Abstract
Chemokine (C-X-C motif) receptor 4 (CXCR4) is the receptor for chemokine (C-X-C motif) ligand 12 (CXCL12, also known as stromal derived factor-1, Sdf1). CXCR4, a protein consisting 352 amino acids, is known to transduce various signals such as cell differentiation, cell survival, cell proliferation, cell chemotaxis and apoptosis [1, 2]. The expression of CXCR4 is observed in embryonic stem cells, blood cells, haematopoietic stem cells, endothelial cells, angioblasts and smooth muscle cells [3-9]. The CXCL12-CXCR4 signaling pathway has very important roles in the embryonic development. Mutant mice for CXCL12 or CXCR4 genes showed lethality due to defects in neurogenesis, angiogenesis, cardiogenesis, myelopoiesis, lymphopoiesis and germ cell development [10-13]. Recently, we reported that CXCL12-CXCR4 signaling pathway has a crucial role in regional specification of the gut endoderm during early development [14]. Here, we would like to focus on the role of CXCL12-CXCR4 signaling pathway in pancreatic development and summarize recent findings of its role in the induction of the pancreatic progenitor cells.
Keywords: CXCL12, CXCR4, signaling pathway
An overview of early pancreas development
The earliest pancreatic marker gene, pancreatic and duodenal homeobox 1 (Pdx1) starts to be expressed in the pre-pancreatic endoderm at the 9-somite stage (ss), corresponding to embryonic day 8.5 (E8.5) in the mouse and at 8 ss in the chick 14-17. Pdx1 is also expressed in the stomach and duodenal endoderm. The pancreas is derived from the dorsal and ventral endoderm, which give rise from two distinct origins. In the mouse, the pancreatic bud emerges from E9.5. Fate map studies revealed that while the dorsal pancreas progenitor cells originated at the 3-6 somite levels at 7-9 ss in the mouse embryo, the ventral pancreatic progenitors are closely associated with hepatic progenitors and that they originates at the 1 ss at the lateral endoderm posterior to the anterior intestinal portal (AIP) lip 18. Later on, at 3-4 ss, they were observed at the outer side at the AIP lip.
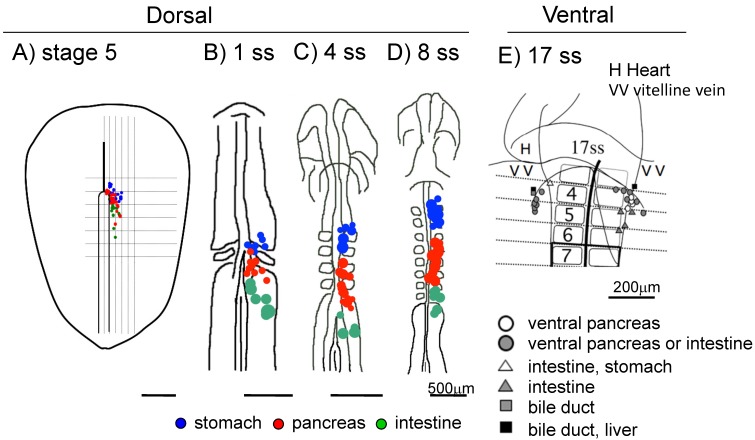
In the chick, the dorsal pancreas progenitors were first observed near the hensen's node at late gastrula stage (stage 5), which then migrate to the 3-6 somite levels at 6 ss, then to the 4-7 somite levels at 8 ss 19. The ventral pancreas progenitor cells were first observed lateral to the 2 somite level at 10 ss, which then migrate to a site lateral to the 4 somite level at 17 ss 20 (Figure 1).
Figure 1.
Fate maps of the early chick endoderm. A-D: Fate maps of the dorsal pancreas at different embryonic stages. Blue: The pre-stomach endoderm, red: pre-pancreas endoderm, green: pre-intestine endoderm. E: Fate map of the ventral pancreas. The pre-ventral pancreas resides at the 4 -somite level in the lateral endoderm near the vitelline vein at 17 ss. A: stage 5, B: 1 ss, C: 4 ss, D: 8 ss E: 17 ss. Scale bar: A-D 500μm, E 200μm.
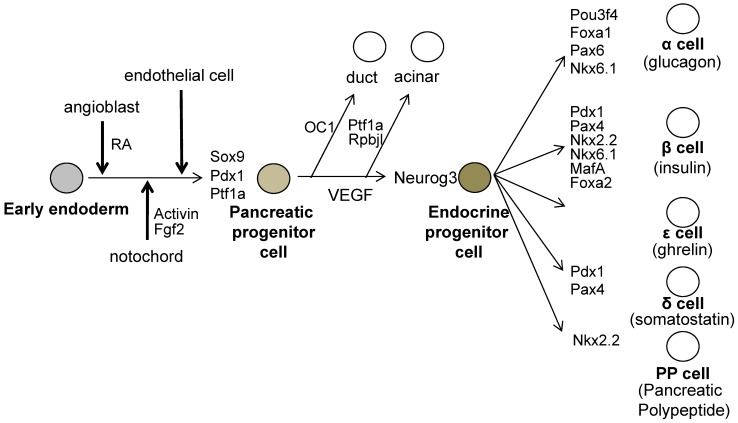
All mature pancreatic cell types are derived from the common pancreatic progenitors, which expresses Pdx1 and pancreas specific transcription factor 1a (Ptf1a) and SRY-box containing gene 9 (Sox9) 21-23. Gu et al. have shown that PDX1-expressing precursor cells give rise to all pancreas cell types, namely, the endocrine, exocrine and duct cells by Cre / LoxP lineage tracing experiments (Figure 2) 23.
Figure 2.
The molecules involved during pancreatic development. The pre-pancreatic endoderm receives the Pdx1 inducing signals from the angioblasts (One of the candidate signal is Retinoic Acid; RA). And next, Pdx1-expressing pre-pancreas endoderm receives the pancreatic maintenance signals sequentially from the notochord, then from the endothelial cells. The pancreatic progenitor cells are induced and these cells express Pdx1, Sox9 and Ptf1a. All endocrine cells are generated from neurogenin 3 (Neurog3)- expressing endocrine progenitor cells, which then differentiate into endocrine cells expressing specific set of transcription factors.
Analyses of the Pdx1 homozygous null mutations have shown that PDX1 have a key role in the pancreas development: the initial pancreatic bud formation and early insulin and glucagon expression are observed, but further development is arrested. However, overexpression of Pdx1 did not induce an ectopic pancreas in the pre-stomach or pre-intestine region in the chick embryo, therefore, Pdx1 alone does not act as a pancreatic determinant 24.
Activin and fibroblast growth factor 2 (Fgf2, also known as bFGF), which are secreted from notochord, are candidate factors for maintaining the early pancreatic related genes through repression of sonic hedgehog (Shh) expression in the pre-pancreatic endoderm 25. However, the notochord signals (Activin and Fgf2) are considered as permissive signals rather than instructive signals, because the notochord does not induce the pancreas in the posterior non-pancreatic endoderm 26. Next, the dorsal aorta replaces the notochord and comes into close contact with the pre-pancreas endoderm and gives the signals that enhance the pancreas differentiation. The dorsal aorta is necessary for dorsal pancreas development, but the molecular nature of these signals is still unknown. The induced β cells produce vascular endothelial growth factor (VEGF), then attract blood vessels, and potentiate insulin expression 27.
On the other hand, the ventral pancreas does not need the notochord signals for further development. The pre-ventral pancreas region is away from the notochord (Figure 1E). When the notochord is removed surgically, the pancreas related genes are down regulated in the dorsal pancreas but not in the ventral pancreas 18, 20, 26, 28.
The ventral pancreas and liver reside at the AIP lip region very near to each other 18, 20, 26, 28. It is proposed that they derived from bipotential progenitor cells in the ventral foregut endoderm. One of the key molecules from the cardiac mesoderm to induce the liver is FGF. When the FGF signaling was suppressed in the ventral foregut region, pancreas developed in the expense of the liver 29-31.
Role of the chemokine signals in pancreatic development: regionalization of the early endoderm
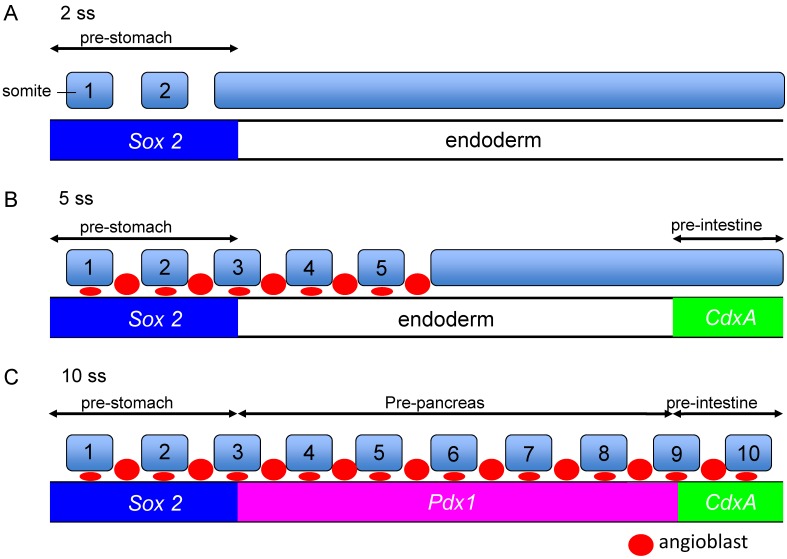
It was reported that activin and bFGF signals from the notochord are permissive signals for pancreatic development, but up to date, there is little information on the inductive signal, by which the pre-pancreas region is defined in the early endoderm. Previously, we have reported in a fate map study, which was based on transplant experiments, that regional-specific endodermal fates are specified in a chronological order of the stomach (SRY-box containing gene 2; Sox2 expression, 2 ss), intestine (caudal type homeobox 1; CdxA expression, 5 ss) and then pancreas (Pdx1 expression, 8 ss) (Figure 3) 19, 32.
Figure 3.
Competency of the endoderm for the Pdx1-inducing signals emitted from the angioblasts. A-C Regional specific endodermal fates are specified in a chronological order: the stomach (Sox2, 2ss), intestine (CdxA, 5ss) and then pancreas (Pdx1, 8ss). The angioblast emit Pdx1 inducing signals. At 5ss, although the angioblasts reside at the pre-stomach region, they cannot induce Pdx1 expression in the pre-stomach endoderm because the pre-stomach endoderm is already determined to express Sox2 at 2ss, and they lost the competency to respond to Pdx1 inducing signals from the angioblasts at 5 ss. The pre-pancreatic endoderm has the competency to respond to signals from the angioblasts. On the other hand, the pre-intestine endoderm is determined to express CdxA at 5 ss, and lost their competency to respond to Pdx1-inducing signals from the angioblasts at the time they reach the pre-intestine at 10ss.
More recently, we revealed that the angioblasts have a crucial role in inducing the Pdx1 expression in the pre-pancreas region in the chick embryo. We found that Cxcl12 is expressed in the endoderm and Cxcr4 is expressed in the angioblasts, which reside in the lateral plate mesoderm and the early endoderm, before it starts to express Pdx1 14.
At 8 ss, Pdx1 starts to be expressed in the inter-somite endoderm in the pre-pancreas region (4-7 somite levels). Later, the Pdx1 expression extends to the endoderm underneath the somites. This Pdx1 expression pattern correlates closely with the spreading of the angioblasts at between the somites, as well as with the migration of the endoderm. The Pdx1-expressing pre-pancreas endoderm lies in contact with the angioblasts, which express angioblast markers such as LIM domain only 2 (Lmo2), T-cell acute lymphocytic leukemia 1 (Tal1), kinase insert domain protein receptor (Kdr, also known as Flk1) and CD34 antigen (Cd34) 14.
The angioblasts reside in the pre-stomach and pre-pancreas, but not in the pre-intestine region at early somitic stages, coinciding with pancreas inducing signals existing in the pre-stomach and pre-pancreas region at these stages 19. The angioblasts begin to appear around the pre-stomach region at 4 ss, in contact with the endoderm. However, these angioblasts do not have the ability to induce the Pdx1 expression in the pre-stomach region. Since the pre-stomach is already committed to express Sox2 (stomach marker gene) at 2 ss, it lost the competency to the Pdx1 inducing signals emitted from the angioblasts. Similarly, the angioblasts appear at the pre-intestine region at 10 ss, but are not able to induce Pdx1 expression in the pre-intestine region. Since the pre-intestine region is already committed to express CdxA (intestine marker gene) at 5 ss, it lost the competency to the Pdx1 inducing signals emitted from the angioblasts (Figure 3) 14, 19.
Ectopic overexpression of Cxcl12 in the early endoderm attracted the Lmo2-expressing cells (angioblast), which then induced the Pdx1 and Sox9 expressing pancreatic progenitors and resulted in generating a bigger pancreas and expanded insulin-expressing area. Conversely, inhibition of CXCR4 by AMD3100 treatment disturbed the migration of angioblasts to the proximity of the endoderm and delayed the timing of the aorta formation. This caused a reduction in Pdx1- and Sox9-expressing pancreatic progenitors and led to a smaller pancreas with a smaller insulin-expressing area. These results suggest that the correct timing that angioblasts come in contact with the endoderm is a key to induce the Pdx1 expression (the induction of a pancreatic fate) in the pre-pancreas region. And this timing is finely controlled by CXCL12-CXCR4 signaling pathway (Figure 3) 14.
The role of the chemokine signals in the early pancreatic differentiation
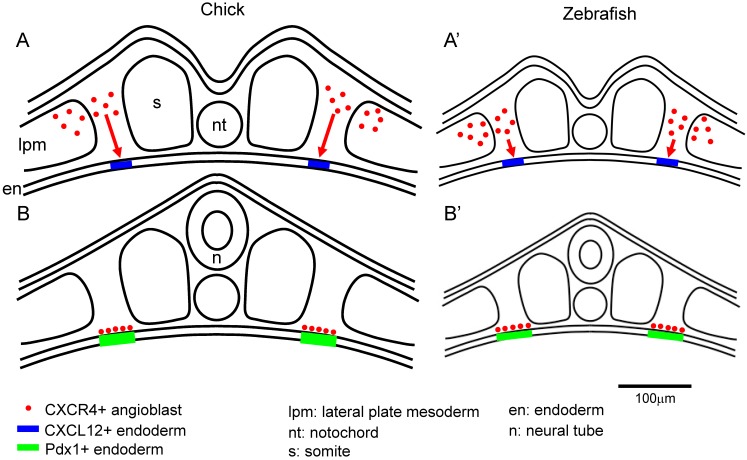
The CXCL12-CXCR4 signaling pathway has key roles in the vascular formation. Cxcl12 or Cxcr4 mutant embryos die before birth. And there are defects in the vasculature of the gastrointestinal and nerve systems 11-13, 33. It is known that CXCL12 and CXCR4 are expressed in the mouse islets. And it is also known that CXCR4 is expressed in the proliferating duct epithelium in the regenerating pancreas of non-obese diabetic (NOD) mice engineered to express interferon (IFN)-γ driven by the rat insulin promoter (RIP). In the IFN-γ transgenic mice, the inhibition of CXCR4 function caused the defects in cell proliferation and increases apoptosis in the pancreatic duct cells 34. In the RIP-CXCL12 transgenic mice, the development of diabetes by the streptozotocin treatment is rescued. In this report, it is also revealed that six hours after streptozotocin administration, a pro-proliferative signal, phospho-Akt, was activated in the α cells, but not in the β cells. In the control mice, β cells were replaced by the α cells two weeks after streptozotocin treatment. In contrast, in the RIP-CXCL12 transgenic mice, many β cells persisted or regenerated 35. These data suggest that a cross-talk mechanism might exist between the α and β cells. It is reported that CXCL12 shows cytoprotective effects on the β cells, and controls the β cell survival not only through the pro-survival kinase Akt, but also through the activation and stabilization of β-catenin/transcription factor 7 like 2, T cell specific, HMG box (TCF7L2) transcriptional activators 36. It is also reported that CXCL12 expression is re-induced in the β cells by treatment with streptozotocin, thapsigargin and cytokines in the adult islet and in the rat insulinoma INS-1 cells. CXCR4 is expressed in both the α and β cells. The paracrine signal of CXCL12 from β cells activates in α cells the protein kinase Akt and induces the production of prohormone convertase (PC) 1/3 and glucoincretin hormone glucagon-like peptide-1 (GLP-1). CXCL12 also shows anti-apoptotic activity. Finally, GLP-1 signal from α cells, in combination with CXCL12 are shown to control the growth and viability of β cells 37. Recently, it was also reported that Epithelial progenitor 1 (EP1), which is related to the uPAR/CD59/Ly-6/snake toxin family that contains cysteine-rich domains, was detected in the duct cells of regenerating pancreas of the IFN-γ transgenic mice, but not in the embryonic stages, or in the wild type mice. Flow cytometry analysis revealed that the EP-1 positive cells in the above INF-γ transgenic mice, also expressed CXCR4. In the RIP-CXCL12 transgenic mice, upon cerulein treatment EP-1 were expressed in the epithelial cells in a scattered manner throughout the pancreas. These data suggest that EP1-expressing cells respond to CXCL12 and are involved in epithelial cell expansion. Until now, there is not much information on how EP1 regulates the pancreatic regeneration through the CXCL12-CXCR4 axis. Further analysis is required to identify the function of EP1 in the pancreatic regeneration process 38. CXCL12 also control the morphological branching in the developing pancreas 39. However, the defect in branching was observed in the ventral but not in the dorsal pancreas in the Cxcl12 mutant mice 39. In the zebrafish, inhibition of cxcl12b and cxcr4a caused the duplication of pancreas 40. This is compatible with a scenario in which CXCL12-CXCR4 axis affects the migration of the angioblasts, which induced the Pdx1 expression in early endoderm in the chick embryo. This discrepancy can be explained by the difference in the size between the chick and zebrafish embryos. We hypothesize that because the zebrafish embryo is much smaller, the cxcr4a- and Lmo2-positive mesodermal cells (angioblasts), which originated from the lateral plate mesoderm, do not have to travel a long distance to reach the site at the pre-pancreatic endoderm border compared to the case of the chick embryo. However, without guidance by the CXCL12-CXCR4 signaling, the migration of the angioblasts to the correct region (pre-pancreatic region) in the zebrafish might be disturbed, thereby generating a duplicated pancreas. Another possibility is that in smaller size embryos (for example, zebrafish), at conditions without the CXCL12-CXCR4 signaling, a lower number of angioblasts might have reached the pre-pancreas endoderm, compared with the wild type embryos. This resulted in the induction of Pdx1 in a patchy fashion in the endoderm, which then yielded a duplicated pancreas. Furthermore, cxcr4a is also important for the migration of endoderm cells themselves 40, 41. Therefore, pancreas formation might also be affected in the CXCR4 inhibited embryos. In contrast, in the chick embryo, because of its large size, Cxcr4 and Lmo2-positive mesodermal cells (angioblasts) have to cover a larger distance to contact the pre-pancreas region at a correct timing. We hypothesize that when lacking the CXCL12-CXCR4 signaling guidance in the chick embryos, the directed migration fails to occur properly (Figure 4) 14. It is also reported that endothelial cells are generated from cxcr4a positive anterior mesoderm and cxcl12b is expressed in the endoderm underlying the lateral aorta in the zebrafish embryos. It is also reported that Cxcl12b-Cxcr4a signaling has crucial roles in the lateral aorta formation in the zebrafish 42. This results support our observations in the early chick embryos 14.
Figure 4.
The size of the embryo affects the migration distance of the angioblasts from the lateral plate mesoderm to the endoderm. A-B: The angioblasts at the lateral plate mesoderm migrate to the correct side of the endoderm, being attracted by the CXCR12-CXCR4 signaling. Since the chick embryo is bigger than the zebrafish, the angioblasts in the chick embryos have to travel a longer distance from the lateral plate mesoderm to the endoderm, compared to that in the zebrafish (A). In the zebrafish, the angioblasts migrate to the endoderm through a comparatively shorter distance (A'). After arriving at the endoderm, the angioblast induce Pdx1 expression in the pre-pancreas region (B,B'). Scale bar: 100μm.
Conclusion
We revealed the mechanism by which the early pancreas regionalization is controlled in the early endoderm. CXCL12-CXCR4 signaling pathway controls the spatiotemporal migration of the angioblasts, which has the pancreatic inducing activity. CXCL12-CXCR4 signaling does not directly induce the expression of Pdx1 (Pancreas) in the early pancreas, but rather through an indirect way, by controlling the migration of the angioblasts. The understanding of the nature of normal pancreatic development is a key to succeed the regeneration of β cells from pluripotent stem cells to treat the diabetes mellitus 43, 44.
References
- 1.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S. et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 2.Schober A, Karshovska E, Zernecke A, Weber C. SDF-1alpha-mediated tissue repair by stem cells: a promising tool in cardiovascular medicine? Trends Cardiovasc Med. 2006;16:103–8. doi: 10.1016/j.tcm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B. et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–91. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S. et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 5.Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008;26:1464–73. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 6.Moll NM, Ransohoff RM. CXCL12 and CXCR4 in bone marrow physiology. Expert Rev Hematol. 2010;3:315–22. doi: 10.1586/ehm.10.16. [DOI] [PubMed] [Google Scholar]
- 7.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R. et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–7. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 9.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 10.Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94:400–7. doi: 10.1093/cvr/cvs132. [DOI] [PubMed] [Google Scholar]
- 11.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 13.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 14.Katsumoto K, Kume S. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development. 2011;138:1947–55. doi: 10.1242/dev.058719. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida T, Murata K, Shiraki N, Kume K, Kume S. Analysis of gene expressions of embryonic stem-derived Pdx1-expressing cells: implications of genes involved in pancreas differentiation. Dev Growth Differ. 2009;51:463–72. doi: 10.1111/j.1440-169X.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–9. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 17.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA. et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 18.Miki R, Yoshida T, Murata K, Oki S, Kume K, Kume S. Fate maps of ventral and dorsal pancreatic progenitor cells in early somite stage mouse embryos. Mech Dev. 2012;128:597–609. doi: 10.1016/j.mod.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Katsumoto K, Fukuda K, Kimura W, Shimamura K, Yasugi S, Kume S. Origin of pancreatic precursors in the chick embryo and the mechanism of endoderm regionalization. Mech Dev. 2009;126:539–51. doi: 10.1016/j.mod.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura K, Katsumoto K, Fukuda K, Kume K, Kume S. Conserved origin of the ventral pancreas in chicken. Mech Dev. 2009;126:817–27. doi: 10.1016/j.mod.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 22.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R. et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–70. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 24.Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–54. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–13. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–52. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 27.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–7. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 28.Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Zaret KS. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet. 2008;9:329–40. doi: 10.1038/nrg2318. [DOI] [PubMed] [Google Scholar]
- 30.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–4. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–81. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 32.Katsumoto K, Shiraki N, Miki R, Kume S. Embryonic and adult stem cell systems in mammals: ontology and regulation. Dev Growth Differ. 2010;52:115–29. doi: 10.1111/j.1440-169X.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 33.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–85. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 34.Kayali AG, Van Gunst K, Campbell IL, Stotland A, Kritzik M, Liu G. et al. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–69. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yano T, Liu Z, Donovan J, Thomas MK, Habener JF. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes. 2007;56:2946–57. doi: 10.2337/db07-0291. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Habener JF. Stromal cell-derived factor-1 promotes survival of pancreatic beta cells by the stabilisation of beta-catenin and activation of transcription factor 7-like 2 (TCF7L2) Diabetologia. 2009;52:1589–98. doi: 10.1007/s00125-009-1384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Stanojevic V, Avadhani S, Yano T, Habener JF. Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia. 2011;54:2067–76. doi: 10.1007/s00125-011-2181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kritzik MR, Lago CU, Kayali AG, Arnaud-Dabernat S, Liu G, Zhang YQ. et al. Epithelial progenitor 1, a novel factor associated with epithelial cell growth and differentiation. Endocrine. 2010;37:312–21. doi: 10.1007/s12020-009-9297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hick AC, van Eyll JM, Cordi S, Forez C, Passante L, Kohara H. et al. Mechanism of primitive duct formation in the pancreas and submandibular glands: a role for SDF-1. BMC Dev Biol. 2009;9:66. doi: 10.1186/1471-213X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008;135:2521–9. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- 42.Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23:2272–7. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kume S. Stem-cell-based approaches for regenerative medicine. Dev Growth Differ. 2005;47:393–402. doi: 10.1111/j.1440-169X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 44.Kume S. The molecular basis and prospects in pancreatic development. Dev Growth Differ. 2005;47:367–74. doi: 10.1111/j.1440-169X.2005.00813.x. [DOI] [PubMed] [Google Scholar]