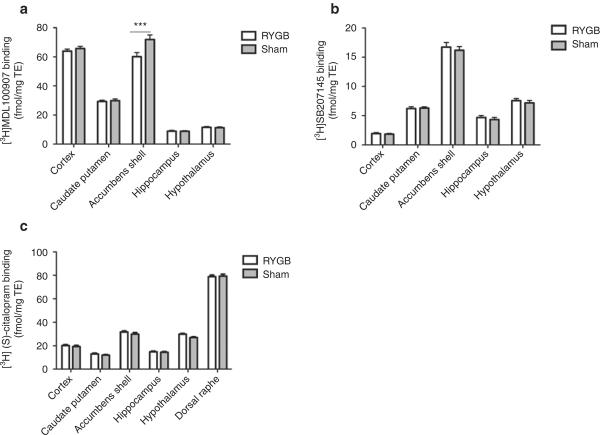
RYGB and sham-operated rats undergoing 12-h fasting before termination were pooled with rats that were not fasted under the assumption that receptor and SERT binding were independent of fed state (29). Surgery had a main effect on 5-HT2A receptor binding (P = 0.007), but not on 5-HT4 receptor (P = 0.11) or SERT binding (P = 0.052). Post hoc analyses indicated a downregulation in 5-HT2A receptor binding in RYGB rats in the NAcS (P < 0.001), whereas all other brain regions examined displayed no change in 5-HT2A receptor binding (Figure 4a). No regional differences in 5-HT4 receptor binding (Figure 4b) or SERT binding (Figure 4b) were observed. Thus, the RYGB procedure affected only 5-HT2A receptors in the NAcS.
Figure 4.
Brain regional 5-HT receptor and transporter binding in a rat model of Roux-en-Y gastric bypass. (a) 5-HT2A receptor-specific binding, (b) 5-HT4 receptor-specific binding, and (c) SERT-specific binding in RYGB and sham-operated rats. Fasted and nonfasted animals were pooled. Data was tested with two-way ANOVA with Bonferroni-corrected post-tests, ***P < 0.001. RYGB: n = 9–10, sham-operated: n = 10–12 per group. RYGB, Roux-en-Y gastric bypass; SERT, serotonin transporter.
DISCUSSION
We here present the first comparative study on cerebral 5-HT markers across different rat models of obesity and body weight loss. Irrespective of dietary intake, pgDIO rats exhibited increased 5-HT2A and 5-HT4 receptor binding, and lower SERT binding as compared to pgDR rats. Thus, it was not unexpected that no differences in these markers were found between outbred DIO rats fed either chow or HF diet. Neither did the 5-HT markers in these rats differ from chow-fed controls. On the other hand, RYGB and subsequent weight loss were associated with reduced 5-HT2A receptor binding selectively in the NAcS. However, there were no effects on either 5-HT4 or SERT binding in any brain region. These results indicate that a genetic predisposition to obesity is associated with upregulation of 5-HT receptors and downregulation of SERT across several brain areas, whereas obesity induced by HF diet is not a determinant of brain 5-HT markers. On the contrary, RYGB and/or its associated weight loss selectively downregulates only specific 5-HT markers in select brain regions.
The results obtained from the pgDIO and pgDR rats are in line with recent neuroimaging studies in obese humans especially for the 5-HT4 receptor. Accordingly, obese individuals show an increase in cerebral 5-HT2A receptor binding (10), a decrease in cerebral SERT binding (12), and an increase in cerebral 5-HT4 receptor binding (11).
The upregulation of the 5-HT2A and 5-HT4 receptor binding in the NAcS in pgDIO rats could suggest a role of these receptors in regulating reward aspects of feeding behavior, which is also supported by other studies. Local application of the 5-HT4 receptor agonist BIMU8 in the NAc reduces the drive to eat in both fed and food-deprived mice. In contrast, 5-HT4 receptor antagonism in the NAc using RS39604 induces hyperphagia in fed but not food-deprived mice (7). In addition, intra-accumbal application of the 5-HT2A/2C receptor antagonist ketanserin stimulates food intake in rats (30). Thus, although the association between the 5-HT system and feeding regulation previously has focused largely on homeostatic regulation in the hypothalamus (31), there is evidence for a role of these receptors in regulating reward aspects of feeding behavior, which results from the present study supports.
From the current data, we cannot, however, infer whether the modulation of SERT and 5-HT2A and 5-HT4 receptor binding in pgDIO rats compared with pgDR rats is a result of direct or indirect effects. Receptor and SERT levels could be altered primarily due to genetic factors or they could be secondary reflecting a decrease in 5-HT turnover (5-hydroxyindoleacetic acid (5-HIAA)/5-HT). Previous studies have indicated decreased 5-HT activity in the obese state, e.g., obese women have decreased levels of the 5-HT metabolite 5-HIAA in their cerebrospinal fluid (32), and chow-fed DIO-susceptible rats have disturbances in their diurnal 5-HT turnover as well as decreased 5-HT activity in response to fasting when compared to chow-fed diet-resistant rats (33). Further, the possibility that the increased 5-HT4 and 5-HT2A receptor binding in pgDIO rats compared with pgDR rats could be mediated through low extracellular 5-HT levels is supported by the anorexigenic response to stimulated 5-HT4 and 5-HT2A receptor activity. Accordingly, 5-HT4 receptor activation suppresses basal food intake and mediates stress- and MDMA-induced anorexia in the NAc (7–9) and systemic administration of the 5-HT2A receptor agonists 2,5-dimethoxy-4-iodoamphetamine and TCB-2 ((4- bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide) reduces food intake (2,3). Thus, if receptor changes were primary and causally related to high body weight gain one would rather have expected lower 5-HT4 and 5-HT2A receptor binding in pgDIO rats compared with pgDR rats.
Several observations suggest that altered 5-HT turnover can modulate SERT and 5-HT2A and 5-HT4 receptor levels. First, it has been suggested that extracellular brain 5-HT levels are inversely correlated to 5-HT2A and 5-HT4 receptor levels, as 5-HT4 receptor binding is downregulated in response to chronic paroxetine treatment, which increases the extracellular 5-HT levels (13). In reverse fashion, 5-HT4 receptor binding is upregulated in response to 5-HT depletion (16). Likewise, 5-HT depletion causes an upregulation of the 5-HT2A receptor binding (13,14), while chronic citalopram or paroxetine treatment decreases 5-HT2A receptor binding (13,15). Thus, low extracellular 5-HT levels are believed to trigger a compensatory upregulation of 5-HT2A and 5-HT4 receptor binding. One could argue that a more global upregulation of the 5-HT4 and 5-HT2A receptor binding would be expected, if low 5-HT levels were the underlying cause. However, the significant main effect of genotype (which is suggested to decrease 5-HT levels) on 5-HT2A and 5-HT4 receptor binding and lack of interaction between genotype and brain region indicate that genotype affected receptor binding in a similar manner in all brain regions, although only high-binding regions reached statistical significance after Bonferroni corrections for multiple comparisons. Further, other studies examining 5-HT4 and 5-HT2A receptor binding after 5-HT depletion indicate that 5-HT2A and 5-HT4 receptors respond differentially to 5-HT depletion depending on brain regions (13,14). Importantly, 5-HT4 receptors in the NAc showed the largest degree of upregulation in a rat study where 5-HT neurons in the raphe nuclei were lesioned (16), and the NAcS was the only region where a significant 5-HT4 receptor upregulation was found after p-chlorophenylalanine-induced 5-HT depletion in pigs (34).
Decreased 5-HT turnover has also been associated with reduced SERT binding. For example, p-chlorophenylalanine treatment causes a downregulation in SERT binding (35,36) and 5-HT depletion caused by a high dose of dexfenfluramine likewise decreases SERT binding, although this could be due to neurotoxic effects causing a decrease in 5-HT fibers rather than a decrease in SERT protein per fiber (36).
Rather than being secondary to the suggested decreased 5-HT turnover, SERT binding could be reduced primarily due to genetic factors. The decrease in dorsal raphe SERT binding in pgDIO rats might reflect that pgDIO rats primarily have fewer 5-HT fibers than pgDR rats, which could explain the hypothesized decreased 5-HT output in obesity. However, decreased SERT binding in other brain regions such as the NAcS would be expected to accompany reduced SERT binding in the principal 5-HT nuclei in the brain stem, since the receptor binding data indicate that the NacS had the most pronounced 5-HT receptor changes. Alternatively, the decrease in dorsal raphe SERT binding could reflect a primary decrease in SERT protein per terminal in this region. Low SERT levels in the dorsal raphe nucleus is anticipated to increase the extracellular 5-HT availability in this region, which in turn is expected to negatively feedback onto the activity of the 5-HT neurons through the inhibitory 5-HT1A autoreceptor. This could cause the suggested low 5-HT activity in the projection fields of the forebrain areas. Thus, we suggest that the upregulation of the 5-HT2A and 5-HT4 receptor binding in pgDIO rats compared with pgDR rats is caused by a genetically inherited decrease in 5-HT activity in forebrain areas, which may be caused by a primary reduction in dorsal raphe SERT levels. However, it must be considered that the decrease in dorsal raphe SERT binding in pgDIO rats was modest and therefore other factors are likely to contribute to the suggested low 5-HT activity in forebrain areas.
While RYGB rats had lower 5-HT2A receptor binding in the NAcS as compared to sham-operated rats, no differences in 5-HT4 receptor and SERT binding were found. The downregulation of 5-HT2A receptors in the NAcS could either be a response to altered 5-HT activity or a specific response of this receptor in this region to the RYGB procedure. Whether the 5-HT turnover is affected by the RYGB procedure is currently unexplored, but if the 5-HT turnover is reduced in the obese state as hypothesized, body weight loss after RYGB could lead to normalization of 5-HT function. Nevertheless, if changes in 5-HT tonus were responsible for the downregulation of the 5-HT2A receptor in the NAcS, then more widespread alterations in the 5-HT system would be anticipated, thus making it presently uncertain whether RYGB surgery procedures have a robust effect on central 5-HT neurotransmission. The observation that the RYGB rats exhibited a substantial body weight loss postsurgery without showing robust changes in 5-HT markers support that even a robust weight loss per se does not trigger significant changes in the 5-HT system. However, the basis for this interpretation is limited since the RYGB rats in the present study were not made DIO prior to surgery. Optimally, RYGB surgery should be performed in pgDIO rats to examine if any of the observed obesity-associated changes in the 5-HT system are reversible with surgery-induced weight loss. It should also be considered that the downregulation of the 5-HT2A receptor binding in the NAcS in RYGB rats could potentially be a direct effect of the RYGB surgical procedure. Although of indirect evidence only, a previous study showed increased 5-HT1B receptor staining in hypothalamic areas in obese rats post-RYGB surgery, as compared to both sham-operated and pair-fed controls (37). Interestingly, DIO rats also have increased 5-HT1B receptor binding in the hypothalamus compared to lean controls (5). Thus, obesity-associated upregulation of 5-HT1B receptors seem to increase further after RYGB surgery, which point to a possible direct effect of the RYGB procedure on the 5-HT system independent of body weight. Hence, we cannot rule out that the RYGB procedure could potentially also directly affect other 5-HT receptor systems, including accumbal 5-HT2A receptors. A lowered preference for palatable foods after RYGB surgery has been reported in both humans (38) and rats (39,40). This suggests that RYGB-induced changes influence hedonic and rewards circuits in the brain and this could implicate the 5-HT system, including 5-HT2A receptor function. Finally, it must be considered that the increase in 5-HT2A receptors in the NAcS in animals that are rapidly gaining weight (pgDIO), and a decrease in animals that are rapidly losing weight (RYGB) with no change in animals that are stably obese (DIO) could also be interpreted differently: changes in NAcS 5-HT2A receptor binding may reflect changes in body weight rather than genetics in the pgDIO rats and a response to the RYGB procedure in RYGB rats. However, the exact mechanism behind the observed changes in 5-HT2A receptor binding remains to be determined.
In conclusion, the upregulation of the 5-HT2A and 5-HT4 receptor binding and the downregulation of SERT binding in pgDIO compared with pgDR rats reflect similar findings in obese humans. The upregulation of the 5-HT2A and 5-HT4 receptor binding in pgDIO rats suggests an adaptive mechanism counteracting the distorted energy balance in obesity. It is speculated that this effect could be driven by an underlying genetically inherited reduction in the 5-HT activity in pgDIO rats. The importance of the genetic component is also supported by results from the DIO and RYGB outbred rat models, as DIO or surgery-induced weight loss did not generally alter the 5-HT system. The upregulation of the 5-HT2A and 5-HT4 receptor binding in pgDIO rats in the NAcS suggests a role of these receptors in regulating reward and hedonic aspects of food intake and implies that central 5-HT perturbations contributing to obesity involve reward circuits.
ACKNOWLEDGMENTS
This work was financially supported by Copenhagen University Hospital, Rigshospitalet, and the Lundbeck Foundation. We also thank Christer Halldin (Karolinska Institute, Stockholm, Sweden), GSK (Brentford, UK), and Lundbeck A/S (Copenhagen, Denmark) for providing the radioligands [3H]MDL100907, [3H]SB207145, and (S)-[N-methyl-3H]citalopram, respectively.
Footnotes
DISCLOSURE The authors declared no conflict of interest.
See the online ICMJE Conflict of Interest Forms for this article.
REFERENCES
- 1.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 2.De Vry J, Schreiber R. Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24:341–353. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 3.Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) 2010;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- 4.Currie PJ, Coiro CD, Niyomchai T, Lira A, Farahmand F. Hypothalamic paraventricular 5-hydroxytryptamine: receptor-specific inhibition of NPY-stimulated eating and energy metabolism. Pharmacol Biochem Behav. 2002;71:709–716. doi: 10.1016/s0091-3057(01)00671-2. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Harrold JA, Widdowson PS, Williams G. Increased binding at 5-HT(1A), 5-HT(1B), and 5-HT(2A) receptors and 5-HT transporters in diet-induced obese rats. Brain Res. 1999;847:90–97. doi: 10.1016/s0006-8993(99)02055-7. [DOI] [PubMed] [Google Scholar]
- 6.Huang XF, Huang X, Han M, et al. 5-HT2A/2C receptor and 5-HT transporter densities in mice prone or resistant to chronic high-fat diet-induced obesity: a quantitative autoradiography study. Brain Res. 2004;1018:227–235. doi: 10.1016/j.brainres.2004.05.093. [DOI] [PubMed] [Google Scholar]
- 7.Jean A, Conductier G, Manrique C, et al. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compan V, Zhou M, Grailhe R, et al. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis HM, Kraushaar NJ, Hunt LR, Cornish JL. Serotonin 5-HT4 receptors in the nucleus accumbens are specifically involved in the appetite suppressant and not locomotor stimulant effects of MDMA (`ecstasy') Psychopharmacology (Berl) 2011;213:355–363. doi: 10.1007/s00213-010-1982-9. [DOI] [PubMed] [Google Scholar]
- 10.Erritzoe D, Frokjaer VG, Haugbol S, et al. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Haahr ME, Rasmussen PM, Madsen K, et al. Obesity is associated with high serotonin 4 receptor availability in the brain reward circuitry. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.03.050. in press. [DOI] [PubMed] [Google Scholar]
- 12.Erritzoe D, Frokjaer VG, Haahr MT, et al. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52:284–289. doi: 10.1016/j.neuroimage.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 13.Licht CL, Marcussen AB, Wegener G, et al. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem. 2009;109:1363–1374. doi: 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed] [Google Scholar]
- 14.Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology (Berl) 2007;190:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- 15.Günther L, Liebscher S, Jähkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Compan V, Daszuta A, Salin P, et al. Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci. 1996;8:2591–2598. doi: 10.1111/j.1460-9568.1996.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 17.Speakman J, Hambly C, Mitchell S, Król E. The contribution of animal models to the study of obesity. Lab Anim. 2008;42:413–432. doi: 10.1258/la.2007.006067. [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Sullivan AC. Glucose-induced sympathetic activation in obesity-prone and resistant rats. Int J Obes. 1989;13:235–246. [PubMed] [Google Scholar]
- 19.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 20.Madsen AN, Hansen G, Paulsen SJ, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- 21.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 22.Bueter M, Löwenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Axel AM, Mikkelsen JD, Hansen HH. Tesofensine, a novel triple monoamine reuptake inhibitor, induces appetite suppression by indirect stimulation of alpha1 adrenoceptor and dopamine D1 receptor pathways in the diet-induced obese rat. Neuropsychopharmacology. 2010;35:1464–1476. doi: 10.1038/npp.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bueter M, Löwenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson J. The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego; Elsevier: 2005. [Google Scholar]
- 26.Parker CA, Brown J, Gee A. Saturation binding of [3H]SB207145 and [3H] GR113808 to 5-HT4 receptors in rat and pig brain: an autoradiographical study. J Cereb Blood Flow Metab. 2003;23:692. [Google Scholar]
- 27.Thomsen C, Helboe L. Regional pattern of binding and c-Fos induction by ®- and (S)-citalopram in rat brain. Neuroreport. 2003;14:2411–2414. doi: 10.1097/00001756-200312190-00024. [DOI] [PubMed] [Google Scholar]
- 28.Elfving B, Madsen J, Knudsen GM. Neuroimaging of the serotonin reuptake site requires high-affinity ligands. Synapse. 2007;61:882–888. doi: 10.1002/syn.20443. [DOI] [PubMed] [Google Scholar]
- 29.Lauzurica N, García-García L, Pinto S, Fuentes JA, Delgado M. Changes in NPY and POMC, but not serotonin transporter, following a restricted feeding/repletion protocol in rats. Brain Res. 2010;1313:103–112. doi: 10.1016/j.brainres.2009.11.075. [DOI] [PubMed] [Google Scholar]
- 30.Soria-Gómez E, Márquez-Diosdado MI, Montes-Rodríguez CJ, Estrada-González V, Prospéro-García O. Oleamide administered into the nucleus accumbens shell regulates feeding behaviour via CB1 and 5-HT2C receptors. Int J Neuropsychopharmacol. 2010;13:1247–1254. doi: 10.1017/S1461145710000702. [DOI] [PubMed] [Google Scholar]
- 31.Heisler LK, Cowley MA, Kishi T, et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 32.Strömbom U, Krotkiewski M, Blennow K, et al. The concentrations of monoamine metabolites and neuropeptides in the cerebrospinal fluid of obese women with different body fat distribution. Int J Obes Relat Metab Disord. 1996;20:361–368. [PubMed] [Google Scholar]
- 33.Hassanain M, Levin BE. Dysregulation of hypothalamic serotonin turnover in diet-induced obese rats. Brain Res. 2002;929:175–180. doi: 10.1016/s0006-8993(01)03387-x. [DOI] [PubMed] [Google Scholar]
- 34.Ettrup A, Kornum BR, Weikop P, Knudsen GM. An approach for serotonin depletion in pigs: effects on serotonin receptor binding. Synapse. 2011;65:136–145. doi: 10.1002/syn.20827. [DOI] [PubMed] [Google Scholar]
- 35.Rattray M, Baldessari S, Gobbi M, et al. p-Chlorphenylalanine changes serotonin transporter mRNA levels and expression of the gene product. J Neurochem. 1996;67:463–472. doi: 10.1046/j.1471-4159.1996.67020463.x. [DOI] [PubMed] [Google Scholar]
- 36.Rothman RB, Jayanthi S, Wang X, et al. High-dose fenfluramine administration decreases serotonin transporter binding, but not serotonin transporter protein levels, in rat forebrain. Synapse. 2003;50:233–239. doi: 10.1002/syn.10266. [DOI] [PubMed] [Google Scholar]
- 37.Romanova IV, Ramos EJ, Xu Y, et al. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J Am Coll Surg. 2004;199:887–895. doi: 10.1016/j.jamcollsurg.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Miras AD, le Roux CW. Bariatric surgery and taste: novel mechanisms of weight loss. Curr Opin Gastroenterol. 2010;26:140–145. doi: 10.1097/MOG.0b013e328333e94a. [DOI] [PubMed] [Google Scholar]
- 39.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajnal A, Kovacs P, Ahmed T, et al. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]