Abstract
Vesicle trafficking and new membrane addition at the cleavage furrow have been extensively documented. However, less clear is the old idea that expansion at the cell poles occurs during cytokinesis. We find that new membrane is added to the cell poles during anaphase, causing the plasma membrane to expand coincident with the constriction of the contractile ring and may provide a pushing force for membrane ingression at the furrow. This membrane addition occurs earlier during mitosis than membrane addition at the furrow and is dependent on actin and astral microtubules. The membrane that is added at the polar regions is compositionally distinct from the original cell membrane in that it is devoid of GM1, a component of lipid rafts. These findings suggest that the growth of the plasma membrane at the cell poles during cell division is not due to stretching as previously thought, but due to the addition of compositionally unique new membrane.
Keywords: Membrane dynamics, polar expansion, cytokinesis, membrane addition
Introduction
The study of cytokinesis has occupied the interests of cell biologists since the 19th century. While the motile force for cytokinesis has been identified as the acto-myosin based contractile ring, there has been a long history of thought that membrane expansion plays a key role in the process [Wilson,1928; Swann and Mitchison, 1958]. Studies involving the adherence of kaolin or charcoal particles to the surface of a dividing a cell, which were then tracked by eye, suggest a stretching of the plasma membrane at the polar regions of the cell [Dan et al., 1937; Dan et al., 1938; Ishizaka, 1958]. The observation that this membrane movement occurred at the same time as a shrinking of the membrane at the furrow region led to the idea that these events were a driving force behind cell division [Swann and Mitchison, 1958; Wolpert, 1960]. These findings led to Wolpert’s “polar relaxation” theory, which proposes that the expansion seen at the poles is caused by a signal from astral microtubules and that this relaxation at the poles allows for the contraction of membrane at the furrow region, thus initiating the ingression of the cleavage furrow [Wolpert, 1960]. More recent studies have confirmed the movement of proteins anchored in the plasma membrane inward toward the furrow during cell division [Wang et al., 1994; Fishkind et al., 1996]. In fact, there are also reports on the expansion of the cortex as well as the membrane at the poles and movement of cortical actin toward the forming contractile ring [Dan 1954; Cao and Wang, 1990]. Subsequently, the experiments of Rappaport supporting the competing equatorial stimulation model shifted the discussion of cell division [Rappaport, 2005]. Currently, it is believed that both polar relaxation and equatorial stimulation work in conjunction to drive cytokinesis.
Recent work on membrane trafficking during cytokinesis has focused on the addition of new plasma membrane at the advancing cleavage furrow late in cytokinesis and on abscission events [for reviews see McKay and Burgess, 2010; Neto et al., 2011; Albertson et al., 2005; Albertson et al., 2008; Montagnac et al., 2008]. Early work using electron microscopy of furrows in amphibian embryos and mammalian cells show the accumulation of vesicles at the ingressing furrow during the later stages of cytokinesis, similar to what is observed in plant cell division [Buck and Tisdale, 1962; Bluemink and De Laat, 1973; Porter and Caulfield, 1958]. New membrane addition in the furrow as a late event has also been observed in echinoderm embryos as well as in C. elegans and mammalian cells [Bluemink and De Laat, 1973; Shuster and Burgess 2002; Gromley et al., 2005; Wilson et al., 2005; Skop et al., 2001]. Subsequent work on genetic model systems has confirmed and expanded these studies to reveal the roles of both Golgi trafficking and the recycling endosome in the trafficking of vesicles to the cleavage furrow [for reviews see McKay and Burgess, 2010; Albertson et al., 2005; Neto et al., 2011]. Thus, while new membrane addition at the furrow has been established, the existence of membrane expansion at the polar regions during cytokinesis remains unexplored.
Here, we revisit the question of polar expansion during cytokinesis. Our previous studies showed dramatic movements of membrane rafts containing signaling molecules toward the furrow [Ng et al., 2005]. We wished to reconcile our earlier studies on the movement of lipid raft containing membrane to the furrow with the lack of mobility of lipid rafts observed in FRAP experiments [Alford et al., 2009; Ng et al., 2005]. Using cholera toxin subunit B (CTB) as a marker for the plasma membrane lipid rafts, we now document the expansion of the plasma membrane at the polar regions of the cell during division, likely due to new membrane being inserted into the plasma membrane at the poles. New membrane addition at the poles is dependent on anaphase onset and astral microtubules, which are known to reach the poles prior to their arrival at the equatorial cortex. This new polar membrane is compositionally unique from the original zygotic membrane and is added significantly earlier during cytokinesis than the new membrane that is added after mitotic exit at the cleavage furrow. Our findings suggest that polar expansion via the addition of new membrane forces the original cell membrane containing lipid rafts to flow into the furrow during cytokinesis. It is likely that the inward inflection of the furrow is due to the joint action of the contractile ring and the pushing forces of the plasma membrane from the pole toward the equatorial region.
Results
Lipid Rafts Are Stabilized within the Plasma Membrane
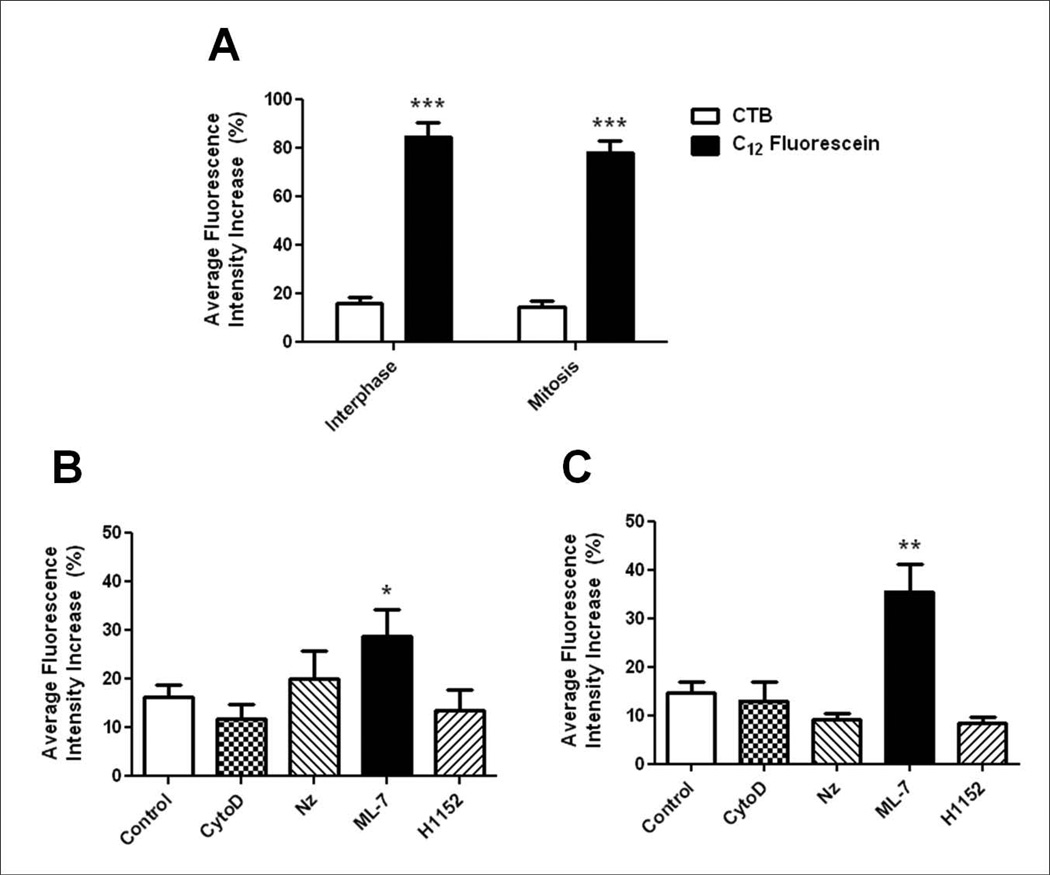
Previous studies have shown that CTB-labeled GM1-containing membrane is dynamic during cytokinesis, accumulating at the equatorial cell surface and moving into the cleavage furrow during ingression, then migrating back out after the completion of cytokinesis due to new membrane addition in the late furrow [Ng et al., 2005; Alford et al., 2009]. We quantified the mobility of GM1 throughout the cell cycle in cleavage stage sea urchin embryos using FRAP in zygotes and 2-cell stage blastomeres stained with 488-CTB, a molecule known to bind to the ganglioside GM1, which is a component of lipid rafts [Badizadegan et al., 2000]. CTB-labeled cells show staining around the entire cell surface of zygotes (Figure S1 in supplemental material), representing labeling of large aggregates of the nanoscale membrane rafts. GM1 was found to be highly immobile within the membrane, having an average fluorescence intensity increase (AFI) of 16.29±2.42% during interphase (Figure 1A and Figure S1 in supplemental material). Surprisingly, we found GM1 to be similarly static during mitosis (metaphase to late anaphase, AFI = 14.76±2.13%) when compared to cells in interphase (Figure 1A and Figure S1 in supplemental material), suggesting that previous observations of GM1 movement into the furrow is specific to cytokinesis [Alford et al., 2009]. It was not possible to perform accurate FRAP experiments during the later stages of cell division when the furrow was ingressing due to the rapid bulk movement of the membrane toward the furrow. As a control, when compared to the fluorescent membrane dye C12-fluorescein, GM1 was immobile. Rapid and complete recovery (84.79±5.98% AFI during interphase and 78.3±4.65% AFI during mitosis) of C12-fluorescein was observed within 2 minutes of bleaching (Figure 1A and Figure S1 in supplemental material). Therefore, our results show that GM1 is not able to freely diffuse and is stabilized within the plasma membrane during both interphase and mitosis.
Figure 1.
Lipid rafts are immobile throughout the cell cycle and sensitive to MLCK inhibition. (A) Lipid rafts labeled with 488-CTB showed an average fluorescence intensity increase (AFI) in the ROI of 16.29±2.42% (n=13) during interphase and 14.76±2.13% (n=17) during mitosis over 5 minutes. The diffusible lipid dye C12-Fluorescein showed rapid recovery to the ROI during both interphase and mitosis (84.79±5.98%, n=9 and 78.3±4.65%, n=8 respectively; ***p<0.0005). Error bars represent SEM. (B) Average fluorescence intensity (AFI) increase in the ROI of 488-CTB labeled cells in interphase upon treatment with cytoskeletal inhibitors. The AFI increase in the ROI of control cells (n=13) was compared to embryos treated with CytoD (n=8), Nz (n=8), ML-7 (n=9), and H1152 (n=8) (*p<0.05). (C) AFI increase in the ROI of 488-CTB labeled cells in mitosis. The AFI increase of control cells (n=17) was compared to embryos treated with CytoD (n=10), Nz (n=9), ML-7 (n=9), and H1152 (n=9) (**p<0.001). Error bars represent SEM.
Disruption of MLCK Increases GM1 Mobility
Components of the cytoskeleton are known to effect the organization of lipid rafts, which are otherwise immobile in the plasma membrane. Actin is necessary for the clustering of lipid rafts in both model membranes and mammalian cells, and has also been shown to affect the mobility of raft markers [Rodgers and Zavzavadjian, 2001; Liu and Fletcher, 2006; Chichili and Rodgers, 2007]. In addition, actin has been observed in lipid rafts fractions from sea urchin embryos [Belton Jr et al., 2001]. Nonmuscle myosin II and myosin light chain kinase (MLCK) have also been found to associate with lipid rafts in mammalian cells [Ishmael et al., 2007; Zhao et al., 2009]. In order to further investigate the how GM1 is anchored within the plasma membrane, CTB-labeled sea urchin embryos were treated with small molecule inhibitors of cytoskeletal proteins prior to FRAP experiments. Surprisingly, depolymerization of actin during interphase using Cytochalasin D (CytoD) showed no effect on the mobility of GM1 (Figure 1B, AFI = 11.88±2.8%). Disruption of microtubules using Nocodazole (Nz) also showed no significant effect on GM1 mobility (Figure 1B, AFI = 20.16±5.67%). In contrast, inhibition of MLCK by treatment with ML-7 showed a significant increase in the recovery of CTB-labeled GM1 to the bleached region (Figure 1B, AFI = 28.91±5.46%). In order to indirectly disrupt myosin phosphorylation, embryos were treated with the Rho kinase (ROCK) inhibitor H1152. This treatment had no effect on the mobility of lipid rafts during interphase (Figure 1B, AFI = 13.42±4.41%), suggesting that the stabilization of rafts within the plasma membrane is specifically MLCK dependent.
Similar results were observed during mitosis. ML-7 treatment led to an increase in the recovery of GM1 to the bleached region (Figure 1C, AFI = 35.54±5.55%) when compared to control cells during mitosis (Figure 1C, AFI = 14.76±2.13%). Inhibitors of actin and microtubule polymerization, as well as ROCK inhibition showed a slight, but not significant decrease in GM1 mobility during mitosis (Figure 1C). This decrease is likely due to the fact that cytokinesis is inhibited in these embryos.
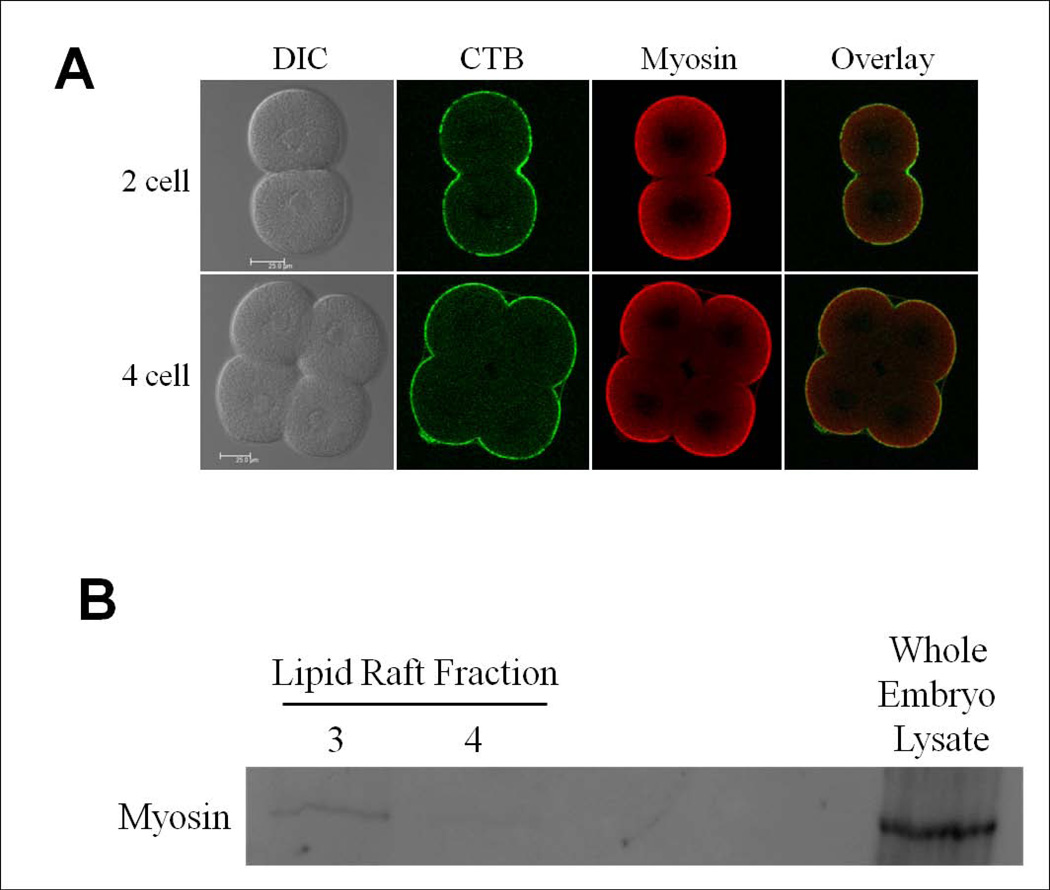
Myosin light chain kinase is necessary for the phosphorylation of myosin light chain, leading to the formation of bipolar myosin filaments. Due to the observation of a disruption in GM1 stability upon ML-7 treatment, the association of myosin and lipid rafts was analyzed in two ways. Myosin and CTB-labeled lipid rafts were colocalized at the surface of zygotic embryos (data not shown). After cytokinesis, GM1 as well as myosin is concentrated on the apical, or free cell surface [as defined by Alford et al., 2009] of the two and four cell stage embryo (Figure 2A). Second, lipid rafts were isolated from sea urchin embryos by sucrose fractionation and analyzed (see Figure S2 in supplemental material) [Belton Jr et al., 2001; Ng et al., 2005]. Myosin was found to fractionate along with a subpopulation of GM1-containing lipid rafts isolated from two cell stage embryos, however MLCK was not found to be present in isolated rafts (Figure 2B, data not shown). These results suggest that the role of MLCK in stabilizing lipid rafts in the plasma membrane is likely via the role of MLCK in the formation of bipolar myosin filaments.
Figure 2.
Myosin is associated with GM1-containing lipid rafts. (A) Immunofluorescence localization of myosin and CTB staining of two and four cell stage embryos. Myosin colocalized with 488-CTB labeled lipid rafts at the apical cell surface. Scale bar represents 25µm. (B) Myosin was detected by Western blot in GM1-containing lipid raft fractions (unlabeled lanes left blank). See also Figure S2 in supplemental material.
New membrane addition occurs at poles during cytokinesis
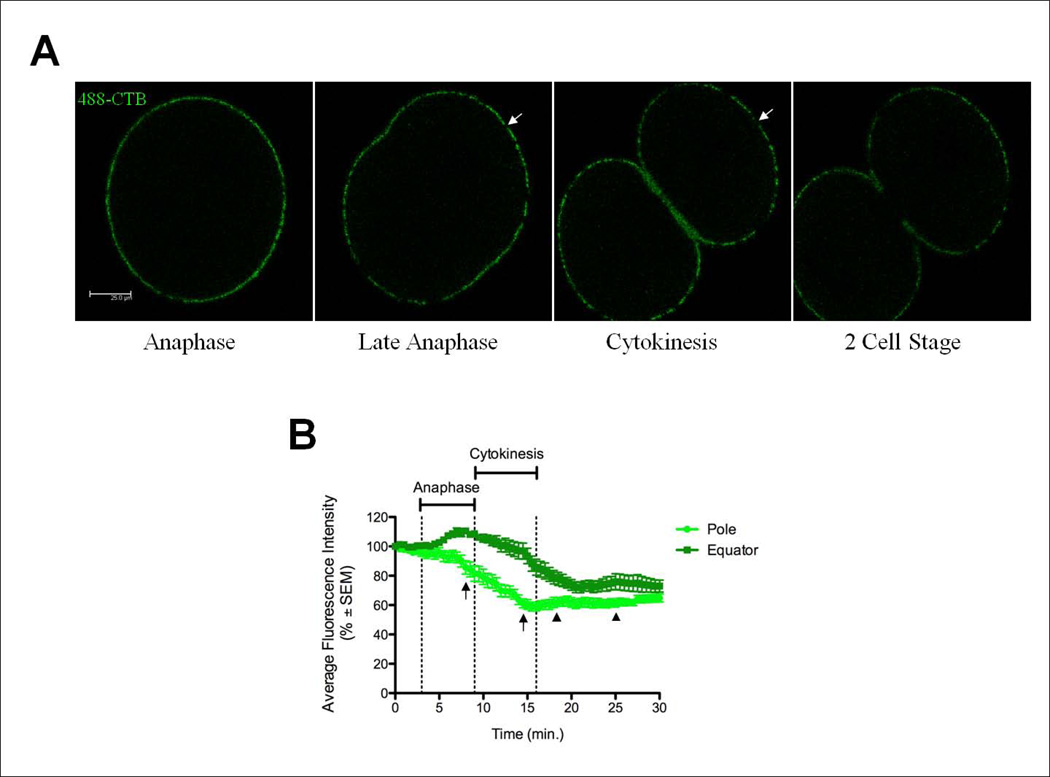
In order to reconcile the facts that GM1-containing membrane flows to the forming furrow and yet it is immobile within the plasma membrane, we further analyzed membrane movements and addition during cell division. Since we were unable to perform FRAP experiments during cytokinesis due to the movement of the plasma membrane, Z-stack time-lapse series were taken from metaphase through the completion of cell division in order to observe the movement of CTB-labeled membrane during cytokinesis. As previously reported, CTB-labeled lipid rafts were observed moving into the ingressing cleavage furrow, then migrating out of the furrow after ingression was completed due to new membrane being inserted into the late furrow region (Figure 3A, Movie S1 in supplemental material) [Alford, et al., 2009].
Figure 3.
The plasma membrane expands at the polar regions while compressing at the equatorial regions. (A) New membrane was added at the polar regions as shown by the expansion of unstained regions (arrows). Scale bar represents 25µm. See also Movie S1 in supplemental material. (B) Fluorescence intensity values were calculated at the pole or equator for zygotes pulse stained with 488-CTB for 20 minutes prior to mitosis. The AFI decreased 49.6±2.2% at the poles (n=12 embryos, 14 regions) during anaphase, reaching the maximum decrease at full furrow ingression. At the equator (n=12 embryos, 23 regions), fluorescence intensity increased 27±2.9% prior to cytokinesis, then decreased during furrow invagination. Time is presented in minutes after metaphase onset. Arrows represent the timing of new membrane addition at the poles. Arrowheads represent the duration of new membrane addition at the furrow.
Older studies were interpreted to mean that the plasma membrane stretched or thinned at the cell poles during cytokinesis [Dan et al., 1937; Dan et al., 1938; Dan and Dan, 1940; Mitchison, 1952; Dan and Ono, 1954; Wolpert, 1960]. Upon further observation of time-lapse z-stacks of CTB-labeled embryos, it was found that large patches of membrane devoid of CTB staining appeared and expanded at the cell pole. This new membrane was labeled with C12-fluorescein. This addition of new membrane devoid of GM1-containing lipid rafts caused a decrease in the intensity of fluorescence at the cell pole, allowing the amount of polar expansion to be quantified by measuring the change in fluorescence intensity at the cell pole throughout the cell cycle. The fluorescence intensity decreased from early anaphase through late telophase, increasing slightly after the completion of abscission (Figure 3B). This decrease in fluorescence intensity at the poles occurred simultaneously with the accumulation of CTB-labeled lipid rafts at the furrow during late anaphase (Figure 3B) [Ng et al., 2005]. Interestingly, the decrease in fluorescence during the cell cycle was not due to diffusion or dilution of GM1 as the membrane stretched, but rather the addition of membrane at the poles completely devoid of 488-CTB staining (Figure 3A arrows). These regions expanded during furrow ingression, suggesting continued membrane addition, and appeared to force the original cell membrane marked by 488-CTB to accumulate and migrate into the cleavage furrow (Figure 3A, Movie S1 in supplemental material). These findings suggest that there is not a global stretching of the plasma membrane as previously theorized, but rather a targeted addition of new membrane at the polar regions that is the source of an increase in surface area required for cytokinesis, as well as the movement of CTB-labeled lipid rafts towards the cleavage furrow.
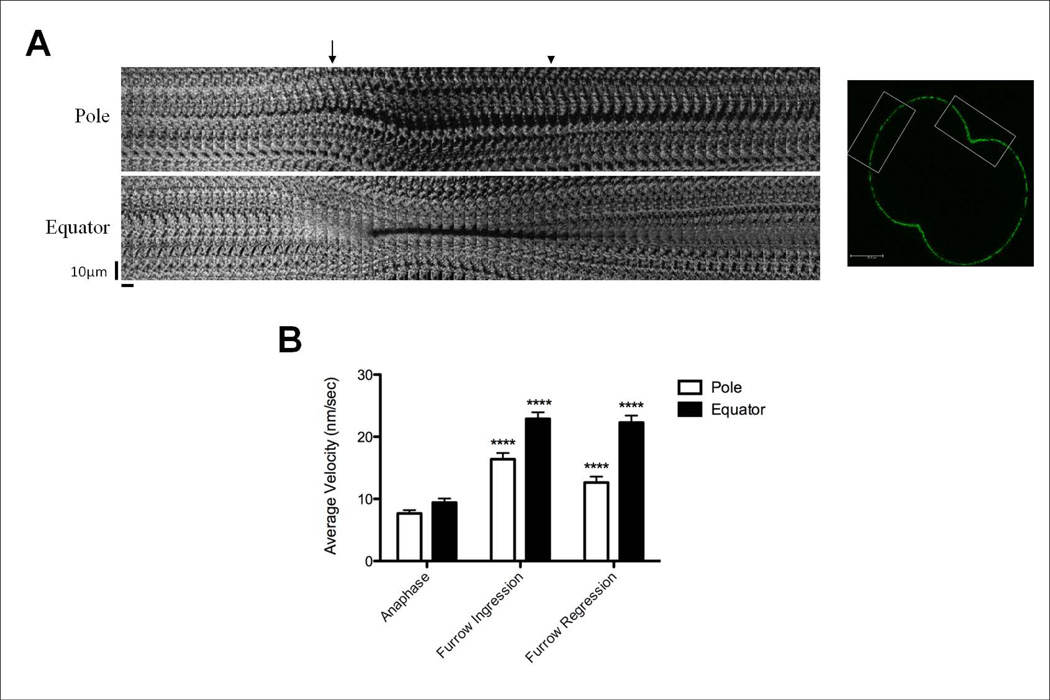
In order to quantitate the movement of lipid rafts caused by the addition of new membrane, spots of CTB-labeled membrane were tracked during cytokinesis at the cell poles as well as the equator. Kymographs were made for the polar and equatorial regions of the zygote and individual spots of CTB staining that remained in this plane for the majority of the time-lapse were tracked. As the cell elongated prior to furrow inflection, new membrane was added to the polar region, forcing CTB-labeled domains outwards towards the equator of the cell (Figure 4A). Once the furrow had ingressed completely and new membrane began to be added to the cleavage plane, the spots moved back towards the pole (Figure 4A). The velocity of movement increased during cytokinesis, from 7.7±0.5nm/sec during anaphase to 16.4±1nm/sec during furrow ingression, then decreased slightly to 12.7±0.9nm/sec during the phase in which new membrane is added to the late furrow (referred to here as furrow regression) (Figure 4B). At the equator, CTB-labeled membrane domains moved inward as the furrow ingressed during cytokinesis, which correlated with the expansion of the polar regions (Figure 4A, Movie S1 in supplemental material). The velocity of these domains increased significantly during cytokinesis as well, from 9.4±0.7nm/sec during anaphase to 22.9±1nm/sec during furrow ingression, remaining high, 22.3±1.1nm/sec, during furrow regression when new membrane was added to the cleavage furrow during the later stages of cytokinesis (Figure 4B). These results suggest that the addition of new membrane at the poles forces original cell membrane away from the poles toward the furrow, causing the movement of GM1-containing lipid rafts to the furrow as previously described [Ng et al., 2005]. During furrow regression, the original GM1-containing membrane is pushed back out of the cleavage furrow as new membrane devoid of GM1 is added at the tip of the cleavage furrow upon the completion of cytokinesis. However, even after completion of cytokinesis, there remain patches of new membrane in the polar region (Figure 3A, Movie S1 in supplemental material).
Figure 4.
Rate of movement of CTB stained lipid rafts increases during early cytokinesis and is higher in the equator than at the poles. (A) Pole: Kymographs from a z-stack time-lapse of a 488-CTB pulse labeled zygote show CTB stained domains moving outward from the pole (arrow identifies onset of cytokinesis), retracting back in the final stages of cell division (arrowhead). Equator: CTB-labeled domains migrated into the ingressing furrow coincident with migration of rafts from the poles. Spots were pushed back outwards toward the poles when new membrane addition began late in furrowing at the tip of the ingressed furrow. Scale bar represents 10 µm. Each section (horizontal bar) is a maximum projection of the z-stack at that 30 second timepoint over the course of 30 minutes. Regions of the cell made into kymographs are shown as white boxes in the image on the right (scale bar represents 25µm). See also Movie S1 in supplemental material. (B) Average velocity values for individual CTB-labeled spots at the cell pole or equator tracked during cell division (n=14 cells). The velocity of the CTB-labeled spots increased significantly during furrow ingression (furrow formation to full ingression) and remained high during furrow regression (full ingression to 2 cells stage) compared to anaphase. (****p<0.0001). Error bars represent SEM.
Polar membrane expansion began at anaphase at a time when astral microtubules reach the poles, and continued through telophase (Figure 3, Movie S1 in supplemental material). The timing of new membrane addition was calculated for each experiment and then averaged. The duration of this membrane addition event is significantly shorter (5.8±0.3 min., Figure 3B arrows) than new membrane addition at the furrow in late cytokinesis (6.9±0.4 min., Figure 3B arrowheads). A 4 minute time span was observed between the time when polar expansion ended and new membrane addition to the furrow began. These results show that there are two spatially and temporally separate membrane addition events that occur during cytokinesis.
Polar membrane addition is dependent on actin and astral microtubules
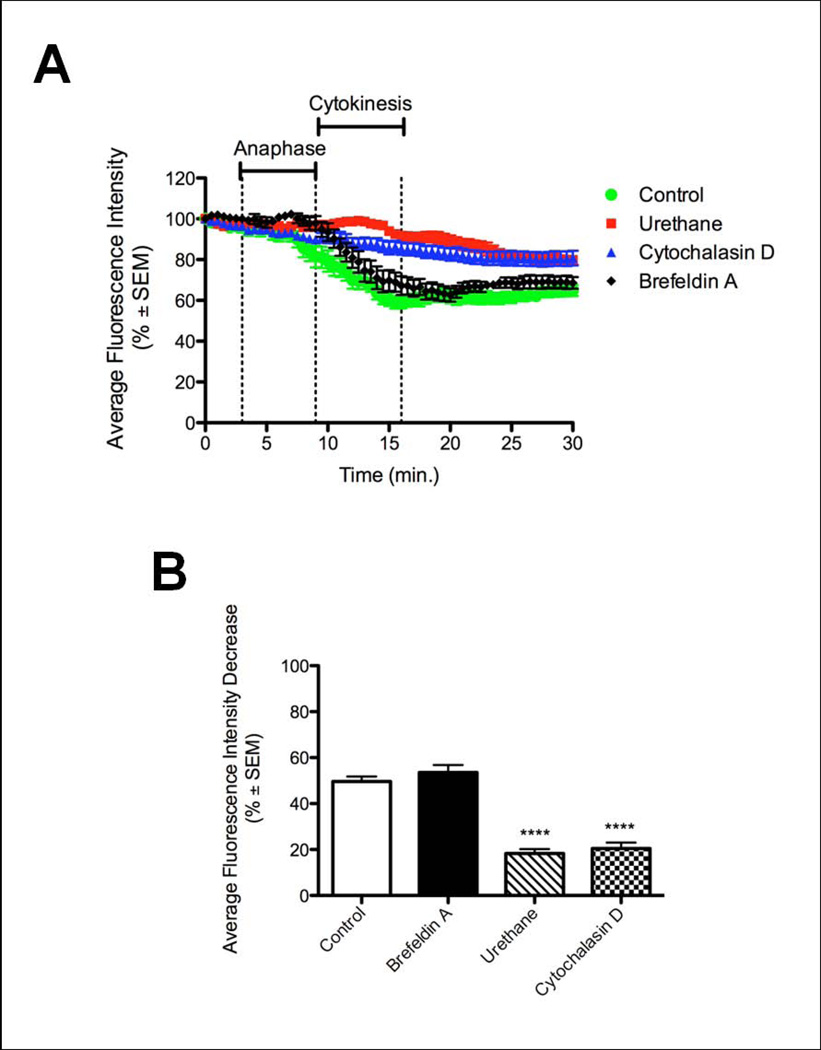
Membrane addition at the furrow during cytokinesis has been shown to be dependent on astral microtubules [Shuster and Burgess, 2002]. To determine whether astral microtubules were required for polar membrane expansion, embryos labeled with 488-CTB were treated with urethane and observed for 45 minutes beginning at the start of metaphase. Urethane has been shown to increase catastrophe of microtubules preventing them from reaching the cell cortex and inducing a furrow, but still allows the cell cycle to progress and mitosis to occur [Strickland et al., 2005; Rappaport, 1971]. Zygotes treated with urethane show significantly less new membrane addition at the poles as exhibited by a decrease in average fluorescence intensity of 18.3±2%, while control zygotes have an average fluorescence intensity decrease of 49.6±2.2% (Figure 5), and the patches of unlabeled membrane appearing at the poles were much smaller. Actin was also found to be necessary for polar membrane addition, as zygotes treated with 5ug/ml CytoD prior to mitosis showed less new membrane addition at the poles, with a fluorescence decrease of only 20.5±2.6% (Figure 5). This data suggests that both astral microtubules and actin are necessary for new membrane addition at the poles during anaphase.
Figure 5.
New membrane addition at the poles is dependent on actin and astral microtubules. A) Control embryos showed a decrease in fluorescence intensity at the poles representing new membrane addition (green). Mitotic zygotes lacking astral microtubules (red) or actin (blue) showed significantly less new membrane addition at the polar regions. Time is presented in minutes after metaphase onset. B) New membrane addition was quantified by decrease in fluorescence at the polar regions during cell division. Control embryos showed a decrease of 49.6±2.2% due to new membrane being added that is devoid of CTB staining. Zygotes treated with urethane or cytochalasin D showed significantly less decrease in fluorescence at the poles (18.3±2% and 20.5±2.6% respectively, ****p<0.0001) during division.
Brefeldin A (BFA), an inhibitor of vesicle trafficking from the endoplasmic reticulum (ER) to the Golgi apparatus, treated zygotes successfully divided and showed similar polar membrane addition (decrease in average fluorescence intensity of 53.5±3.3%) to controls (Figure 5). These results are similar to those observed for membrane addition at the cleavage furrow in sea urchin embryos [Shuster and Burgess, 2002]. Therefore, both membrane addition events during cytokinesis are independent of ER to Golgi vesicle trafficking.
New membrane is distinct from old membrane
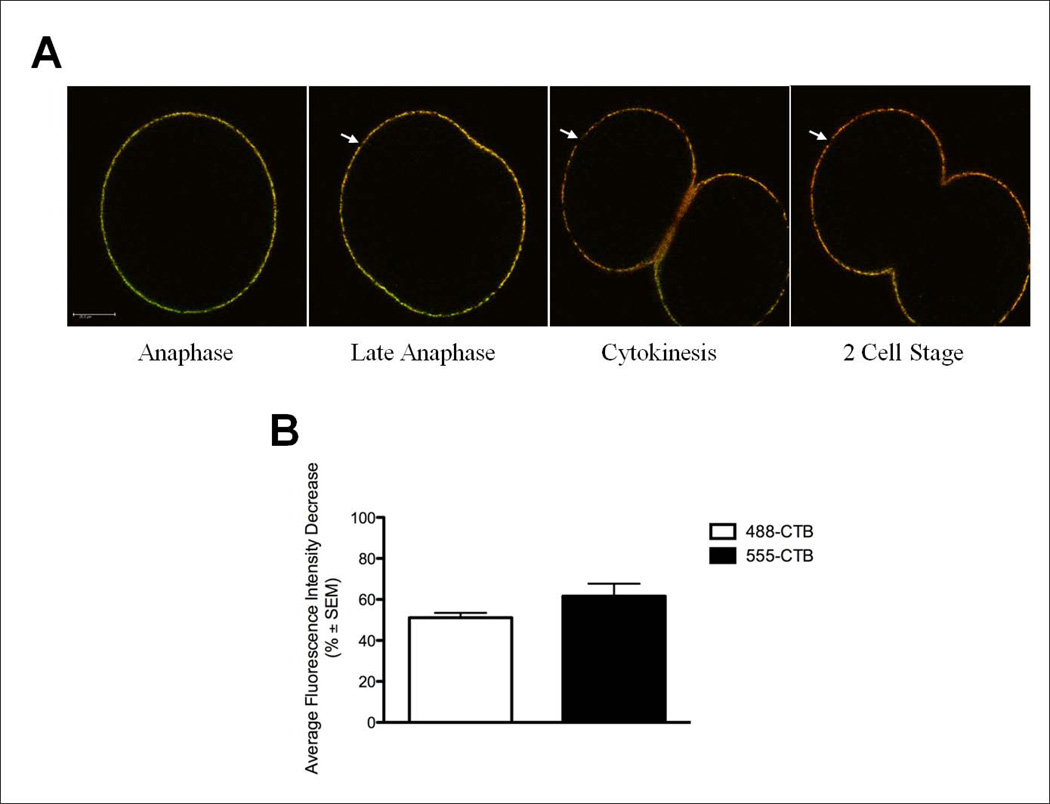
To determine if the new membrane added at the poles during cytokinesis contained membrane rafts, cells were initially pulse labeled with 488-CTB as above, and then continuously labeled with CTB conjugated to an Alexa 555 fluorophore (555-CTB) during cytokinesis. If the new membrane inserted at the polar regions originated from a population of previously endocytosed membrane containing GM1 or new GM1-containing membrane appearing via exocytosis, we would predict membrane domains solely labeled with 555-CTB and the intensity of 555-CTB to remain stable or increase throughout cytokinesis. However, if the membrane is from internal membrane stores devoid of GM1, the intensity of 555-CTB would be similar to that of 488-CTB – decreasing during anaphase when new membrane is added. Likewise, if there were new GM1 containing membrane added during cell division, it would be labeled only with 555-CTB. Prior to anaphase, we found the plasma membrane is co-labeled with both 488-CTB and 555-CTB (Figure 6A). Areas devoid of both 488 and 555-CTB labeling were observed at the poles and the decrease in fluorescence intensity at the poles of 555-CTB was similar to that seen with 488-CTB (51.1±2.3% and 61.6±6.1% respectively, Figure 6B arrows). The similarity in the fluorescence decrease between the pulse labeled 488-CTB and the continuously labeled 555-CTB show that the decrease in polar fluorescence seen in pulse labeling experiments is not due to a bleaching of the fluorescent signal or loss labeling over time, but a result of the addition of new, unlabeled membrane. While the majority of new membrane was devoid of CTB staining, there was some addition of new GM1-containing membrane during cytokinesis as detected by staining with 555-CTB only, especially in the new furrow membrane (Figure 6A arrowhead, Movie S2 in supplemental material). These findings suggest that the majority of new membrane that is added during cytokinesis is devoid of GM1, thus being distinct from the pre-mitotic cell plasma membrane in its composition and origin.
Figure 6.
New membrane is distinct from original cell membrane. Zygotes were pulse labeled with 488-CTB during interphase, then continuously labeled with 555-CTB throughout cell division. A) Addition of new membrane at the cell pole distinct from the original cell membrane is evidenced by the expansion of areas devoid of 488-CTB (green) and 555-CTB (red) staining (arrows). Some new membrane containing GM1 (red) was seen at the ingressing furrow during late cytokinesis (arrowhead). Scale bar represents 25µm. See also Movie S2 in supplemental material. B) Quantification of the decrease in fluorescence intensity at the polar regions during anaphase and cytokinesis. Both the pulse-labeled 488-CTB staining and the 555-CTB staining equally decreased at the cell poles (51.1±2.3% and 61.6±6.1% respectively) during cell division.
Discussion
The surface area of a dividing cell must increase in order for the successful division of one cell into two daughter cells, however the mechanism of this expansion was uncertain [Dan and Ono, 1954; Wolpert 1960]. In spherical, non-adherent cells, such as echinoderm embryos, the cell surface must increase 28% in order to divide into two spherical cells maintaining the same volume [Wolpert, 1960]. Recent studies strongly suggest a major source of this new membrane is due to membrane addition in the furrow region [McKay and Burgess, 2010; Albertson et al., 2005; Montagnac et al., 2008; Schiel and Prekeris, 2010; Neto et al., 2011]. In sea urchin embryos, this addition is dependent on microtubules, thought to be used in trafficking vesicles to the furrow as well as syntaxins necessary for vesicle docking and fusion [Conner and Wessel, 1999; Shuster and Burgess, 2002]. Largely ignored however, is the older idea that the membrane at the polar cell surface expands during cell division, resulting in movement of the membrane and cortex toward the equatorial zone. Our results showed an addition of new membrane at the poles early in cytokinesis, accounting for the expansion of the plasma membrane during mid- to late-anaphase and coincident with bulk movement of the membrane toward the advancing furrow (Figure 3, Movies S1 and S2 in supplemental material). This new polar membrane was easily observed as it does not contain CTB-labeled lipid rafts and thus is compositionally distinct from the original, post fertilization membrane (Figure 3, Movie S1 in supplemental material). The addition of membrane at the poles occurs significantly earlier in cell division than membrane addition at the furrow and thus constitutes a spatially as well as temporally distinct membrane addition event.
Membrane addition in mammalian cells remains unclear. In most adherent mammalian cells, the cell surface area must decrease as the cell rounds up in preparation for division [Schweitzer et al., 2005; Boucrot and Kirchhausen, 2007]. In HeLa cells, the loss of cell surface area occurs via decreased exocytosis early in mitosis, causing the formation of an internal store of membrane vesicles. One report suggests that during anaphase and telophase these vesicles are inserted into the polar regions [Boucrot and Kirchhausen, 2007]. However, another study found that the plasma membrane area decreases due to endocytosis at the polar regions of the cell during anaphase and telophase, and the vesicles are then shuttled to the ingressing cleavage furrow late in cytokinesis [Schweitzer et al., 2005]. PtK2 cells, on the other hand, remain flattened until telophase. These cells exhibit a loss of microvilli at the polar regions upon the completion of cytokinesis, when the cells flatten once again, suggesting that the expansion at the poles necessary for such changes in cell shape may be due to the flattening of microvilli on the polar cell surface [Sanger et al., 1984]. Sea urchin embryos provide a unique opportunity to study the role of polar expansion during cell division in a cell which does not undergo dramatic changes in cell shape prior to mitotic entry. We have found that the addition of new membrane at the cell poles, not membrane stretching, enables the cell to elongate in preparation for the successful completion of cytokinesis and the formation of two, equally sized daughter cells.
In addition, our results show that membrane addition at the pole may provide a pushing force to the original, CTB-labeled cell membrane towards the cleavage furrow (Figure 4A, Movie S1 in supplemental material), possibly delivering or reorienting actin, myosin, or signaling molecules associated with the membrane cortex for the formation and action of the contractile ring. Such a pushing mechanism is possible due to the finding that the rafts themselves are immobile in the membrane and thus are forced to move in bulk toward the furrow. The original raft-containing plasma membrane is then forced out of the furrow during late telophase due to new membrane addition via exocytosis in the furrow leaving the new, basolateral cell surface facing daughter cells free of CTB labeling (Figure 6A) [Alford et al., 2009]. Since the large raft free patches in the poles diminish in size during the phase where the membrane is added to the furrow, it is likely that there is a period of compensatory endocytosis of the raft free patches at the poles.
Whereas previous work suggests that actin is the main cytoskeletal element controlling lipid raft formation and organization within the plasma membrane, we find the mobility of GM1-containing lipid rafts in sea urchin embryos to be controlled by the activity of myosin light chain kinase (Figure 1B and C) [Kwik et al., 2003; Liu and Fletcher, 2006; Chichili and Rodgers, 2007]. This is most likely through the phosphorylation of myosin regulatory light chain by MLCK, leading to the formation of bipolar myosin filaments, as myosin was found to be associated with lipid rafts, while MLCK was not observed in isolated raft fractions (Figure 2, data not shown). However, we cannot rule out the possibility that myosin bipolar filaments affect lipid raft mobility by altering the dynamics of actin. In mammalian cells, inhibition of myosin ATPase activity by treatment with Blebbistatin increased the recovery time of actin at the equatorial cortex, the polar cortex, and the contractile ring [Guha et al., 2005; Murthy and Wadsworth, 2005]. Surprisingly, neither disruption of actin nor microtubules caused a change in the mobility of lipid rafts within the membrane (Figure 1B and C).
This work suggests that the original hypotheses that membrane expansion and relaxation of the poles are an important component for cytokinesis warrant further consideration. Results presented here suggest that bulk movement of the membrane from the poles toward the furrow, driven by insertion of large patches of new membrane at the poles, may be one driving force for cytokinesis. This microtubule and actin-dependent membrane expansion at the polar regions occurs after anaphase onset and happens significantly earlier in cell division than membrane addition at the furrow. How this addition is temporally and spatially regulated awaits further analysis.
Materials and Methods
Culturing of Embryos
Lytechinus pictus gametes were collected by intracoelomic injection of 0.5M KCl. Eggs were collected in artificial sea water (ASW) and swirled twice to expand the jelly coat. Sperm was collected dry and diluted 1:1,000 in ASW prior to addition to eggs. Eggs were fertilized in ASW with 3 drops of diluted sperm and the fertilization envelopes were removed by two passages through 118µm nytex. Fertilized embryos were cultured in calcium free sea water (CaFSW) at 15°C. Embryos were treated with ML-7 (100µM), H1152 (2.5µM, Alexis Biochemicals), Cytochalasin D (5µg/ml or 10µg/ml), Nocodazole (0.01µM), urethane (40mM), or Brefeldin A (BFA, 15µg/ml) directly prior to imaging. All inhibitors were purchased from Sigma-Aldrich unless otherwise noted.
Cholera Toxin Labeling
At 20 minutes post-fertilization, embryos were labeled with 1µg/ml Alexa 488-conjugated cholera toxin subunit B (CTB; Life Technologies) in CaFSW for 20 minutes. Prior to imaging and drug treatment, the embryos were washed three times with CaFSW. For double labeling experiments, embryos were labeled as above with 488-CTB in CaFSW for 30 minutes, washed three times with CaFSW, and then labeled with 1µg/ml Alexa 555-conjugated CTB. Z-stack images (0.5µm slices for 10µm) were taken on a Leica DM I 6000 inverted microscope equipped with the Leica TCSSP5 confocal system every 30 seconds through completion of mitosis. Fluorescence intensities were obtained using the LAS AF software and analyzed using GraphPad Prism software. Kymographs were made of a 28 × 56µm region encompassing the cell pole or equator using Metamorph software (Molecular Devices).
Fluorescence Recovery After Photobleaching
Embryos were stained with 488-CTB as described above. For control experiments, 5-dodecanoylaminofluorescein (C12-Fluorescein, 0.5ug/ml; Life Technologies) was added to live embryos for 10 minutes. The embryos were then rinsed three times with CaFSW. Prior to experiments, the embryos were stained with Hoeschst (1:10,000 dilution; AnaSpec Inc.). Fluorescence recovery after photobleaching (FRAP) experiments were conducted on a Leica DM I 6000 inverted microscope equipped with the Leica TCSSP5 confocal system using the FRAP Wizard on the LAS AF software. A region of interest (ROI) of 10µm × 5µm was bleached and recovery monitored by time-lapse microscopy over 5 minutes. The fluorescence recovery was low and therefore analysis did not yield discernible curves from which to obtain t1/2 or mobile fraction data. Therefore, fluorescence intensities were analyzed for each time point, and values were normalized as previously described [Goodwin and Kenworthy, 2005]. The AFI was calculated by taking the average of the fluorescence intensity at tmax minus the fluorescence intensity at t0 for each embryo. Analysis of FRAP data was performed in Microsoft Excel and GraphPad Prism. An unpaired t-test was used to evaluate statistical significance and a p-value of ≤ 0.05 was considered significant.
Isolation of Lipid Rafts and Western Blot Analysis
Embryos were grown in CaFSW until the two cell stage. Detergent resistant lipid rafts were obtained as previously described [Belton Jr et al., 2001; Ng et al., 2005]. After centrifugation in a sucrose gradient, 1ml fractions were collected from the top of the gradient. Based on previous work [Belton Jr et al., 2001] and dot blot analysis (Figure S2 in supplemental material), the lipid raft fractions 3 and 4 were run on a SDS-PAGE gel and transferred to an Immobilon-P membrane (Millipore) for western blot analysis. Blots were blocked with 5% non-fat dry milk in TBS with 0.1% Tween-20 (TBS-T), and then incubated with a 1:500 dilution of rabbit-anti-S. purpuratus egg myosin II in 5% milk in TBS-T at room temperature for one hour. Blots were washed three times with PBS with 0.1% TritonX-100 (PBT) prior to incubation with a 1:10,000 dilution of HRP conjugated donkey-anti-rabbit (Amersham Bioscience) in 5% non-fat dry milk in TBS-T for one hour. Blots were washed three times in PBT before addition of the HRP substrate.
Fixation and Immunofluorescence
Embryos were labeled with 488-CTB as described above and raised to the desired stage in CaFSW. The embryos were then incubated for 45 minutes in fixation buffer (3.2% formaldehyde, 0.125% glutaraldehyde, 0.2M NaH2PO4H2O, 0.136M NaCl). Fixed embryos were permeabilized in fixation buffer with 0.1% NP-40 for 20 minutes, then incubated with 50mM glycine for 15 minutes and washed three times with PBS. Immunostaining with rabbit-anti-S. purpuratus egg myosin II (1:250) was added to embryos in PBS, 0.1% Triton-X100 (PBT) and were rotated overnight at 4°C and washed three times for 20 minutes with PBT. Alexa 555 goat anti-rabbit (Life Technologies) was used at a 1:1000 dilution in PBT for 2 hours and washed three times for 20 minutes with PBT prior to imaging.
Supplementary Material
Acknowledgements
We would like to thank Josh Rosenberg for his help with the FRAP analysis and microscopy support as well as Dr. Fred Chang for his helpful comments. This work was supported in part by NIH grant GM093978 to DB.
References
- Albertson R, Cao J, Hsieh T, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol. 2008;181:777. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Alford LM, Ng MM, Burgess DR. Cell polarity emerges at first cleavage in sea urchin embryos. Dev Biol. 2009;330:12–20. doi: 10.1016/j.ydbio.2009.02.039. [DOI] [PubMed] [Google Scholar]
- Badizadegan K, Wolf AA, Rodighiero C, Jobling M, Hirst TR, Holmes RK, Lencer WI. Floating cholera toxin into epithelial cells: functional association with caveolae-like detergent-insoluble membrane microdomains. International journal of medical microbiology. 2000;290:403–408. doi: 10.1016/S1438-4221(00)80052-1. [DOI] [PubMed] [Google Scholar]
- Belton RJ, Jr, Adams NL, Foltz KR. Isolation and characterization of sea urchin egg lipid rafts and their possible function during fertilization. Mol Reprod Dev. 2001;59:294–305. doi: 10.1002/mrd.1034. [DOI] [PubMed] [Google Scholar]
- Bluemink JG, De Laat SW. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis. J Cell Biol. 1973;59:89. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proceedings of the National Academy of Sciences. 2007;104:7939. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Tisdale JM. An electron microscopic study of the development of the cleavage furrow in mammalian cells. J Cell Biol. 1962;13:117–125. doi: 10.1083/jcb.13.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wang Y. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J Cell Biol. 1990;111:1905–1911. doi: 10.1083/jcb.111.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichili GR, Rodgers W. Clustering of membrane raft proteins by the actin cytoskeleton. J Biol Chem. 2007;282:36682. doi: 10.1074/jbc.M702959200. [DOI] [PubMed] [Google Scholar]
- Conner SD, Wessel GM. Syntaxin is required for cell division. Mol Biol Cell. 1999;10:2735. doi: 10.1091/mbc.10.8.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan K. The cortical movement in Arbacia punctulata eggs through cleavage cycles. Embryologia. 1954;2:115–122. [Google Scholar]
- Dan K, Dan JC. Behavior of the Cell Surface during Cleavage: III. On the Formation of New Surface in the Eggs of Strongylocentrotus Pulcherrimus. Biol Bull. 1940;78:486–501. [Google Scholar]
- Dan K, Ono T. A method of computation of the surface area of the cell. Embryologia. 1954;2:87–99. [Google Scholar]
- Dan K, Dan JC, Yanagita T. Behaviour of the cell surface during cleavage. II. Cytologia. 1938;8:521–531. [Google Scholar]
- Dan K, Yanagita T, Sugiyama M. Behavior of the cell surface during cleavage. I. Protoplasma. 1937;28:66–81. [Google Scholar]
- Fishkind DJ, Silverman JD, Wang Y. Function of spindle microtubules in directing cortical movement and actin filament organization in dividing cultured cells. J Cell Sci. 1996;109:2041–2051. doi: 10.1242/jcs.109.8.2041. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Kenworthy AK. Photobleaching approaches to investigate diffusional mobility and trafficking of Ras in living cells. Methods. 2005;37:154–164. doi: 10.1016/j.ymeth.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Guha M, Zhou M, Wang Y. Cortical actin turnover during cytokinesis requires myosin II. Current biology. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Ishizaka S. Surface Characters of Dividing Cells I. J Exptl Biol. 1958;35:396. [Google Scholar]
- Ishmael JE, Safic M, Amparan D, Vogel WK, Pham T, Marley K, Filtz TM, Maier CS. Nonmuscle myosins II-B and Va are components of detergent-resistant membrane skeletons derived from mouse forebrain. Brain Res. 2007;1143:46–59. doi: 10.1016/j.brainres.2007.01.061. [DOI] [PubMed] [Google Scholar]
- Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4, 5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AP, Fletcher DA. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys J. 2006;91:4064–4070. doi: 10.1529/biophysj.106.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay HF, Burgess DR. ‘Life is a Highway’: Membrane Trafficking During Cytokinesis. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J. Cell membranes and cell division. 1952;6:105–127. [Google Scholar]
- Montagnac G, Echard A, Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr Opin Cell Biol. 2008;20:454–461. doi: 10.1016/j.ceb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Current Biology. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Neto H, Collins LL, Gould GW. Vesicle trafficking and membrane remodelling in cytokinesis. Biochem J. 2011;437:13–24. doi: 10.1042/BJ20110153. [DOI] [PubMed] [Google Scholar]
- Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Developmental cell. 2005;9:781–790. doi: 10.1016/j.devcel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Porter KR, Caulfield JB. The formation of the cell plate during cytokinesis in Allium cepa L. Proc 4th Internat Conf Electron Micr. 1958;2:503. [Google Scholar]
- Rappaport R. Reversal of chemical cleavage inhibition in echinoderm eggs. J Exp Zool. 1971;176:249–255. doi: 10.1002/jez.1401760210. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in animal cells. New York, New York: Cambridge University Press; 2005. p. 386. [Google Scholar]
- Rodgers W, Zavzavadjian J. Glycolipid-enriched membrane domains are assembled into membrane patches by associating with the actin cytoskeleton. Exp Cell Res. 2001;267:173–183. doi: 10.1006/excr.2001.5253. [DOI] [PubMed] [Google Scholar]
- Sanger JM, Reingold AM, Sanger JW. Cell surface changes during mitosis and cytokinesis of epithelial cells. Cell Tissue Res. 1984;237:409–417. doi: 10.1007/BF00228425. [DOI] [PubMed] [Google Scholar]
- Schiel JA, Prekeris R. Making the final cut-mechanisms mediating the abscission step of cytokinesis. Scientific World Journal. 2010;10:1424–1434. doi: 10.1100/tsw.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JK, Burke EE, Goodson HV, D'Souza-Schorey C. Endocytosis resumes during late mitosis and is required for cytokinesis. J Biol Chem. 2005;280:41628. doi: 10.1074/jbc.M504497200. [DOI] [PubMed] [Google Scholar]
- Shuster C, Burgess D. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proceedings of the National Academy of Sciences. 2002;99:3633. doi: 10.1073/pnas.052342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Current Biology. 2001;11:735–746. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland LI, Donnelly EJ, Burgess DR. Induction of cytokinesis is independent of precisely regulated microtubule dynamics. Mol Biol Cell. 2005:E05. doi: 10.1091/mbc.E05-04-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann M, Mitchison J. The mechanism of cleavage in animal cells. Biological Reviews. 1958;33:103–135. [Google Scholar]
- Wang Y, Silverman JD, Cao L. Single particle tracking of surface receptor movement during cell division. J Cell Biol. 1994;127:963–971. doi: 10.1083/jcb.127.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB. The Cell in Development and Heredity. New York: Macmillan Company; 1928. [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. The mechanics and mechanism of cleavage. Int Rev Cytol. 1960;10:163–216. [Google Scholar]
- Zhao J, Singleton PA, Brown ME, Dudek SM, Garcia JGN. Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement. Cell Signal. 2009;21:1945–1960. doi: 10.1016/j.cellsig.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.