Abstract
Background
We have noticed changes in paediatric anaphylaxis triggers locally in Singapore.
Objective
We aimed to describe the demographic characteristics, clinical features, causative agents and management of children presenting with anaphylaxis.
Methods
This is a retrospective study of Singaporean children presenting with anaphylaxis between January 2005 and December 2009 to a tertiary paediatric hospital.
Results
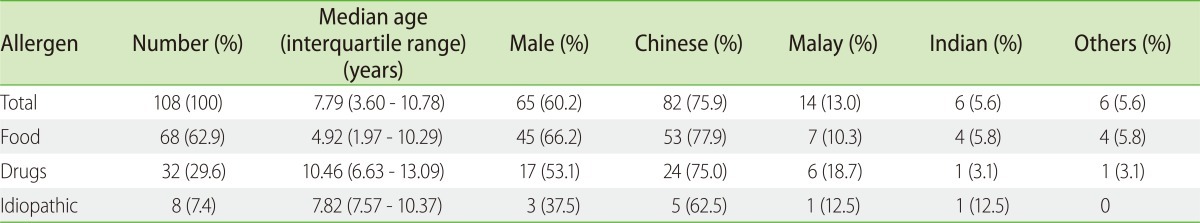
One hundred and eight cases of anaphylaxis in 98 children were included. Food was the commonest trigger (63%), followed by drugs (30%), whilst 7% were idiopathic. Peanut was the top food trigger (19%), followed by egg (12%), shellfish (10%) and bird's nest (10%). Ibuprofen was the commonest cause of drug induced anaphylaxis (50%), followed by paracetamol (15%) and other nonsteroidal anti-inflammatory drugs (NSAIDs, 12%). The median age of presentation for all anaphylaxis cases was 7.9 years old (interquartile range 3.6 to 10.8 years), but food triggers occurred significantly earlier compared to drugs (median 4.9 years vs. 10.5 years, p < 0.05). Mucocutaneous (91%) and respiratory features (88%) were the principal presenting symptoms. Drug anaphylaxis was more likely to result in hypotension compared to food anaphylaxis (21.9% vs. 2.7%, Fisher's exact probability < 0.01). There were 4 reported cases (3.6%) of biphasic reaction occurring within 24 h of anaphylaxis.
Conclusion
Food anaphylaxis patterns have changed over time in our study cohort of Singaporean children. Peanuts allergy, almost absent a decade ago, is currently the top food trigger, whilst seafood and bird's nest continue to be an important cause of food anaphylaxis locally. NSAIDs and paracetamol hypersensitivity are unique causes of drug induced anaphylaxis locally.
Keywords: Anaphylaxis, Drug allergy, Food allergy, Paediatrics
INTRODUCTION
It is more than a decade since the first paediatric food anaphylaxis series in Singapore highlighted the unique bird's nest allergen locally [1]. Of significance, there were no peanut or tree nut food anaphylaxis cases reported in that series conducted between 1992 and 1996. In recent years, we have noted a changing pattern of food sensitization in children locally [2], and that peanut allergy has become a significant food trigger, with an estimated prevalence of 0.47-0.64% [3] in schoolchildren. Whilst a recent Epinephrine auto-injectors prescription survey [4] showed that the most common food trigger is peanut allergy in children < 15 years old, the patients were mostly non-Singaporean children. In addition, non-food anaphylaxis cases in children have not been studied locally, but we have previously reported cross-reactive paracetamol and non-steroidal anti-inflammatory drug (NSAID) hypersensitivity amongst local children [5], and some complicated by anaphylaxis [6].
Singapore's population demographics had changed significantly in the past 2 decades, with a significant increase of non-residents from 10.2% in 1990, to 18.7% in 2000 to 25.7% in 2010 [7]. As anaphylaxis triggers differ between populations, we decided to study Singaporean residents only so as to examine if food anaphylaxis triggers has changed. In addition, we aim to describe the demographic characteristics, clinical features, causative agents, and management of Singaporean children presenting with anaphylaxis to one of the two tertiary paediatric facilities in the country.
MATERIALS AND METHODS
Anaphylaxis cases were identified by: 1) discharge codes of hospital admissions; 2) discharge codes from the Department of Children's Emergency (CE); and 3) the referrals made to the Allergy service outpatient clinics in KK Women's and Children's Hospital, between January 2005 and December 2009. The study was approved by the Institution's Review Board.
All records were reviewed by the attending allergists to ensure that they meet the inclusion clinical criteria of anaphylaxis defined by the Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium [8]. Allergen triggers were determined by the attending allergist from the history account, and verified by a positive skin prick test or allergen-specific IgE performed at least 6 weeks after the anaphylaxis event. Exceptions to this rule include previously reported drug (eg, NSAIDs) with non-IgE mechanism (anaphylactoid reactions). The allergen triggers were independently determined by the principal investigator allergist at data entry.
We have included only Singapore citizens and permanent residents in this study. Non-residents, tourists and short-term visitors receiving intermittent care in Singapore were excluded.
Skin prick tests (SPT)
All patients presenting with a history of food anaphylaxis underwent a SPT from commercially available extracts of the implicated food or prick-prick food tests if commercial extract unavailable. A standardized food panel comprising of egg whole, egg white, milk, soy, sesame, fish, wheat, shellfish and peanut were performed when the implicated trigger was unclear. In addition, an environmental panel was included if the patient presented with a history of respiratory allergies: The panel of aeroallergens include house dust mite mix (Dermatophagoides farinae 5,000 AU/mL/Dermatophagoides pteronyssinus 5,000 AU/mL, standardized), Blomia tropicalis 0.2 mg/mL, cockroach mix (Periplaneta americana, Blattella germanica), Candida albicans, cat hair (standardized cat hair, Felis domesticus 10,000 BAU/mL), dog epithelia (Canis familiaris), grass mix (nine southern grass mix, standardized) and a mold mix (Alternaria alternata, Aspergillus niger, Bipolaris sorokiniana, Cladosporium sphaerospermum, Penicillium chrysogenum). Drug SPTs were performed using the parenteral or oral form of the drug diluted to non-irritant concentrations. All SPTs were performed in the allergy laboratory by a team of experienced technicians using the Greer-Pick SPT device (Greer Laboratories, USA) applied to the forearm or the back (children aged less than 5 years). Prick-prick food tests using either fresh, cooked or canned food, and drug SPT were performed by the attending paediatric allergist. Wheal size was recorded at 15 min. Results were regarded as positive if the mean wheal diameter was at least 3 mm greater than that for the negative control. Histamine (10 mg/mL) was used as a positive control. The allergen extracts included in our panel were commercially produced by Greer (Greer Laboratories, USA), except for the B. tropicalis extract, produced by the Allergy and Molecular Immunology Laboratory, National University of Singapore, Singapore.
Allergen specific IgE were performed if SPT were not able to be done. Measurements of allergen specific IgE levels were carried out in an accredited allergy laboratory using the Pharmacia CAP system (Pharmacia Corporation, USA).
Statistical methods
Statistical analysis was performed using SPSS for Windows version 14. Continuous data was described as either mean (SD) or median (interquartile range) if not normally distributed. Differences between groups for categorical variables were determined using either the Chi-Square analysis or Fisher's exact test. The Mann-Whitney U test or Student's t test was used for comparisons between non-parametric and parametric continuous variables respectively. p-values <0.05 were considered statistically significant.
RESULTS
One hundred and eighteen paediatric anaphylaxis events occurred between January 2005 and December 2009. Ten anaphylaxis events were recurrent events triggered by the same allergen were excluded from this report. One hundred and eight cases of anaphylaxis in 98 children were included for analysis.
Food was the commonest trigger (63%), followed by drugs (30%), whilst 7% were idiopathic (Table 1). There was no insect sting or exercise-induced anaphylaxis cases. There is a higher preponderance (60.2%) for males to develop anaphylaxis. Whilst the median age of presentation for all anaphylaxis cases was 7.9 years old (interquartile range 3.6 to 10.8 years), food triggers occurred significantly earlier compared to drug (median age 4.9 years vs. 10.5 years, p < 0.05). Sixty-eight cases (75%) occurred in Chinese; 13 (11.6%) in Malays; 7 (6.3%) in Indians; and 7 (6.3%) in other races, similar to Singapore's racial distribution published in a recent population census [7].
Table 1.
Breakdown of total anaphylaxis events by allergen triggers, median age on presentation, sex and race
Muco-cutaneous (91%) and respiratory features (88%) were the principal presenting symptoms. Drug anaphylaxis was more likely to result in hypotension compared to food anaphylaxis (21.9% vs. 2.7%, Fisher's exact probability < 0.01).
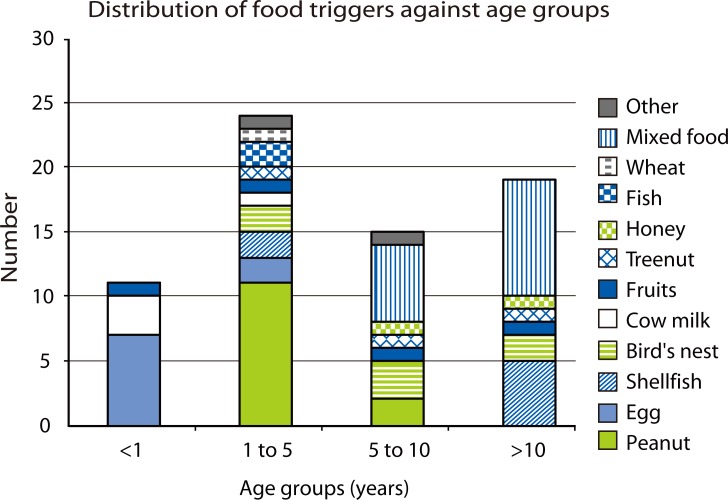
Peanut was the top food trigger (19%), followed by egg (12%), shellfish (10%) and bird's nest (10%). Less common food triggers include cow's milk, tropical fruits (banana, jackfruit and mango), fish and tree nuts (Table 2). There were 3 cases diagnosed retrospectively to be likely secondary to ingesting house-dust mite contaminated flour, after this phenomenon was described locally [9]. Of the 13 peanut anaphylaxis patients, 11 were Singaporean Chinese born locally, one was a Eurasian Chinese born in Canada, and one was a Caucasian born in Singapore. Food triggers differ between the various age groups, with egg, peanut, mixed food and shellfish being the top trigger in age groups of less than 1 year old, 1 to 5 year old, 5 to 10 years old, and greater than 10 year old respectively (Fig. 1).
Table 2.
Causes of anaphylaxis in our cohort of Singaporean children
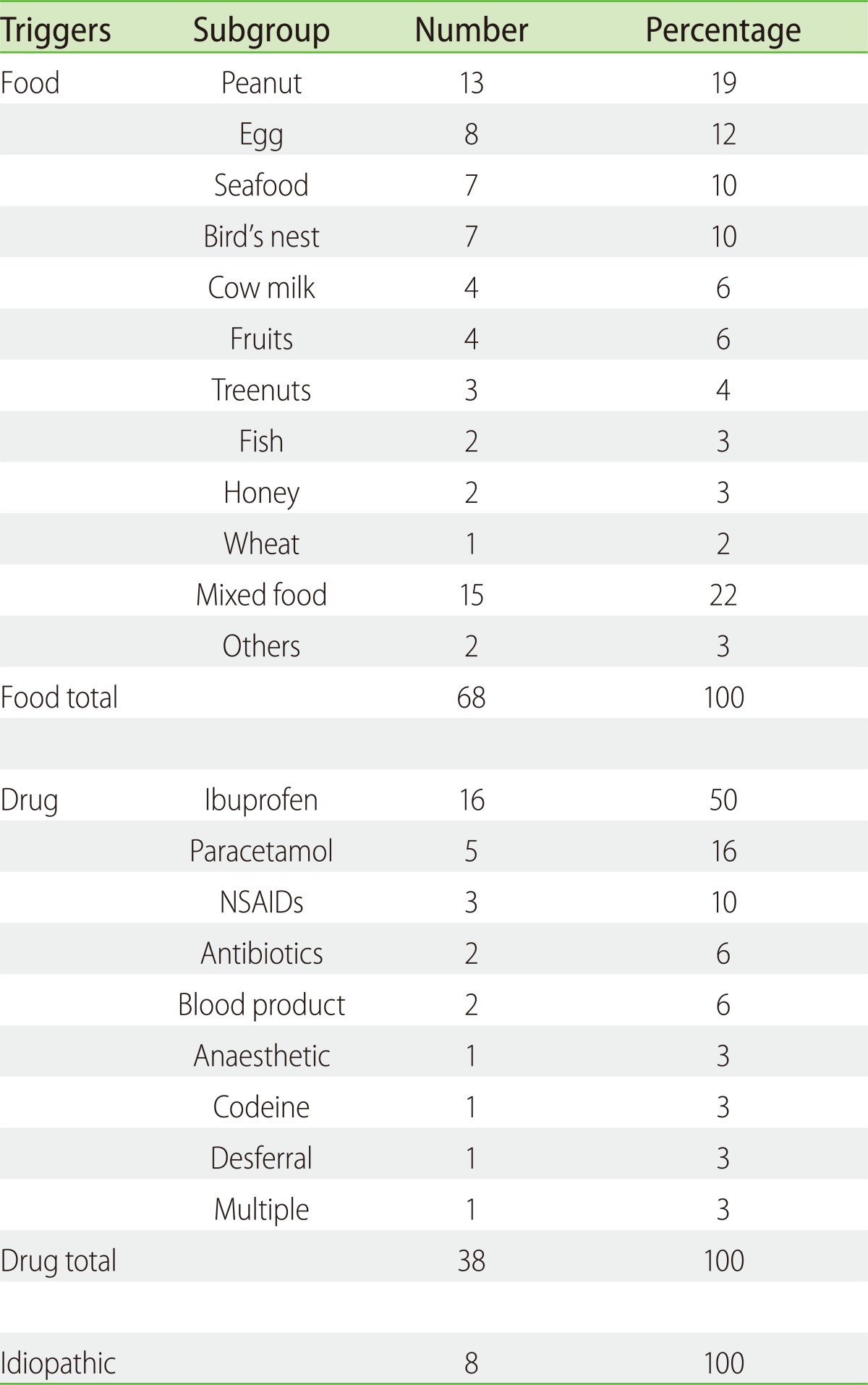
Fig. 1.
Distribution of food triggers against age groups.
Ibuprofen was the commonest cause of drug induced anaphylaxis (50%), followed by paracetamol (15%) and other NSAIDs (12%). There were 2 cases of antibiotics (beta-lactams, 6%), 2 blood products (fresh frozen plasma and immunoglobulin G, 6%) and 1 anesthetic agent (Atracurium) trigger (Table 2). Of the 19 patients with ibuprofen/NSAID anaphylaxis, 7 (36.8%) reported a history of milder hypersensitivity reaction to paracetamol; Of the 5 patients with paracetamol anaphylaxis, 1 reported a history of milder hypersensitivity reaction to ibuprofen. In addition, 20.8% of NSAID and paracetamol hypersensitivity anaphylaxis has recurrent idiopathic urticaria. This is in contrast to 0% in food anaphylaxis cases and 33% of idiopathic anaphylaxis cases. The incidence of other atopic diseases like asthma, allergic rhinitis and atopic dermatitis were similar amongst the 3 groups.
Seventy-nine cases (70%) were acute episodes managed in the CE, whereby 73% were subsequently admitted for observation. All of these patients were subsequently reviewed by an allergist either as an inpatient and/or at Specialist outpatient clinics upon discharged for ward or CE. The median length of stay was 1 day (range 1-5 days). Forty-eight children (43%) received adrenaline in the CE, predominantly via the intramuscular route (69%). Seventy-eight cases (70%) received corticosteroids and sixty-two (55%) received antihistamines.
There were 4 reported cases (3.6%) of biphasic reaction occurring within 24 h of anaphylaxis. One was of similar severity to the original reaction, whilst 3 were milder. Three patients required intensive care unit (ICU) stay for hypotension at presentation, which resolved after adrenaline and intravenous fluid. None of the ICU patients required continued inotropes or ventilatory support. There were no deaths or long-term morbidity sequelae from anaphylaxis.
DISCUSSION
We have identified a changing pattern of food anaphylaxis in our cohort of Singaporean children, with peanut emerging tops, whilst seafood and bird's nest continue to be an important cause of food anaphylaxis locally. This is in contrast to zero peanut food triggers 15 years ago [1], and that bird's nest allergy was the commonest and unique food trigger locally. Of note, the definition of anaphylaxis used previously was different, with a significant proportion of cases having generalized allergic reaction (personal communication). Whilst the total number of anaphylaxis over a 5 year period is low, our hospital is the largest paediatric tertiary referral centre in Singapore. We estimate that paediatric anaphylaxis incidence is at least 2.5 per 100,000 children (Singapore residents ≤ 18 years [7]) per year in Singapore.
This is the first report that confirms that peanut anaphylaxis is the top food trigger in a cohort of Singaporean children. Whilst a recent epinephrine auto-injector prescription survey [4] has suggested that they are prescribed most commonly for peanut allergy in children < 15 years old in Singapore, the patients are mainly non-Singaporean children.
Whilst the reasons for the change in food induced anaphylaxis trends are unknown, migration and genetic predisposition are unlikely to account for this as we have excluded non-Singaporean children and the short time span between the 2 comparative periods. Anecdotally, we have observed that more children consumed boiled peanuts in porridge or soup as their first peanut exposure years ago, in contrast to processed peanut butter recently. We hypothesize that the changing food ingestion patterns and the peanut avoidance advice for allergy prevention, may have contributed to increased peanut sensitization, allergy and anaphylaxis. And although the prevalence of peanut allergy is still somewhat lower than that seen in Western countries [3], once sensitized, ubiquitous exposure, low public awareness and almost absent labeling and regulation, makes for significant morbidity, with a measurably increased risk of anaphylactic reactions. Shellfish and bird's nest anaphylaxis continues to be an important food trigger in our local children consistent with the previous survey [1]. The food triggers for each age group are different, with egg, cow's milk predominantly less than 1 year old, peanut between 1 to 5 years old, and shellfish after 10 year old. Some food triggers eg. bird's nest and fruits span most age groups.
NSAIDs, especially ibuprofen hypersensitivity is fairly common in our pediatric population [10], and the commonest cause of drug induced anaphylactic reactions in our series. A significant proportion of these children also have cross-reactive paracetamol hypersensitivity, thus posing limited options for anti-pyretics use. As opposed to patients with the traditional aspirin triad, our patients do not have nasal polyps, but have association with recurrent idiopathic urticaria and angioedema, and suboptimal controlled allergic rhinitis. The incidence of drug anaphylaxis secondary to beta-lactam antibiotics, contrast media and anesthetic agents is relatively low in our population.
Compared to other recently published paediatric anaphylaxis series [11-19], we have a significant proportion of drug triggers, low proportion of idiopathic triggers, and have not recorded insect sting triggers and food dependent exercise-induced anaphylaxis in our series. This may be in part due to our urbanized city thus limiting bee and wasp population to forested areas, with insect sting anaphylaxis affecting mostly adult military personel [20]. As food dependent exercise-induced anaphylaxis has been reported locally [21], we have reviewed our food and idiopathic anaphylaxis cases to ensure we do not miss this less common entity.
The strengths of our series lie with the multisource identification and definition of anaphylaxis cases. Anaphylaxis epidemiology derived from such series would likely be a more accurate representative of the total anaphylaxis cases, as opposed to data obtained from a single source eg. emergency department anaphylaxis attendances. This is especially so in countries where anaphylaxis recognition and diagnosis is low, and allergy clinics referrals would capture those not treated at the emergency department. In our series 70% of cases attended the children's emergency, whist 30% were referred directly to the allergy outpatient clinics from other physicians. In addition, the perceived trigger or diagnosis is sometimes inaccurate during the initial assessment and an outpatient review by an allergist, coupled with further investigations including skin prick tests or food-specific IgE further validate the diagnosis and allergen involved in the cases included. This may partially explain the low proportion of idiopathic anaphylaxis in our series. Adrenaline auto-injector prescription surveys may be biased by factors including awareness of anaphylaxis diagnosis and treatment, varying physician thresholds, varying patients' care requirement, and healthcare resources limitations. They are also not usually prescribed for drug triggers and hence not a good reflection of drug anaphylaxis patterns.
The limitation of our study is the retrospective design, and unavoidable biases, such as referral bias (milder cases of anaphylaxis, may have received care in the community and not referred.)
In conclusion, food anaphylaxis patterns have changed over time in our study cohort of Singaporean children. Peanut allergy, almost absent a decade ago, is currently the top food trigger, whilst seafood and bird's nest continue to be an important cause of food anaphylaxis locally. NSAIDs and paracetamol hypersensitivity are unique causes of drug induced anaphylaxis locally.
References
- 1.Goh DL, Lau YN, Chew FT, Shek LP, Lee BW. Pattern of food-induced anaphylaxis in children of an Asian community. Allergy. 1999;54:84–86. doi: 10.1034/j.1398-9995.1999.00925.x. [DOI] [PubMed] [Google Scholar]
- 2.Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007;37:1055–1061. doi: 10.1111/j.1365-2222.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 3.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, Ma S, Lee BW. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. 331.e1–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Tham EH, Tay SY, Lim DL, Shek LP, Goh AE, Giam YC, Chng HH, Lee BW. Epinephrine auto-injector prescriptions as a reflection of the pattern of anaphylaxis in an Asian population. Allergy Asthma Proc. 2008;29:211–215. doi: 10.2500/aap.2008.29.3102. [DOI] [PubMed] [Google Scholar]
- 5.Kidon MI, Liew WK, Chiang WC, Lim SH, Goh A, Tang JP, Chay OM. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007;144:51–56. doi: 10.1159/000102614. [DOI] [PubMed] [Google Scholar]
- 6.Kang LW, Kidon MI, Chin CW, Hoon LS, Hwee CY, Chong NK. Severe anaphylactic reaction to ibuprofen in a child with recurrent urticaria. Pediatrics. 2007;120:e742–e744. doi: 10.1542/peds.2006-2634. [DOI] [PubMed] [Google Scholar]
- 7.Singapore Department of Statistics. Census of Population 2010 Advance Census Release. Singapore: Department of Statistics, Ministry of Trade & Industry, Republic of Singapore; 2010. [Google Scholar]
- 8.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, Brown SG, Camargo CA, Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD, Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 9.Yi FC, Chen JY, Chee KK, Chua KY, Lee BW. Dust mite infestation of flour samples. Allergy. 2009;64:1788–1789. doi: 10.1111/j.1398-9995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 10.Kidon MI, See Y. Adverse drug reactions in Singaporean children. Singapore Med J. 2004;45:574–577. [PubMed] [Google Scholar]
- 11.de Silva IL, Mehr SS, Tey D, Tang ML. Paediatric anaphylaxis: a 5 year retrospective review. Allergy. 2008;63:1071–1076. doi: 10.1111/j.1398-9995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 12.Melville N, Beattie T. Paediatric allergic reactions in the emergency department: a review. Emerg Med J. 2008;25:655–658. doi: 10.1136/emj.2007.054296. [DOI] [PubMed] [Google Scholar]
- 13.Hoffer V, Scheuerman O, Marcus N, Levy Y, Segal N, Lagovsky I, Monselise Y, Garty BZ. Anaphylaxis in Israel: experience with 92 hospitalized children. Pediatr Allergy Immunol. 2011;22:172–177. doi: 10.1111/j.1399-3038.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Hompes S, Köhli A, Nemat K, Scherer K, Lange L, Rueff F, Rietschel E, Reese T, Szepfalusi Z, Schwerk N, Beyer K, Hawranek T, Niggemann B, Worm M. Provoking allergens and treatment of anaphylaxis in children and adolescents--data from the anaphylaxis registry of German-speaking countries. Pediatr Allergy Immunol. 2011;22:568–574. doi: 10.1111/j.1399-3038.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 15.Silva R, Gomes E, Cunha L, Falcão H. Anaphylaxis in children: a nine years retrospective study (2001-2009) Allergol Immunopathol (Madr) 2012;40:31–36. doi: 10.1016/j.aller.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Solé D, Ivancevich JC, Borges MS, Coelho MA, Rosário NA, Ardusso L, Bernd LA. Anaphylaxis in Latin American children and adolescents: The Online Latin American Survey on Anaphylaxis (OLASA) Allergol Immunopathol (Madr) 2012;40:331–335. doi: 10.1016/j.aller.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Calvani M, Cardinale F, Martelli A, Muraro A, Pucci N, Savino F, Zappalà D, Panetta V. Risk factors for severe pediatric food anaphylaxis in Italy. Pediatr Allergy Immunol. 2011;22:813–819. doi: 10.1111/j.1399-3038.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 18.Orhan F, Canitez Y, Bakirtas A, Yilmaz O, Boz AB, Can D, Kuyucu S, Harmanci K, Tahan F, Reisli I, Karakas T, Baki A, Cokugras H, Cakir M, Yuksel H. Anaphylaxis in Turkish children: a multi-centre, retrospective, case study. Clin Exp Allergy. 2011;41:1767–1776. doi: 10.1111/j.1365-2222.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Chawla K, Järvinen KM, Nowak-Węgrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012;129:162–168.e1-3. doi: 10.1016/j.jaci.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thong BY, Cheng YK, Leong KP, Tang CY, Chng HH. Anaphylaxis in adults referred to a clinical immunology/allergy centre in Singapore. Singapore Med J. 2005;46:529–534. [PubMed] [Google Scholar]
- 21.Teo SL, Gerez IF, Ang EY, Shek LP. Food-dependent exercise-induced anaphylaxis - a review of 5 cases. Ann Acad Med Singapore. 2009;38:905–909. [PubMed] [Google Scholar]