Abstract
Purpose
This review provides researchers and practitioners with an overview of the physical activity and pregnancy literature to promote prenatal physical activity, improve measurement, further elucidate the role of activity in reducing maternal health complications, and inform future research.
Methods
We examined past and present physical activity and pregnancy studies and highlight key papers with a particular focus on maternal health outcomes to best inform physical activity promotion efforts.
Results
This review discusses: (a) historical overview of prenatal physical activity with a specific focus on the physical activity guidelines, how they have changed over time, and how evidence of the effect of prenatal activity on maternal/fetal health outcomes has impacted clinical recommendations; (b) existing tools and challenges associated with measuring prenatal physical activity; (c) empirical evidence on the multi-level determinants of prenatal activity to help guide future intervention work; (d) empirical evidence of prenatal activity on adverse maternal outcomes (gestational diabetes mellitus, preeclampsia, excessive gestational weight gain) from observational and intervention studies; and (e) summary/recommendations for future research and practice.
Conclusions
The physical activity and pregnancy literature has evolved over the past 50 years and there is currently sufficient empirical evidence to support the promotion of moderate to vigorous prenatal physical activity for maternal health benefits. Future studies and interventions should be carefully-designed, theoretically driven, and include validated and reliable measures of activity. Researchers and practitioners should also consider the multifaceted determinants and outcomes of prenatal physical activity and intervening to promote physical activity before, during, and after pregnancy.
Keywords: prenatal, exercise, review
Pregnancy is a time in women’s lives that is associated with considerable physiological and psychological changes which may promote sedentary behaviors and/or low levels of physical activity (PA). Such behaviors have been associated with elevated risk of gestational diabetes, pregnancy-induced hypertension, high gestational weight gain, and the long-term risk for overweight/obesity development, Type 2 diabetes, and cardiovascular disease (United States (US) Department of Health and Human Services [USDHHS], 2008). Recent epidemiological data based on the National Health and Nutrition Examination Survey (NHANES) indicates that only 15% of pregnant women meet the minimum national recommendations of 150 minutes of moderate-intensity PA per week (Evenson & Wen, 2010b; USDHHS, 2008). Also, until recently, intervention efforts to promote prenatal PA have been relatively sparse and those which have been conducted are typically limited to small samples of predominantly non-Hispanic, White women, have varied in effectiveness, and/or have had limited translatability to clinical practice. One reason for this is likely due to the challenges associated with intervening during pregnancy (e.g., nausea, fatigue, increased body size, low motivation, pregnancy complications) and the varied clinical and research perspectives regarding how much PA in pregnancy should be recommended. Moreover, measuring PA in pregnancy has been plagued with methodological challenges (e.g., lack of valid measures, limited objective PA data), making it difficult at best to obtain accurate estimates of PA volume and understanding the true impact of prenatal PA on maternal and ultimately infant health outcomes. Considering these issues, it is not surprising that researchers, clinicians, and interventionists are often reluctant to make recommendations about how to promote and effectively, efficiently, and safely increase prenatal PA.
The objectives of this review are to provide researchers and practitioners with a comprehensive overview of the PA and pregnancy literature in an effort to promote prenatal PA, improve pregnancy-related PA measurement, further elucidate the role of PA in reducing maternal prenatal health complications, and inform future study and intervention design. We examined past and present PA and pregnancy studies and highlighted key papers with a particular focus on maternal health outcomes to best inform PA promotion efforts. To this end, we first provide a historical overview of the PA guidelines in pregnancy, how they have changed over time, and how the accumulating evidence of the effect of prenatal PA on maternal and fetal outcomes has impacted clinical guidelines. Second, we discuss measuring pregnancy-related PA and its associated challenges. The third section provides an overview of the empirical evidence on the multi-level determinants of prenatal PA to help guide future intervention work. The fourth section focuses on the empirical evidence of prenatal PA on adverse maternal outcomes (e.g., gestational diabetes mellitus, preeclampsia, excessive gestational weight gain). The review concludes with a summary and recommendations for future research and practice.
Historical Overview of Prenatal Physical Activity
The earliest recommendations for prenatal PA largely reflected the cultural and social norms of the times, rather than scientific evaluation (Mittelmark & Gardin, 1991). In the 18th Century, while maternal PA was viewed favorably and associated with easier labor and reduced fetal size (Kerr, Johnstone, & Phillips, 1954), a number of PA limitations were promulgated (e.g., avoidance of dancing and horseback riding). The first scientific studies on the relationship between maternal PA and birth outcomes were published in the late 19th and early 20th Centuries (Briend, 1980) and focused on determinants of birth weight and attributed lower birth weights to increased levels of occupational and household PA; and, in turn, attributed higher birth weights to prescribed maternal rest. In the beginning of the 20th Century, ‘moderate PA’ was defined as a daily walk of at least 2–6 miles. In the 1920’s and 1930’s, the prenatal exercise program was introduced in the US with the goal of easing labor/delivery and prescribed breathing patterns and PA for improving muscle tone, diminishing labor pain, improving fetal oxygenation, and facilitating postpartum weight loss (Mittelmark & Gardin, 1991).
In 1949, the US Children’s Bureau issued a standard recommendation for prenatal PA: in the absence of maternal complications, pregnant women can continue housework, gardening, daily walks (up to 1-mile in several short bouts), and even swim occasionally but should avoid sports participation (Federal Security Agency and Social Security Administration, 1949; Sternfeld, 1997). Such recommendations for ‘moderate PA’ formed the basis of prenatal exercise programs of the 1970s and 1980s which were highly specific and focused mainly on improving maternal fitness and easing labor/delivery. In 1985, ACOG issued the first guidelines for prenatal PA. They were based on the consensus opinion of a panel of obstetricians, endorsed the safety of most aerobic PA but advised caution with high impact activities such as running and included restrictions for duration (no longer than 15 minutes for strenuous PA), heart rate (no greater than 140 beats/minute), and core body temperature (no greater than 100.4°F/38°C).
Over the following decade, several epidemiologic studies evaluated the association between prenatal PA and maternal/fetal outcomes. In a key scientific study, Clapp and Capeless (1990) found that pregnant women who continued a program of moderate-to-high intensity exercise experienced a 300–500 gram reduction in birth weight compared to sedentary controls or women who reduced their prenatal PA. This reduction in birth weight, however, was still within normal range and there was no difference in risk of preterm birth between the groups. Generalizability of study findings were limited by a small sample size and restricting the study population to athletes. These findings were not confirmed in larger prospective epidemiologic studies which found, overall, either neutral or beneficial effects of exercise during pregnancy on birth weight (Hatch et al., 1993; Schramm, Stockbauer, & Hoffman, 1996; Sternfeld, Quesenberry, Eskenazi, & Newman, 1995). In fact, studies with more rigorous measures of total activity (e.g., sports/exercise, household/childcare, occupational, active living) were more likely to find neutral or beneficial effects (Chasan-Taber, Evenson, Sternfeld, & Kengeri, 2007). The lack of evidence for any harmful effects of prenatal PA on pregnancy outcomes suggested that for healthy women, prenatal PA was safe and subject to few limits (Sternfeld, 1997). This conclusion was reflected in the revised 1994 ACOG guidelines which placed almost no restriction on maternal PA and eliminated parameters for heart rate and exercise duration.
In 2002, ACOG released updated guidelines that recommended 30 minutes of moderate- intensity PA during most days of the week for pregnant women without medical/obstetrical complications and suggested that participating in a wide-range of recreational PA is safe (ACOG, 2002; Pate et al., 1995). Consistent with ACOG, the USDHHS released in 2008 the “Physical Activity Guidelines for Americans” which recommended at least 150 minutes of moderate-intensity PA per week for pregnant women without obstetric/medical complications. This report also put forward, for the first time, guidelines for vigorous-intensity aerobic PA (pregnant women who habitually engage in vigorous-intensity aerobic PA can continue vigorous PA as long as they discuss with their healthcare provider how and when activity should be modified over time). This report provided strong scientific evidence for the safety of moderate-intensity PA; stating that it does not elevate the risk for low birth weight, preterm delivery, or early pregnancy loss. Moreover, it highlighted the growing evidence that PA reduces the risk of pregnancy complications (e.g., preeclampsia, gestational diabetes mellitus) and the length of labor, however, these guidelines also noted the evidence as of 1998 was not conclusive.
It is important to note that international guidelines for prenatal PA are relatively consistent with US recommendations. For example, the Netherlands has generally adopted the USDHHS (2008) PA guidelines for pregnancy. Also, the Canadian National Guidelines (Wolf, Mottola, & MacKinnon, 2003) and recommendations for the UK and Australia supported by The Royal College of Obstetrician and Gynecologists (RCOG, 2006) state that all women without contraindications should be encouraged to participate in aerobic and strength-conditioning PA as part of a healthy lifestyle. The Canadian Academy of Sports Medicine further specifies that pregnant women who have been previously active may continue PA in the first trimester to a maximum of 30–40 minutes at a frequency of 3–4 times per week as tolerated (Alleyne, 2008).
Recent calls have been made for ACOG to update their 2002 guidelines in light of the growing body of research on maternal prenatal PA over the past decade. Such revisions could provide greater specificity by defining moderate-intensity PA, addressing the specific weekly energy expenditure to be attained, as well as clarifying the impact of incorporating vigorous PA on maternal/infant health outcomes. Also, in light of the increasing obesity epidemic worldwide, updated PA guidelines are also needed to address the special issues surrounding obesity in pregnancy. In particular, recent findings indicating that fetal exposure in utero to maternal obesity, excessive gestational weight gain, and abnormal glucose tolerance critically influence the risk of subsequent overweight/obesity in the offspring. This evidence highlights the need for PA guidelines targeted to overweight/obese pregnant women with the goal of reducing the inter-generational impact of obesity (Mottola, 2009).
In summary, beliefs and recommendations regarding prenatal PA have varied widely over the course of history. While initially, the health benefits of prenatal PA were accepted as ‘common sense’, subsequent time periods saw the introduction of the concept of moderation of PA in pregnancy. Formal guidelines for prenatal PA were not introduced in the US until the mid 20th century. In the past 25 years, the body of evidence evaluating the impact of prenatal PA on maternal and fetal outcomes has increased.
Measurement of Prenatal Physical Activity
In this section we provide an overview of measuring prenatal PA and highlight advantages and disadvantages of different methodologies. PA measurement is typically divided into two broad types: self-report and objective assessment, with a summary of examples of each type provided in Table 1. Both types of measures have been used in studies of pregnant women.
Table 1.
Examples of Physical Activity Measures Collected During Pregnancy
Several PA questionnaires have been developed and evaluated for evidence of both validity and reliability among pregnant women, with a recent comprehensive review available elsewhere (Evenson, Chasan-Taber, Symons Downs, & Pearce, in press). For example, the Pregnancy Physical Activity Questionnaire (Chasan-Taber et al., 2004; Ota et al., 2008) is a self-administered questionnaire that includes activities that are important discriminators of PA among pregnant women. The self-administered Kaiser Physical Activity Survey (Schmidt et al., 2006) was modified from its original format (Ainsworth, Sternfeld, Richardson, & Jackson, 2000) for use among pregnant women. Both of these questionnaires assess total PA (e.g., household/childcare, occupational, sports/exercise, transportation) during a trimester. To capture more recent PA, the interviewer-administered third Pregnancy, Infection, and Nutrition Study (PIN-3) PA questionnaire (Evenson & Wen, 2010a) was developed to assess moderate-to-vigorous PA among pregnant women in the past week and perceived intensity of each type of PA. Perceived intensity is captured for each activity mode by asking women “considering their breathing and heart rate, how hard did the activity feel” with response options of fairly light, somewhat hard, hard, and very hard. For all three questionnaires, absolute intensity can be assigned to modes of activities using metabolic equivalent (MET) values (Ainsworth et al., 2011). However, one challenge of relying on metabolic tables to assign intensity of PA is that the energy cost of a moderate-to-vigorous PA performed in the identical manner likely increases through pregnancy. In support of this, one study found that pregnant women walked more slowly than non-pregnant women, indicating that pregnant women may compensate for the increased energy costs of an activity by performing it more slowly (Lof, 2011). Also, the compendium of physical activities used to assign MET values to activities is based on adults and does not account for pregnancy (Ainsworth et al., 2011). Thus, it may be valuable to also collect perceived intensity during the PA recall.
A science-base is also developing around non-exercise activity thermogenesis (NEAT), a distinct form of exercise that includes daily activities such as sitting, standing, and walking (Levine, 2004; Levine, Vander Weg, Hill, & Klesges, 2006). NEAT can vary by up to 2000 kilocalories/day between persons (Levine, 2006). A combination of accelerometers and inclinometers can assess NEAT, while also distinguishing sitting from standing and sedentary behavior from walking. This is an area for future exploration as it pertains to pregnant women because few, if any, studies have documented NEAT during pregnancy.
Studies often use PA self-reported measures from large US surveillance data collection efforts such as the Behavioral Risk Factor Surveillance System (Evenson, Savitz, & Huston, 2004), the National Health and Nutrition Examination Survey (NHANES; Evenson & Wen, 2010b), and the Pregnancy Risk Assessment Monitoring System (PRAMS; Bovbjerg & Siega-Riz, 2009). Recently accelerometry, an objective measure of PA, was added to the NHANES and included a sufficient number of pregnant women who wore the ActiGraph accelerometer affording an opportunity to examine objective PA assessments (Evenson & Wen, 2011).
Several advantages to questionnaires include reduced participant burden, inexpensive, and can assess type of PA (e.g., exercise, household, transportation) and perceptions of intensity. Questionnaire options usually include self- or interviewer-administered, although results may differ if mode of administration is mixed within the same study population. A major disadvantage to both questionnaires and diaries is the potential for recall bias. However, recent studies have found reasonable reliability and validity for such measures and significant correlations with pregnancy outcomes (Pivarnik, et al., 2006; USDHHS, 2008). Moreover, keeping a diary may lead to changes in a woman’s behavior (i.e., reactivity) and in fact, diaries have been used in prior studies for self-monitoring and goal-setting as intervention tools for behavior change (Lindseth & Vari, 2005).
The advantages of using objective PA measures include a more precise estimate of PA volume and eliminating or reducing recall bias, literacy, and cultural differences. Disadvantages include cost, dependency on participant to wear the monitor, and difficulty in assessing long-term PA patterns (e.g., most monitors store data for limited periods of time). Also, the monitors cannot be worn during water activities, which limits the ability to assess activity output from swimming. The monitors may also be inadvertently worn incorrectly if placed at the hip, due to changes in the pregnant woman’s girth (Connolly, Coe, Kendrick, Bassett, & Thompson, 2011; DiNallo, Symons Downs, & Le Masurier, 2012). To date, many of the accelerometers used in pregnancy have been only uniaxial in nature, and therefore are not able to accurately represent PA in dimensions other than the vertical plane. Accelerometers also often require calibration studies to determine how to interpret PA intensity. Such calibration studies have not been performed among pregnant women, and are complicated by the fact that the resultant values may be dependent on the time course of pregnancy. Several studies have noted that compliance with wearing accelerometers declines in pregnancy (McParlin, et al., 2010; Rousham, Clarke, & Gross, 2005), which may be due to increased discomfort with waist-worn devices over the course of pregnancy as well as increasing periods of sleep (a time during which participants are typically instructed to remove the monitor).
Furthermore, heart rate monitors can be problematic, since heart rate is variable across pregnancy and it can be affected by other exposures besides PA. Also, these monitors are often bulky and the chest strap can be uncomfortable to wear, particularly for pregnant women who experience breast tenderness. Although pedometers have been used in some prenatal studies to assess locomotion type activities (i.e., running, walking), they have not been able to differentiate intensity level (e.g., mild from moderate PA). However, with newer technology and continually updated devices available on the market, these limitations will likely be overcome.
PA among pregnant women will continue to be ascertained using both self-reported and objectively measured assessments. Challenges to self-reported measures include recall bias, cultural adaptation issues, and the potential for differential recall over time for the same activity. Challenges to objective measurement include cost, burden, and accuracy of hip-worn devices with changing girth due to the woman’s pregnancy. Continued research is needed to improve both self-reported and objective PA assessments among pregnant women. Improving PA measurement will help researchers more accurately assess PA as both an exposure and an outcome and better clarify any existing dose response relationships.
Empirical Evidence: Determinants of Prenatal Physical Activity
The next section reviews determinants of prenatal PA to help guide future intervention work. Given the low rates of prenatal PA (Evenson et al., 2004; Evenson & Wen, 2010b), coupled with the many benefits associated with PA (USDHHS, 2008), there is a need to develop effective strategies to promote PA and reduce obesity-related health issues among pregnant women.
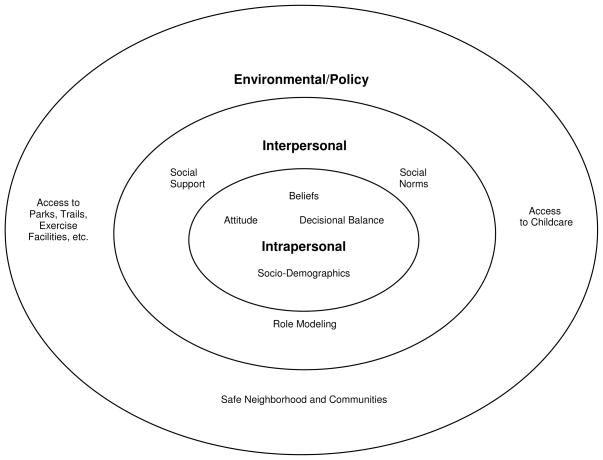
In addition to understanding the effects of sociodemographic influences, identifying what factors motivate or prevent pregnant women from engaging in prenatal PA is of upmost importance in guiding intervention planning and programming. These motivating factors and barriers can be conceptualized on a multi-level socio-ecological framework that purports that there are interwoven relationships between an individual and their environment (Glanz, Rimer, & Viswanath, 2008). Using a multi-level socio-ecological model, PA motivating factors and barriers can be intrapersonal, interpersonal, environmental, and policy-related (see Figure 1).
FIGURE 1.
Multi-level Determinants of Prenatal Physical Activity.
Evidence suggests that intrapersonal factors strongly influence PA beliefs and behaviors (Evenson, Moos, Carrier, & Siega-Riz, 2008). In general, study findings suggest that there are positive beliefs that prenatal PA contributes to overall well-being (Doran & O’Brien, 2007), stress relief (Duncombe, Wertheim, Skouteris, Paxton, & Kelly, 2007; Rich, Currie, & McMahon, 2004), easier labor/delivery (Rutkowska & Lepecka-Klusek, 2002), reduced depressive/anxiety symptoms (Leiferman, Swibas, Koiness, Marshall, & Dunn, 2011), enjoyment of PA (Jukic et al., in press), and after the baby is born, an enhanced maternal-infant relationship (Rich et al., 2004). Sociodemographic factors known to influence PA beliefs and behaviors include education and income (Evenson et al., 2004; Grace et al., 2006; Ning et al., 2003), age (PA frequency/duration declines with advancing maternal and gestational age; Haakstad, Voldner, Henriksen, & Bo, 2009), and parity (first-time mothers more likely to engage in PA than multiparous mothers; Cramp & Bray, 2009; Grace, Williams, Stewart, & Franche, 2006; Ning et al., 2003). Also, pre-pregnancy body mass index (BMI) is negatively associated with PA. However, it is positively associated with increasing PA after becoming pregnant (Hinton & Olson, 2001), although some researchers suggest that pregnant women of higher pre-pregnancy BMIs (i.e., >25 kg/m2) are more likely to stop exercising by the third trimester (Mottola & Campbell, 2003). Since little is known about potential unique determinants of prenatal PA among overweight and obese women, more research is warranted to inform effective intervention development and implementation in this special population.
At the interpersonal level, consistent with extant evidence, social role models and/or support systems influence PA (Haakstad et al., 2009; Thornton et al., 2006). Having access to informational (e.g. how to safely and effectively engage in prenatal PA) and emotional support (e.g. perceived sense of accountability, support from friends and family) motivates individuals to engage in PA (Leiferman et al., 2011). At the environmental level, perceived community safety, the use of PA as a means of transportation, access to childcare, and access to trails, parks, and built environments that are conducive to PA are also commonly cited motivating factors (Kieffer, Willis, Arellano, & Guzman, 2002; Thornton et al., 2006). Given this knowledge, intervention strategies should target personal, social, and environmental factors to facilitate behavioral change and promote prenatal PA. Furthermore, within the socio-ecological framework, it can be assumed that a woman is more likely to engage in prenatal PA if she has positive beliefs about the importance of prenatal PA, has the knowledge and skills to engage in prenatal PA, and has adequate support to be active during pregnancy at the intrapersonal, interpersonal, and environmental levels.
However, behavioral change is difficult and often times individuals who have the appropriate knowledge and skills to perform the behavior are still unable to do so due to competing barriers. There is ample evidence providing insight to the PA barriers pregnant women encounter. At the intrapersonal level, perceived barriers to prenatal PA include physical discomfort from nausea, fatigue, shortness of breath, heart burn, leg cramps, and body soreness (Duncombe et al., 2007; Evenson et al., 2008; Kieffer et al., 2002; Marquez et al., 2009; Symons Downs & Hausenblas, 2004; Symons Downs & Ulbrecht, 2006). Additional perceived barriers include beliefs such as embarrassment related to appearance (Kieffer et al., 2002), uncertainty about how to exercise safely during pregnancy (Doran & O’Brien, 2007; Kieffer et al., 2002), concern about injury (Vladutiu, Evenson, & Marshall, 2010), and surprisingly, lack of or incorrect information from healthcare providers (Clarke & Gross, 2004; Doran & O’Brien, 2007; Duncombe et al., 2007; Rutkowska & Lepecka-Klusek, 2002). Not surprisingly, another commonly reported prenatal PA barrier is a perceived lack of time, especially due to childcare commitments (Leiferman et al., 2011; Symons Downs & Hausenblas, 2004; Symons Downs & Ulbrecht, 2006). Interventionists should focus on providing accurate information to dispel popular myths about prenatal PA as well as highlight the positive effects of PA on reducing many physical discomforts associated with pregnancy. Moreover, helping pregnant women with time management and competing self-endeavors can help facilitate behavioral change when perceived available time is limited (Ewart, 2009). Interpersonal barriers include a lack of social support (Doran & O’Brien, 2007; Kieffer et al., 2002), role modeling, and social norms that encourage PA (Leiferman et al., 2011). Some studies reveal potential cultural and/or ethnic differences that warrant further investigation. For example, among pregnant Latina women, Thornton et al. (2006) found that social support systems (i.e., husbands, other family members) were integral to women’s adherence to and belief in the importance of PA. In contrast, Esperat, Feng, Zhang, and Owen (2007) found a significant, and negative correlation between social support and health-promoting behaviors among low-income African American women (Esperat et al., 2007). Conducting formative work on the target population is critical in identifying the types and amount of social support needed to promote prenatal PA. Last, environmental barriers include lack of access to suitable areas for PA (e.g., safe parks, trails, exercise facilities) and poor weather (Kieffer et al., 2002; Leiferman et al., 2011; Symons Downs & Hausenblas, 2004). Thus, helping pregnant women identify available resources and/or providing environmental resources will help facilitate behavioral change.
Based on the above findings it is clear that there are many multi-level factors that either facilitate or prevent women’s engagement in prenatal PA. The importance of this evidence is underscored given that many current health behavior theories purport that a critical construct in changing one’s behavior is based on the ‘decisional balance’. The decisional balance suggests that in considering behavior change, an individual will weigh the pros and the cons prior to deciding to engage in the health behavior. Individuals whose pros outweigh the cons are more likely to make the behavioral change. Thus, implementing intervention strategies that highlight motivating factors and reduce perceived barriers to positively influence decisional balance may further promote behavioral change.
In reviewing the literature, the majority of prenatal PA intervention studies have not applied theoretically-based strategies to promote PA behavior; thus limiting mechanistic insight to intervention successes (Gaston & Cramp, 2011). Of the intervention studies that were theoretically-based, some focused on a primary behavioral change agent, self-efficacy (Cramp & Bray, 2009; Haakstad et al., 2009; Leiferman et al., 2011; Ning et al., 2003), a belief that an individual has the confidence and skills to perform a certain behavior (Bandura, 1986) but did not focus on a theoretical framework. Findings suggest that an individual’s self-efficacy is a strong predictor of PA engagement. Also, some prospective studies have examined the Theory of Planned Behavior for predicting exercise behavior in pregnant women (Hausenblas & Symons Downs, 2004; Symons Downs & Hausenblas, 2003; 2007). The Theory of Planned Behavior suggests that behavior is influenced by a person’s intention, or level of motivation, to perform a behavior and intention, in turn, is influenced by one’s attitude (positive and negative evaluation of the behavior), subjective norm (perceived social pressure from significant others to engage in the behavior), and perceived behavioral control (ease or difficulty in performing the behavior; a concept similar to self-efficacy). Evidence suggests the Theory of Planned Behavior constructs for predicting pregnant women’s PA intention and behavior vary across the trimesters. For example, in the first trimester, the strongest determinant of PA is perceived behavioral control (Hausenblas & Symons Downs, 2004) whereas in the second (Symons Downs & Hausenblas, 2003) and third (Symons Downs & Hausenblas, 2007) trimesters, intention is the strongest predictor of PA. For predicting intention, attitude is the strongest determinant in the first and second trimesters (Hausenblas & Symons Downs, 2004; Symons Downs & Hausenblas, 2003) whereas subjective norm is the strongest predictor in the third trimester (Symons Downs & Hausenblas, 2007). Moreover, another Theory of Planned Behavior study found the subjective benefits of PA, ability to overcome environmental barriers, and ability to overcome personal barriers were strong determinants of PA for pregnant women (Black, Kieffer, Villarruel, & Sinco, 2007). These studies show a relatively consistent theoretical model for predicting PA intention and behavior in pregnant women.
In addition to the Theory of Planned Behavior, Protection Motivation Theory has also been used to examine whether information about PA preventing maternal-fetal disease could be used as a source of PA motivation. Gaston and Pravpavessis (2009) found that using the Protection Motivation Theory model led to significantly higher perceived severity, response efficacy, self-efficacy, goal intention, and increased PA behavior. In sum, designing studies and interventions with theoretical frameworks is strongly encouraged to promote and sustain PA behavior change among pregnant women (Glanz et al., 2008).
Empirical Evidence for Association Between Physical Activity and Maternal Prenatal Outcomes: Gestational Diabetes Mellitus, Preeclampsia, and Excessive Gestational Weight Gain
The following section reviews existing epidemiologic literature on the association between prenatal PA and the following prenatal complications: gestational diabetes mellitus, preeclampsia, and excessive gestational weight gain. Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy with a prevalence rate varying from 1% to above 20% depending on the population studied (American Diabetes Association [ADA], 2011). With the recent adoption of the International Association of Diabetes and Pregnancy Study Groups Consensus Panel (IADPSG) diagnostic criteria, it is estimated that about 18% of all pregnant women will be diagnosed with GDM (IADPSG, 2010). GDM is related to short-term as well as long-term adverse health outcomes for both mothers and their offspring. Women with GDM are at increased risk of maternal hypertensive disorders, cesarean delivery, and have a 7-fold increased risk for future Type 2 diabetes (ADA, 2004; Bellamy, Casas, Hingorani, & Williams, 2009). Even more concerning is that offspring of women with GDM are at increased risk for poor birth outcomes such as large-for-gestational-age birth, neonatal hypoglycemia, and neonatal death (Siega-Riz et al., 2009) and in the long-term, are at increased risk for obesity, glucose intolerance, and Type 2 diabetes (ADA, 2004; Oken, Taveras, Kleinman, Rich-Edwards, & Gillman, 2007; Whitaker, Pepe, Seidel, Wright, & Knopp, 1998).
Hypertensive disorders of pregnancy affect approximately 8% of pregnancies (Roberts, Pearson, Cutler, & Lindheimer, 2003) and include preeclampsia (defined as gestational hypertension with proteinuria) and gestational hypertension (defined as new onset hypertension in pregnancy after 20 weeks gestation). Preeclampsia complicates 2–8% of all pregnancies (Gong, Savitz, Stein, & Engel, 2011), and it is associated with an increased risk of preterm delivery, neonatal intensive-care unit admission, and fetal death (Berg, Mackay, Qin, & Callaghan, 2009). Hypertensive disorders are also the second leading cause of maternal mortality, accounting for 19% of pregnancy-related deaths for women following a live birth and 20% of pregnancy-related deaths for women following a still birth (Kieffer et al., 2001).
Pregnancy has also been proposed as a critical period for the development of maternal overweight and obesity (Rossner & Ohlin, 1995; Siega-Riz, Evenson, & Dole, 2004). Because overweight is characterized by insulin resistance and increased systemic inflammatory response (Bodnar, Catov, Klebanoff, Ness, & Roberts, 2007), it is not surprising that excessive gestational weight gain is associated with increased risk of GDM and preeclampsia (Cedergren, 2004; Crane, White, Murphy, Burrage, & Hutchens, 2009). Indeed, there is evidence that the incidence of GDM may be increasing as the prevalence of obesity among women of reproductive age increases (Cheung & Byth, 2003; Dabelea et al., 2005; Ferrara, Kahn, Quesenberry, Riley, & Hedderson, 2004). Recent studies also indicate a relationship between high gestational weight gain, an abnormal metabolic environment in utero, and increased risk of large-for-gestational-age infants, neonatal death (Siega-Riz et al., 2009), and subsequent childhood adiposity and morbidity (Oken et al., 2007). In light of the increasing prevalence of GDM along with its associated risk factors (e.g., excessive gestational weight gain) and related sequelae (e.g., preeclampsia, long-term obesity), the need for strategies to target modifiable risk factors that may prevent these factors becomes critical. In addition, there are no reliable tools for the early clinical diagnosis of preeclampsia, nor effective treatments (with the exception of delivery of the fetus), further highlighting the need to identify modifiable risk factors.
PA and GDM
Prior observational epidemiologic studies have suggested that pre-pregnancy PA may have a protective role in GDM development. A recent meta analysis found a 55% lower risk of GDM for women in the highest pre-pregnancy PA quartile compared with those in the lowest quartile (pooled odds ratio [OR] = 0.45, 95% confidence interval [CI]: 0.28–0.75; p < .001), as well as a 24% lower risk of GDM for women in the highest PA group during pregnancy compared with those in the lowest PA group (pooled OR = 0.76, 95% CI: 0.70–0.83; p<0.001; Tobias, Zhang, van Dam, Bowers, & Hu, 2011).
In light of the fact that walking is the most popular form of PA in pregnancy (Mottola, 2009), several of these observational studies evaluated the impact of walking independently (Dempsey et al., 2004; Oken et al., 2006; Zhang, Solomon, Manson, & Hu, 2006). Studies were consistent in finding that increased intensity of walking pace was significantly and inversely associated with the risk for GDM, although it was unclear whether walking duration (distance or time) had a similar protective effect. For example, in a prospective cohort study, women reporting a brisk walking pace and longer durations of walking prior to pregnancy had a reduced risk of GDM compared with women reporting a casual usual pace and shorter durations of walking (pooled OR = 0.59, 95% CI: 0.30–0.87; Tobias et al., 2011). However, this association was attenuated and no longer statistically significant in early pregnancy. Also, few studies have addressed the association between sedentary behavior in pre- or early pregnancy and risk of GDM. In general, these studies have been suggestive, but failed to show statistically significant associations between sedentary behavior, as well as hours watching television, and GDM risk (Oken et al., 2006; van der Ploeg, van Poppel, Chey, Bauman, & Brown, 2011; Zhang et al., 2006). However, the hypothesis that sedentary behavior may increase the risk of GDM is supported by evidence in nonpregnant women (Grøntved & Hu, 2011; Hu, Li, Colditz, Willett, & Manson, 2003; Krishnan, Rosenberg, & Palmer, 2009). A recent meta analysis found that greater TV viewing time was associated with a higher risk of Type 2 diabetes (pooled relative risk [RR] = 1.20 per 2 hours of TV viewing time) and a linear dose-response relationship was observed (Grøntved & Hu, 2011). In summary, data from observational epidemiologic studies suggest that pre- and early pregnancy sedentary behavior are important risk factors for GDM, and that the protective effect of PA appears to increase with intensity and time including common activities such as walking.
Furthermore, few intervention studies have tested if PA can prevent GDM among high risk women. However, there is substantial evidence that targeting at-risk groups for Type 2 diabetes prevention is effective if lifestyle changes are made. For example, the Diabetes Prevention Program randomized controlled trial (RCT) found that intensive lifestyle modification over four years with diet and exercise reduced the incidence of Type 2 diabetes by more than 50% (Knowler et al., 2002). In terms of GDM, with few exceptions, current lifestyle intervention studies largely reflect pilot studies or recently initiated trials. Also, a recent review of controlled trials designed to prevent GDM (Oostdam, van Poppel, Wouters, & van Mechelen, 2011) included three studies with a total of 238 participants that compared exercise to usual care (Barakat, Lucia, & Ruiz, 2009; Hopkins, Baldi, Cutfield, McCowan, & Hofman, 2010; Ong et al., 2009). The trials showed no statistical difference in the risk for GDM (RR = −0.05, 95% CI: −0.20–0.10) between pregnant women who received an exercise training program and those who received usual care. Two of the studies, both RCTs (Hopkins et al., 2010; Ong et al., 2009), also reported no group differences in maternal fasting glucose. The review concluded that findings were limited by small sample sizes and the evidence was of low quality (Oostdam et al., 2011). However, encouraging findings from several pilot feasibility studies have been recently published. While these RCTs did not impact risk of GDM, positive effects were observed on maternal glucose tolerance (Barakat, Cordero, Coteron, Luaces, & Montejo, 2011), weight gain (Korpi-Hyövälti et al., 2011), and PA levels (Callaway et al., 2010).
PA and Preeclampsia
The majority of prior epidemiologic studies have observed a protective effect of PA in early pregnancy on preeclampsia (Fortner et al., 2011; Kriketos et al., 2004; Marquez & McAuley, in press; Martin & Brunner Huber, 2010; Ruige et al., 1999), while others have found no association (Hegaard et al., 2010; Osterdal et al., 2009; Vollebregt et al., 2010). In terms of pre-pregnancy PA, two case-control studies (Avery, Leon, & Kopher, 1997; Ruige et al., 1999) suggested a decreased risk of preeclampsia for women who participated in any recreational PA during the year prior to pregnancy; while cohort studies have not supported this association (Braun, Sharoff, Chipkin, & Beaudoin, 2004; Dabelea et al., 2005; King, Haskell, Young, Oka, & Stefanick, 1995; Kriketos et al., 2004).
Limited observational research has evaluated the association between PA such as walking or sedentary behavior and preeclampsia risk. Sorenson et al. (2003) evaluated walking and the risk of preeclampsia in their case-control study of 201 preeclamptic and 383 normotensive pregnant women. Brisk walking (average walking pace ≥3 mile/hour) was associated with a 30–33% reduced risk as compared to no walking at all. In their prospective cohort study, Saftlas, Logsden-Sackett, Wang, Woolson, and Bracken (2004) found that women who were in the low (OR = 0.72) or moderate (OR = 0.72) sitting categories (defined as proportion of time spent on the job sitting) had nonsignificant reduced risks of preeclampsia as compared to women who sat more than two thirds of their time on the job. Thus, the evidence is largely equivocal and warrants further evidence to better understand the impact of PA on preeclampsia.
Furthermore, a Cochrane Review (Meher & Duley, 2006a) included two RCTs (Avery et al., 1997; Yeo et al., 2000) comparing moderate-intensity aerobic exercise with leisure PA during pregnancy in women who were at moderate to high risk of preeclampsia, however, they did not find a significant reduction in risk for preeclampsia. The RCT by Avery et al. (1997) also evaluated the impact on gestational hypertension and found no association. The authors concluded that there was insufficient evidence for reliable conclusions about the effects of PA for preventing preeclampsia. Since the time of the Cochrane review, there have been a small number of additional trials. For example, Yeo et al. (2008) conducted an exercise RCT among 79 women with a history of preeclampsia in a prior pregnancy to evaluate the association between PA and preeclampsia. Women assigned to the exercise group (n = 41) were instructed to walk 40 minutes per day, 5 days per week at moderate-intensity. Higher rates of preeclampsia were found in the exercise group (15%) than in the stretching group (3%) though this difference was not statistically significant (p = .11). Women in the stretching group were at 1.8 times the risk of developing gestational hypertension relative to women in the exercise group, though this increase in risk was also not statistically significant. Limitations of this trial impacting the study findings include the small number of participants restricting statistical power and compliance issues in the intervention group.
Ramírez-Vélez et al. (2011) conducted a double-blinded RCT of 64 primigravid women between 16–20 weeks gestation to evaluate the impact of regular aerobic exercise on endothelium-dependent vasodilation. The exercise group performed aerobic exercise at an intensity of 50–65% of their maximum heart rate for 60 minutes, three times per week, for 16 weeks. The control group was instructed to engage in usual PA. Endothelial function was evaluated by flow-mediated dilatation. At the end of the intervention, the exercise group had a statistically greater flow-mediated dilatation and normalized flow-mediated dilatation than controls. Given findings that endothelial dysfunction is associated with preeclampsia (Chambers et al., 2001), it is reasonable to hypothesize that improvements in flow-mediated dilatation induced by exercise may reduce risks for this disorder.
In terms of sedentary activity, a separate Cochrane Review assessed two small RCTs designed to investigate the impact of rest during pregnancy and preventing preeclampsia (Meher & Duley, 2006b). The first RCT found a significant reduction in the relative risk of preeclampsia with four to six hours of rest per day compared to normal activity (Spinapolice, Feld, & Harrigan, 1983). The second RCT found that rest of 30 minutes per day plus nutritional supplementation was also associated with a 13% reduced risk of preeclampsia as compared to no restriction of activity and placebo pills (Herrera, 1983). The authors concluded, however, that the reported effects may reflect bias or random error rather than a true effect. Current evidence is therefore insufficient to support recommending rest or reduced PA to women for preventing preeclampsia and its complications.
PA and Excessive Gestational Weight Gain
There is observational evidence that PA may be a modifiable risk factor for excessive gestational weight gain (Institute of Medicine [IOM], 2009). In observational studies, vigorous PA, total PA, and walking have been associated with a lower risk of excessive gestational weight gain (Chasan-Taber et al., 2008; Olson & Strawderman, 2003; Stuebe, Oken, & Gillman, 2009). In the prospective cohort study by Olson and Strawderman (2003), women who engaged in less PA during pregnancy as compared to prior to pregnancy were more likely to have excessive gestational weight gain. This is problematic because high gestational weight gain is independently associated with postpartum weight retention and the long-term development of obesity (IOM, 2009). Because PA may be one mechanism to reduce the risk of obesity, understanding its link to gestational weight gain is important for designing obesity-prevention efforts as women transition to motherhood.
While randomized and nonrandomized controlled trials have been designed to reduce excessive gestational weight gain, recent systematic reviews have found the majority of studies demonstrated minimal effectiveness and were limited to small samples, were largely atheoretical, lacked behavioral strategies for PA change, and/or relied on historical control groups (Dodd, Crowther, & Robinson, 2008; Ronnberg & Nilsson, 2010). The content of the intervention in the majority of these trials was dietetic counseling alone, however, those trials which included a PA component in addition to diet, were more likely to observe the suggestion of a protective effect. A meta analysis of PA RCTs found a significant reduction in gestational weight gain in the PA intervention group as compared to the control group (-0.61kg; Streuling et al., 2011).
In one of the largest RCT intervention studies conducted to date, Phelan et al. (2011) randomized 401 pregnant women to a low-intensity behavioral intervention or to standard care. The intervention included one face-to-face visit; weekly mailed materials that promoted appropriate weight gain, healthy eating, and exercise; individual graphs of weight gain; and telephone-based feedback. Normal weight women in the intervention arm were less likely to exceed the IOM gestational weight gain recommendations as compared to women in the standard care group (40% vs. 52%, p < .001); however the intervention did not have a significant impact on gestational weight gain among overweight/obese women. In another recent intervention study, Hui et al. (2012) randomized 190 normal weight pregnant women between 20–36 weeks of gestation to community-based group exercise sessions, instructed home exercise, and dietary counseling or to standard care. Participants in the intervention group were significantly less likely to exceed IOM gestational weight gain guidelines compared with to the control group (35% vs. 55%, p < .01). Because the IOM guidelines for gestational weight gain are BMI-specific, intervention strategies that are tailored to women based on their BMI category are likely needed to effectively moderate women’s weight during pregnancy.
In summary, evidence is relatively sparse, but suggests that future lifestyle interventions that target PA have the potential to prevent gestational diabetes, preeclampsia, and excessive gestational weight gain in pregnant women. In light of the increasing prevalence of these disorders and their relationship to increasing obesity rates, the need to identify strategies that might prevent their onset, as well as the associated short and long term sequelae for both the mother and her offspring, becomes critical. Ongoing and future well-designed, randomized controlled exercise trials will be necessary to develop effective lifestyle modification programs designed to prevent the incidence of gestational diabetes, preeclampsia, and excessive gestational weight gain in women at high risk of these disorders in a broader effort to prevent or reduce long-term obesity development.
Overall Summary and Recommendations for Future Research
Despite substantial advances in scientific evidence and continually evolving guidelines to promote prenatal PA, most pregnant women are not meeting PA guidelines and will continue to be insufficiently active as they transition to the postpartum and interconceptional periods (Evenson et al., 2004; Evenson & Wen, 2010b; IOM, 2009). This is highly problematic because low PA rates are associated with the risk for GDM and subsequent obesity, Type 2 diabetes, and cardiovascular disease. Although the frequency of observational, experimental, and intervention studies examining prenatal PA have increased in recent years, there is still an overwhelming need for more research in this area. This presents a conundrum for researchers, practitioners, and interventionists aiming to increase PA in women of childbearing age. That is, although promoting prenatal PA is a challenge, it is nevertheless a critical public health priority in an effort to understand its impact on maternal and infant health outcomes.
Clinical Implications
While evidence suggests that prenatal PA may positively impact maternal and infant health outcomes, incorporating lifestyle interventions that increase prenatal PA is often difficult to deliver at primary care settings due to lack of time (Ampt et al., 2009; Jansik, Branspenning, Weijden, Elwyn, & Grol, 2010), lack of adequate PA knowledge (Jansik et al., 2010), or negative attitudes toward patient’s ability to increase their PA (Jansik et al., 2010). For example, Ampt et al. (2009) reported that, although primary care providers understood the importance of lifestyle interventions, delivery of such interventions in a form of counseling were influenced by personal interests, level of risk to the patient, capacity of the practice, and availability of time. Similarly, Jansik et al. (2010) found that barriers to successful lifestyle counseling existed at three levels: provider, patient, and practice. At the providers’ level, the main barrier was lack of PA knowledge. At the patients’ level, main barriers were patients’ unwillingness to change their lifestyle and providers’ lack of awareness regarding access to fitness centers in the patient’s neighborhood. At the practice level, barriers were lack of time and poor coordination among care providers. Some issues (e.g., providing effective motivational interviewing or learning about PA) can be addressed by providing educational trainings for clinicians through their continuation education requirement. At the organizational level, agencies can influence clinics and hospitals to promote PA for patients and employees. Thus, below are realistic suggestions that prenatal care providers can adopt immediately in their practice to promote prenatal PA:
After providers have medically pre-screened a pregnant woman for contraindications to prenatal PA (ACOG, 2002), she should be provided with information on prenatal PA guidelines (i.e., ACOG [2002], USDHHS [2008]), and given the opportunity to ask questions about the guidelines; providers should also help their prenatal patients to identify strategies to overcome barriers to adopting and maintaining prenatal PA behaviors. If a space is available at a waiting room, provide posters, handouts, and/or videos about pregnant women’s PA to promote an active lifestyle.
Ask patients about the level of PA at each prenatal care visit as part of the standard of care: when primary care providers ask about PA, patients show much higher motivation to change their behavior, which may not last long if they are asked only once (Meriwether, Lee, Lafleur, & Wiseman, 2008). Also, showing interest in patient’s willingness to increase their PA may have positive effects on patient’s behavior modification when a woman is motivated (Miller & Rose, 2009).
While many pregnant women experience barriers to PA in early (e.g., nausea or fatigue) and late pregnancy (e.g., increased size due to growing baby), explain that engaging in PA and meeting the PA guidelines may actually help to reduce some symptoms; also, replacing more vigorous PA (e.g., running) with moderate PA (e.g., walking, low-impact aerobics, yoga, or elliptical/cycle machines) may relieve some symptoms and be more comfortable to sustain throughout pregnancy (Yeo et al., 2008).
Recommendations for Future Research
This review aimed to provide researchers and practitioners with an overview of the PA and pregnancy literature by focusing on promoting prenatal PA, pregnancy-related PA measurement, and maternal health outcomes. In this remaining section, we offer some recommendations for future research in an effort to better develop PA study design and intervention and promotion efforts:
Studies are needed to better understand the impact of the USDHHS (2008) PA guidelines on PA during pregnancy, with a special emphasis on understanding the impact of the recommendation that healthy women without medical complications can safely engage in vigorous-intensity PA throughout pregnancy.
Improvements in measuring prenatal PA are needed for more accurate estimates of PA mode, intensity, and duration. Although there are several self-report measures of prenatal PA with evidence of validity and reliability, these instruments are nevertheless impacted by recall bias, cultural adaptation and literacy issues, and variability for different recall over time for the same activity. Objectively-measured PA has generally been limited to assessing PA among pregnant women residing in developed countries and thus, studies are needed examining PA with these devices among pregnant women residing in under-developed and developing countries.
Continued advancements in technology and research are needed to develop and provide evidence for valid objective devices that can better estimate the types of activities that pregnant women do including mild-intensity (household chores, childcare) and water-based (swimming, water aerobics) activities as well as reduce participant burden (e.g., devices that accurately estimate PA despite changes in body size, shape, and gait patterns in pregnancy). To date, the accelerometer cut-points used to define sedentary, light, moderate, and vigorous PA are also not known for pregnant women; thus, this is another area in need of future research.
Although advancements have been made in recent years in identifying the determinants of prenatal PA in pregnancy, the bulk of this research is largely atheoretical which limits the ability to understand why certain psychological and motivational factors impact pregnant women’s PA self-efficacy, intention, perceived control, and behavior among other factors. There is an important need for theoretically-driven observational studies to better understand the complex interactions among the psychological, behavioral, and biological determinants of prenatal PA to develop more effective interventions.
Carefully controlled and sufficiently powered experimental/intervention studies are needed to examine and understand dose-response effects of prenatal PA and the impact of different PA prescriptions on maternal/infant outcomes. Mottola (2009) has proposed exercise prescriptions for pregnant women, however, more work on identifying specific intensities of activity associated with the greatest reduction in poor health outcomes is required. Specifically, more studies are needed examining the impact of varied PA intensities on maternal (e.g., GDM, preeclampsia, gestational weight gain) and infant (birth weight, growth, markers for childhood obesity) outcomes.
Evidence-based intervention studies are also needed to evaluate the frequency, intensity, duration, and type of PA necessary to optimize maternal outcomes of pregnancy among women at risk for health complications, particularly those with risk factors for obesity. Programs should also evaluate the effect of PA of different intensities as well as active living (walking, gardening, household activities) on maternal outcomes. Programs are also needed that identify how to effectively motivate pregnant women for PA and should include a better understanding of how behavioral strategies (goal-setting, using technology such as Smart Phones and texting for self-regulation and motivational prompting) may influence pregnant women’s PA motivational determinants.
Given that each subsequent pregnancy is associated with greater postpartum weight retention, coupled with the increasing incidence of maternal obesity in the US, PA guidelines targeted for overweight and obese pregnant women are clearly needed (Mottola, 2009). Similarly, a greater focus should be placed on evaluating the impact of prenatal PA interventions on preventing excessive maternal weight gain. It will also be critical to assess if compliance with the new IOM (2009) recommendations result in lower risk of complications such as GDM and preeclampsia. Research is also warranted examining how providers deliver gestational weight gain and PA recommendations since patient compliance with these guidelines is low.
There is an important need for research to better understand the role that maternal PA plays in infant growth, development, and the onset of diseases such as metabolic syndrome, diabetes, cardiovascular disease, and obesity. The relationship between pregnancy and women’s cardiovascular risk in later life has been the focus of studies in GDM and preeclampsia, however, research is warranted further examining the impact of maternal PA on both maternal and infant future cardiovascular disease risks. The recently proposed fetal origins hypothesis suggests that infant development is impacted by many factors including maternal genetics, behavioral, psychological, and environmental influences (Murphy Paul, 2010). Thus, further studies are needed on the impact of low levels of prenatal PA and sedentary behavior on risk of adverse infant health outcomes (large-for-gestational age births, subsequent childhood adiposity).
A paradigm shift is needed focusing more broadly on promoting PA before, during, and after pregnancy. There is recent evidence from an observational cohort study, the Central Pennsylvania Women’s Health Study, that women meeting PA guidelines during the preconceptional period had reduced odds of excessive pregnancy weight gain (Weisman, Hillemeier, Symons Downs, Chuang, & Dyer, 2010). Also, women participating in a pre- and interconceptional behavioral intervention, Strong Healthy Women, (including a PA component promoting the PA guidelines) that became pregnant after the intervention ended had lower weight and BMI and more appropriate gestational weight gain during pregnancy compared to controls (Symons Downs et al., 2009; Weisman et al., 2011). Although recommendations suggest that women be counseled prior to conception and encouraged to adopt lifestyle changes to minimize the risk of pregnancy issues (Entin & Munhall, 2006), few studies have examined the impact of preconceptional interventions and clinical practice recommendations to promote PA on maternal and infant outcomes.
In conclusion, the PA and pregnancy literature has evolved significantly over the past 50 years and there is currently sufficient empirical evidence to support the promotion of prenatal PA for maternal and infant health benefits. Understanding how to motivate pregnant women to be active and identifying effective strategies for PA adoption and maintenance to improve maternal health and reduce the onset of long-term diseases such as obesity, Type 2 diabetes, and cardiovascular disease is an important focus of future research. Lastly, advancements in this area of research are still needed to identify how to effectively disseminate PA promotion in pregnancy into clinical practice for wide-spread impact.
What Does This Paper Add?
The negative impact of low levels of PA on maternal and infant health outcomes is evident and highly problematic because most pregnant women do not meet PA guidelines and will continue to be insufficiently active as they transition to the postpartum and interconceptional periods. This paper demonstrates the need for researchers and practitioners to understand the impact of the USDHHS (2008) PA guidelines in an effort to promote prenatal PA, improve methods for measuring PA during pregnancy, and further clarify how prenatal PA reduces maternal health complications. This paper highlights that healthy pregnant women can safely engage in moderate to vigorous PA throughout pregnancy and practitioners are encouraged to further disseminate this message. This paper also contributes to the literature by providing particular insight about how to improve future research such as developing carefully-designed studies that are theoretically-driven, include valid and reliable measures of PA, and consider the multifaceted determinants and outcomes of PA across different subpopulations of women. A key point is that a “one size fits all” approach is not the recommended strategy for developing interventions in pregnancy. Instead, researchers and practitioners should take into account the specific needs of the population (e.g., differences across weight status, ethnicity, etc) and consider a paradigm shift in developing interventions that promote PA before, during, and after pregnancy. Lastly, this paper demonstrates a clear need for future research examining the role that maternal PA plays in infant growth, development and the onset of future diseases.
Acknowledgments
The authors would like to acknowledge the following contributions to this review manuscript: Funding for Danielle Symons Downs, Exercise Psychology Laboratory, Department of Kinesiology at The Pennsylvania State University was provided by the Social Science Research Institute; funding for Lisa Chasan-Taber, School of Public Health and Health Sciences at the University of Massachusetts at Amherst was provided by the National Institutes of Health (NIH) NIDDK074876; funding for Kelly R. Evenson, Department of Epidemiology, University of North Carolina, Chapel Hill was provided by NIH/National Cancer Institute CA109804-01; Jenn Leiferman is at the Colorado School of Public Health, University of Colorado; and SeonAe Yeo is at the School of Nursing, University of North Carolina, Chapel Hill. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or University affiliations.
Contributor Information
Danielle Symons Downs, Exercise Psychology Laboratory, Department of Kinesiology, The Pennsylvania State University.
Lisa Chasan-Taber, School of Public Health and Health Sciences, University of Massachusetts at Amherst.
Kelly R. Evenson, Department of Epidemiology, University of North Carolina, Chapel Hill
Jenn Leiferman, Colorado School of Public Health, University of Colorado at Denver.
SeonAe Yeo, School of Nursing, University of North Carolina, Chapel Hill.
References
- Ainsworth B, Sternfeld B, Richardson M, Jackson K. Evaluation of the Kaiser Physical Activity Survey in women. Medicine & Science in Sports & Exercise. 2000;32(7):1327–1338. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Leon AS. 2011 Compendium of Physical Activities: A second update of codes and MET Values. Medicine & Science in Sports & Exercise. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Aittasalo M, Pasanen M, Fogelholm M, Ojala K. Validity and repeatability of a short pregnancy leisure time physical activity questionnaire. Journal of Physical Activity and Health. 2010;7:109–118. doi: 10.1123/jpah.7.1.109. [DOI] [PubMed] [Google Scholar]
- Alleyne J. Canadian Academy of Sports Medicine position statement: Exercise and pregnancy. 2008 Retrieved from: http://www.sirc.ca/newsletters/may08/documents/PregnancyPositionPaper.pdf.
- American College of Obstetricians and Gynecologists. Exercise during pregnancy and the prenatal period. Washington, DC: ACOG; 1985. [Google Scholar]
- American College of Obstetricians and Gynecologists. Technical bulletin number 189: Exercise during pregnancy and the postpartum period. Washington, DC: ACOG; 1994. [Google Scholar]
- American College of Obstetricians and Gynecologists (ACOG) ACOG committee opinion number 267: Exercise during pregnancy and the postpartum period. Obstetrics and Gynecology. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes, 2011. Diabetes Care. 2011;34:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampt AJ, Amoroso C, Harris MF, McKenzie SH, Rose VK, Taggart JR. Attitudes, norms and controls influencing lifestyle risk factor management in general practice. BMC Family Practice. 2009;10:59. doi: 10.1186/1471-2296-10-59. Retrieved from: http://www.biomedcentral.com/1471-2296/10/59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery MD, Leon AS, Kopher RA. Effects of a partially home-based exercise program for women with gestational diabetes. Obstetrics and Gynecology. 1997;89(1):10–15. doi: 10.1016/s0029-7844(97)84256-1. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliff, NJ: Prentice Hall; 1986. [Google Scholar]
- Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn’s birth size: A randomised controlled trial. International Journal of Obesity. 2009;33(9):1048–1057. doi: 10.1038/ijo.2009.150. [DOI] [PubMed] [Google Scholar]
- Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: A randomised controlled trial. British Journal of Sports Medicine. 2011 doi: 10.1136/bjsports-2011-090009. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas J, Hingorani A, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. The Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstetrics and Gynecology. 2009;113:1075–1081. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- Berntsen S, Stafne SN, Morkved S. Physical activity monitor for recording energy expenditure in pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2011;90(8):903–907. doi: 10.1111/j.1600-0412.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- Black JE, Kieffer EC, Villarruel AM, Sinco BR. Predicting the exercise intention of pregnant Latina women. Hispanic Health Care International. 2007;5(1):5–12. [Google Scholar]
- Bodnar L, Catov J, Klebanoff M, Ness R, Roberts J. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18(2):234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- Bovbjerg ML, Siega-Riz AM. Exercise during pregnancy and cesarean delivery: North Carolina PRAMS, 2004–2005. Birth. 2009;36(3):200–207. doi: 10.1111/j.1523-536X.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsaeter AL, Owe KM, Haugen M, Alexander J, Meltzer HM, Longnecker MP. Validation of self-reported recreational exercise in pregnant women in the Norwegian Mother and Child Cohort Study. Scandinavian Journal of Medicine and Science in Sports. 2010;20:e48–e55. doi: 10.1111/j.1600-0838.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun B, Sharoff C, Chipkin SR, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 2004;97(3):991–997. doi: 10.1152/japplphysiol.00231.2004. [DOI] [PubMed] [Google Scholar]
- Briend A. Maternal physical activity, birth weight and perinatal mortality. Medical Hypotheses. 1980;6(11):1157–1170. doi: 10.1016/0306-9877(80)90138-3. [DOI] [PubMed] [Google Scholar]
- Callaway L, Colditz P, Byrne N, Lingwood B, Rowlands I, Foxcroft K, McIntyre HD. Prevention of gestational diabetes: Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33(7):1457–1459. doi: 10.2337/dc09-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergren M. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstetrics and Gynecology. 2004;103(2):219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. Journal of the American Medical Association. 2001;285(12):1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- Chasan-Taber L, Schmidt M, Roberts D, Hosmer D, Markenson G, Freedson P. Development and validation of a pregnancy physical activity questionnaire. Medicine & Science in Sports & Exercise. 2004;36(10):1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- Chasan-Taber L, Evenson KR, Sternfeld B, Kengeri S. Assessment of recreational physical activity during pregnancy in epidemiologic studies of birth weight and length of gestation: Methodologic aspects. Women & Health. 2007;45:85–107. doi: 10.1300/J013v45n04_05. [DOI] [PubMed] [Google Scholar]
- Chasan-Taber L, Schmidt M, Pekow P, Sternfeld B, Solomon C, Markenson G. Predictors of excessive and inadequate gestational weight gain in Hispanic women. Obesity. 2008;16(7):1657–1666. doi: 10.1038/oby.2008.256. [DOI] [PubMed] [Google Scholar]
- Cheung NW, Byth K. Population health significance of gestational diabetes. Diabetes Care. 2003;26(7):2005–2009. doi: 10.2337/diacare.26.7.2005. [DOI] [PubMed] [Google Scholar]
- Clapp JF, 3rd, Capeless EL. Neonatal morphometrics after endurance exercise during pregnancy. American Journal of Obstetrics and Gynecology. 1990;163:1805–1811. doi: 10.1016/0002-9378(90)90754-u. [DOI] [PubMed] [Google Scholar]
- Clarke PE, Gross H. Women’s behaviour, beliefs and information sources about physical exercise in pregnancy. Midwifery. 2004;20:133–141. doi: 10.1016/j.midw.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Cohen TR, Plourde H, Koski KG. Are Canadian women achieving a fit pregnancy? A pilot study. Canadian Journal of Public Health. 2010;101(1):87–91. doi: 10.1007/BF03405570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CP, Coe DP, Kendrick JM, Bassett DR, Jr, Thompson DL. Accuracy of physical activity monitors in pregnant women. Medicine & Science in Sports & Exercise. 2011;43(6):1100–1105. doi: 10.1249/MSS.0b013e3182058883. [DOI] [PubMed] [Google Scholar]
- Cramp AG, Bray SR. A prospective examination of exercise and barrier self efficacy to engage in leisure-time physical activity during pregnancy. Annals of Behavioral Medicine. 2009;37:325–334. doi: 10.1007/s12160-009-9102-y. [DOI] [PubMed] [Google Scholar]
- Crane JMG, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. Journal of Obstetrics and Gynaecology Canada. 2009;31(1):28–35. doi: 10.1016/s1701-2163(16)34050-6. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM screening program. Diabetes Care. 2005;28(3):579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, Williams MA. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Research and Clinical Practice. 2004;66(2):203–215. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- DiNallo J, Le Masurier G, Williams N, Symons Downs D. Walking for health in pregnancy: Assessment by indirect calorimetry and accelerometry. Research Quarterly for Exercise and Sport. 2008;79(1):28–35. doi: 10.1080/02701367.2008.10599457. [DOI] [PubMed] [Google Scholar]
- DiNallo JM, Symons Downs D, Le Masurier GC. Objectively assessing treadmill walking during the second and third pregnancy trimesters. Journal of Physical Activity and Health. 2012;9:21–28. doi: 10.1123/jpah.9.1.21. [DOI] [PubMed] [Google Scholar]
- Dodd J, Crowther C, Robinson J. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: A systematic review. Acta Obstetricia Et Gynecologica Scandinavica. 2008;87(7):702–706. doi: 10.1080/00016340802061111. [DOI] [PubMed] [Google Scholar]
- Doran F, O’Brien AP. A brief report of attitudes towards physical activity during Pregnancy. Health Promotion Journal of Australia. 2007;18:155–158. doi: 10.1071/he07155. [DOI] [PubMed] [Google Scholar]
- Duncombe D, Wertheim EH, Skouteris H, Paxton SJ, Kelly L. Factors related to exercise over the course of pregnancy including women’s beliefs about safety of exercise during pregnancy. Midwifery. 2007;25:430–438. doi: 10.1016/j.midw.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Entin PL, Munhall KM. Recommendations regarding exercise during pregnancy made by private/small group practice obstetricians in the USA. Journal of Sports Science and Medicine. 2006;5:449–458. [PMC free article] [PubMed] [Google Scholar]
- Ewart C. Changing our unhealthy ways: Emerging perspectives from social action theory. In: DiClemente RJ, Crosby RA, Kegler MC, editors. Emerging theories in health promotion practice and research. 2. San Francisco, CA: Jossey-Bass; 2009. pp. 359–389. [Google Scholar]
- Esperat C, Feng D, Zhang Y, Owen D. Health behaviors of low-income pregnant minority women. Western Journal of Nursing Research. 2007;29:284–300. doi: 10.1177/0193945906295532. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among Pregnant women in the US. Paediatric and Perinatal Epidemiology. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Moos MK, Carrier K, Siega-Riz AM. Perceived barriers to physical activity among pregnant women. Maternal and Child Health Journal. 2008;13:364–375. doi: 10.1007/s10995-008-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson K, Wen F. Measuring physical activity in pregnant women using a structured one-week recall questionnaire: Evidence for validity and reliability. International Journal of Physical Activity. 2010a;7:21. doi: 10.1186/1479-5868-7-21. Retrieved from: http://www.ijbnpa.org/content/27/21/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson K, Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Preventive Medicine. 2010b;50:123–128. doi: 10.1016/j.ypmed.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Evenson K, Wen F. National prevalence and correlates of objectively measured physical activity and sedentary behaviors among pregnant women. Preventive Medicine. 2011;53:39–43. doi: 10.1016/j.ypmed.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Chasan-Taber L, Symons Downs D, Pearce EE. Review of self-reported physical activity assessments for pregnancy: Summary of the evidence for validity and reliability. Paediatric and Perinatal Epidemiology. doi: 10.1111/j.1365-3016.2012.01311.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Security Agency and Social Security Administration. Prenatal care. Children’s Bureau Publication; 1949. pp. 25–26. [Google Scholar]
- Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstetrics and Gynecology. 2004;103(3):526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- Fortner R, Pekow P, Whitcomb B, Sievert L, Markenson G, Chasan-Taber L. Physical activity and hypertensive disorders of pregnancy among Hispanic women. Medicine & Science in Sports & Exercise. 2011;43(4):639–646. doi: 10.1249/MSS.0b013e3181f58d3e. [DOI] [PubMed] [Google Scholar]
- Gaston A, Cramp A. Exercise during pregnancy: A review of patterns and determinants. Journal of Science and Medicine in Sport. 2011;14:299–305. doi: 10.1016/j.jsams.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Gaston A, Pravpavessis H. Maternal-fetal disease information as a source of exercise motivation during pregnancy. Health Psychology. 2009;28(6):726–733. doi: 10.1037/a0016702. [DOI] [PubMed] [Google Scholar]
- Glanz K, Rimer BK, Viswanath K. Health behavior and health education. 4. Vol. 1. San Francisco, CA: Jossey-Bass Publishers; 2008. [Google Scholar]
- Gong J, Savitz DA, Stein CR, Engel SM. Maternal ethnicity and pre-eclampsia in New York City, 1995–2003. Paediatric and Perinatal Epidemiology. 2011;26:45–52. doi: 10.1111/j.1365-3016.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SL, Williams A, Stewart DE, Franche RL. Health-promoting behaviors through pregnancy, maternity leave, and return to work: Effects of role spillover and other correlates. Women & Health. 2006;43(2):51–72. doi: 10.1300/J013v43n02_04. [DOI] [PubMed] [Google Scholar]
- Grøntved A, Hu F. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A meta-analysis. Journal of the American Medical Association. 2011;305(23):2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haakstad LA, Gundersen I, Bo K. Self-reporting compared to motion monitor in the measurement of physical activity during pregnancy. Acta Obstetrica Gynecologica Scandinavia. 2010;89(6):749–756. doi: 10.3109/00016349.2010.484482. [DOI] [PubMed] [Google Scholar]
- Haakstad L, Voldner N, Henriksen T, Bo K. Why do pregnant women stop exercising in the third trimester? Acta Obstetrica Et Gynecologica. 2009;88:1267–1275. doi: 10.3109/00016340903284901. [DOI] [PubMed] [Google Scholar]
- Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. International Journal of Behavioral Nutrition and Physical Activity. 2011;8:19. doi: 10.1186/1479-5868-8-19. Retrieved from: http://www.ijbnpa.org/content/8/1/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine & Science in Sports & Exercise. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Hatch MC, Shu XO, McLean DE, Levin B, Begg M, Reuss L, Susser M. Maternal exercise during pregnancy, physical fitness, and fetal growth. American Journal of Epidemiology. 1993;137(10):1105–1114. doi: 10.1093/oxfordjournals.aje.a116614. [DOI] [PubMed] [Google Scholar]
- Hausenblas HA, Symons Downs D, Giacobbi P, Tuccitto D, Cook B. A Multilevel examination of exercise intention and behavior during 1 pregnancy. Social Science and Medicine. 2008;66:2555–2561. doi: 10.1016/j.socscimed.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Hausenblas HA, Symons Downs D. Prospective examination of the Theory of Planned Behavior applied to exercise behavior during women’s first trimester of pregnancy. Journal of Reproductive and Infant Psychology. 2004;3:199–210. [Google Scholar]
- Hegaard HK, Ottesen B, Hedegaard M, Petersson K, Henriksen TB, Damm P, Dykes AK. The association between leisure time physical activity in the year before pregnancy and pre-eclampsia. Journal of Obstetrics and Gynaecology. 2010;30:21–24. doi: 10.3109/01443610903315686. [DOI] [PubMed] [Google Scholar]
- Herrera JA. Nutritional factors and rest reduce pregnancy induced hypertension and pre-eclampsia in positive rollover test primigravidas. International Journal of Gynecology & Obstetrics. 1993;41:31–35. doi: 10.1016/0020-7292(93)90151-l. [DOI] [PubMed] [Google Scholar]
- Hinton PS, Olson CM. Predictors of pregnancy-associated changes in physical activity in a rural white population. Maternal and Child Health Journal. 2001;5(1):7–14. doi: 10.1023/a:1011315616694. [DOI] [PubMed] [Google Scholar]
- Hopkins S, Baldi J, Cutfield W, McCowan L, Hofman P. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. The Journal of Clinical Endocrinology and Metabolism. 2010;95(5):2080–2088. doi: 10.1210/jc.2009-2255. [DOI] [PubMed] [Google Scholar]
- Hu F, Li T, Colditz G, Willett W, Manson J. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. Journal of the American Medical Association. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- Hui A, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean H, Shen G. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG: An International Journal of Obstetrics and Gynaecology. 2012;119:70–77. doi: 10.1111/j.1471-0528.2011.03184.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Nutrition during pregnancy. Washington, DC: National Academy of Sciences; 1990. Subcommittee on Nutritional Status and Weight Gain during Pregnancy. [Google Scholar]
- Institute of Medicine. Weight gain during pregnancy: Reexamining the guidelines. In: Rasmussen KM, Yaktine AL, editors. Committee to reexamine IOM pregnancy weight guidelines. Institute of Medicine, National Research Council; 2009. Retrieved from: http://www.nap.edu/ [PubMed] [Google Scholar]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansik R, Braspenning J, van der Weijden T, Elwyn G, Grol R. Primary care nurses struggle with lifestyle counseling in diabetes care: A qualitative analysis. BMC Family Practice. 2010;11:41. doi: 10.1186/1471-2296-11-41. Retrieved from: http://www.biomedcentral.com/1471-2296/11/41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AMZ, Evenson KR, Daniels J, Herring AH, Wilcox AJ, Hartmann KE. Correlates of physical activity at two time points during pregnancy. Journal of Physical Activity and Health. doi: 10.1123/jpah.9.3.325. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JMM, Johnstone RW, Phillips MH. Historical review of British obstetrics and gynecology. London: E. S. Livingstone; 1954. [Google Scholar]
- Kieffer EC, Carman WJ, Gillespie BW, Nolan GH, Worley SE, Guzman JR. Obesity and gestational diabetes among African-American women and Latinas in Detroit: Implications for disparities in women’s health. Journal of the American Medical Women’s Association. 2001;56(4):181–187. [PubMed] [Google Scholar]
- Kieffer EC, Willis SK, Arellano N, Guzman R. Perspectives of pregnant and postpartum Latino women on diabetes, physical activity, and health. Health Education and Behavior. 2002;29:542–556. doi: 10.1177/109019802237023. [DOI] [PubMed] [Google Scholar]
- King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91(10):2596–2604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi-Hyövälti EAL, Laaksonen D, Schwab U, Vanhapiha T, Vihla K, Heinonen S, Niskanen L. Feasibility of a lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health. 2011;11:179–179. doi: 10.1186/1471-2458-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27(2):629–630. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Rosenberg L, Palmer J. Physical activity and television watching in relation to risk of type 2 diabetes: The black women’s health study. American Journal of Epidemiology. 2009;169(4):428–434. doi: 10.1093/aje/kwn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiferman JA, Swibas T, Koiness K, Marshall JA, Dunn AL. My Baby, My Move: Examination of perceived barriers and motivating factors related to antenatal physical activity. Journal Midwifery Women’s Health. 2011;56:33–40. doi: 10.1111/j.1542-2011.2010.00004.x. [DOI] [PubMed] [Google Scholar]
- Levine J. Non-exercise activity theromogenesis (NEAT): Environment and biology. American Journal of Physiology, Endocrinology, and Metabolism. 2004;286:E675–E685. doi: 10.1152/ajpendo.00562.2003. [DOI] [PubMed] [Google Scholar]
- Levine J, Vander Weg M, Hill J, Klesges R. Non-exercise activity thermogenesis: The crouching tiger hidden dragon of societal weight gain. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;25:2451–2462. doi: 10.1161/01.ATV.0000205848.83210.73. [DOI] [PubMed] [Google Scholar]
- Lindseth G, Vari P. Measuring physical activity during pregnancy. Western Journal of Nursing Research. 2005;27(6):722–734. doi: 10.1177/0193945905276523. [DOI] [PubMed] [Google Scholar]
- Löf M. Physical activity pattern and activity energy expenditure in healthy pregnant and non-pregnant Swedish women. European Journal of Clinical Nutrition. 2011;65(12):1295–1301. doi: 10.1038/ejcn.2011.129. [DOI] [PubMed] [Google Scholar]
- Löf M, Forsum E. Activity pattern and energy expenditure due to physical activity before and during pregnancy in healthy Swedish women. British Journal of Nutrition. 2006;95(2):296–302. doi: 10.1079/bjn20051497. [DOI] [PubMed] [Google Scholar]
- Löf M, Hannestad U, Forsum E. Assessing physical activity of women of childbearing age. Ongoing work to develop and evaluate simple methods. Food Nutrition Bulletin. 2002;23:30–33. [PubMed] [Google Scholar]
- Löf M, Hannestad U, Forsum E. Comparison of commonly used procedures, including the doubly-labeled water technique, in the estimation of total energy expenditure of women with special reference to the significance of body fatness. British Journal of Nutrition. 2003;90(5):961–968. doi: 10.1079/bjn2003975. [DOI] [PubMed] [Google Scholar]
- Lombard C, Deeks A, Jolley D, Teede HJ. Preventing weight gain: The baseline weight related behaviors and delivery of a randomized controlled intervention in community based women. BMC Public Health. 2009;9:2. doi: 10.1186/1471-2458-9-2. Retrieved from: http://www.biomedcentral.com/1471-2458/9/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez DX, Bustamante EE, Bock BC, Markenson G, Tovar A, Chasan-Taber L. Perspectives of Latina and Non-Latina white women on barriers and facilitators to exercise in pregnancy. Women’s Health. 2009;49(6):505–521. doi: 10.1080/03630240903427114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez DX, McAuley E. Does measurement of leisure time physical activity levels reflect accurate physical activity in Latino adults? Annals of Behavioral Medicine (in press) [Google Scholar]
- Martin C, Brunner Huber L. Physical activity and hypertensive complications during pregnancy: Findings from 2004 to 2006 North Carolina pregnancy risk assessment monitoring system. Birth. 2010;37(3):202–210. doi: 10.1111/j.1523-536X.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- McParlin C, Robson SC, Tennant PW, Besson H, Rankin J, Adamson AJ, Bell R. Objectively measured physical activity during pregnancy: A study in obese and overweight women. BMC Pregnancy Childbirth. 2010;10:76. doi: 10.1186/1471-2393-10-76. Retrieved from: http://www.biomedcentral.com/1471-2393/10/76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher S, Duley L. Exercise or other physical activity for preventing pre-eclampsia and its complications. Cochrane Database of Systematic Reviews. 2006a;(2):CD005942–CD005942. doi: 10.1002/14651858.CD005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher S, Duley L. Rest during pregnancy for preventing pre-eclampsia and its complications in women with normal blood pressure. Cochrane Database of Systematic Reviews. 2006b;(2):CD005939–CD005939. doi: 10.1002/14651858.CD005939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriwether RA, Lee JA, Lafleur AS, Wiseman P. Physical activity counseling. American Family Physician. 2008;77(8):1129–1136. [PubMed] [Google Scholar]
- Miller WR, Rose GS. Toward a theory of motivational interviewing. The American Psychologist. 2009;64(6):527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelmark RA, Gardin SK. Historical perspectives. In: Mittelmark RA, Wiswell RA, Drinkwater BL, editors. Exercise in pregnancy. 2. Baltimore, MD: Williams & Wilkins; 1991. pp. 1–8. [Google Scholar]
- Mottola MF. Exercise prescription for overweight and obese women: Pregnancy and postpartum. Obstetrics and Gynecology Clinics of North America. 2009;36(2):301–316. doi: 10.1016/j.ogc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Mottola MF, Campbell MK. Activity patterns during pregnancy. Canadian Journal of Applied Physiology. 2003;28(4):642–653. doi: 10.1139/h03-049. [DOI] [PubMed] [Google Scholar]
- Murphy Paul A. Origins: How the nine months before birth shape the rest of our lives. New York: Free Press; 2010. [Google Scholar]
- Ning Y, Williams MA, Dempsey JC, Sorensen TK, Frederick IO, Luthy DA. Correlates of recreational physical activity in early pregnancy. Journal of Maternal-Fetal Neonatal Medicine. 2003;13:385–393. doi: 10.1080/jmf.13.6.385.393. [DOI] [PubMed] [Google Scholar]
- Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstetrics and Gynecology. 2006;108(5):1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. American Journal of Obstetrics and Gynecology. 2007;196:322.e1–322.e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. Journal of the American Dietetic Association. 2003;103(1):48–54. doi: 10.1053/jada.2003.50001. [DOI] [PubMed] [Google Scholar]
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metabolism. 2009;35(5):418–421. doi: 10.1016/j.diabet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Oostdam N, van Poppel MNM, Wouters MGAJ, van Mechelen W. Interventions for preventing gestational diabetes mellitus: A systematic review and meta-analysis. Journal of Women’s Health. 2011;20:1551–1563. doi: 10.1089/jwh.2010.2703. [DOI] [PubMed] [Google Scholar]
- Osterdal ML, Strom M, Klemmensen AK, Knudsen VK, Juhl M, Halldorsson TI, Olsen SF. Does leisure time physical activity in early pregnancy protect against pre-eclampsia? Prospective cohort in Danish women. British Journal of Obstetrics and Gynaecology. 2009;116(1):98–107. doi: 10.1111/j.1471-0528.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- Ota E, Haruna M, Yanai H, Suzuki M, Anh DD, Matsuzaki M, Murashima S. Reliability and validity of the Vietnamese version of the Pregnancy Physical Activity Questionnaire (PPAQ) Southeast Asian Journal of Tropical Medicine and Public Health. 2008;39:562–570. [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, King AC. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Journal of the American Medical Association. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Phelan S, Phipps M, Abrams B, Darroch F, Schaffner A, Wing R. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The fit for delivery study. The American Journal of Clinical Nutrition. 2011;93(4):772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivarnik JM, Chambliss H, Clapp J, III, Dugan S, Hatch M, Lovelady C, Williams MA. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Medicine & Science in Sports & Exercise. 2006;38(5):989–1006. doi: 10.1249/01.mss.0000218147.51025.8a. [DOI] [PubMed] [Google Scholar]
- Poudevigne MS, O’Connor PJ. Physical activity and mood during pregnancy. Medicine & Science in Sports & Exercise. 2005;37(8):1374–1380. doi: 10.1249/01.mss.0000174907.27818.ff. [DOI] [PubMed] [Google Scholar]
- Ramirez-Velez R, Aguilar de Plata AC, Escudero M, Echeverry I, Ortega J, Salazar B, Lopez-Jaramillo P. Influence of regular aerobic exercise on endothelium-dependent vasodilation and cardiorespiratory fitness in pregnant women. Journal of Obstetrics & Gynaecology Research. 2011;37(11):1601–1608. doi: 10.1111/j.1447-0756.2011.01582.x. [DOI] [PubMed] [Google Scholar]
- Rich M, Currie J, McMahon C. Physical exercise and the lactating women: A qualitative pilot study of mothers’ perceptions and experiences. Breastfeeding Review. 2004;12:11–17. [PubMed] [Google Scholar]
- Roberts J, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension in Pregnancy. 2003;22(2):109–127. doi: 10.1081/PRG-120016792. [DOI] [PubMed] [Google Scholar]
- Ronnberg AK, Nilsson K. Interventions during pregnancy to reduce excessive gestational weight gain: A systematic review assessing current clinical evidence using the grading of recommendations, assessment, development and evaluation (GRADE) system. BJOG: An International Journal of Obstetrics and Gynaecology. 2010;117(11):1327–1334. doi: 10.1111/j.1471-0528.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- Rossner S, Ohlin A. Pregnancy as a risk factor for obesity: Lessons from the Stockholm pregnancy and weight development study. Obesity Research. 1995;3(Suppl 2):267s–275s. doi: 10.1002/j.1550-8528.1995.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Rousham E, Clarke P, Gross H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. European Journal of Clinical Nutrition. 2005;60(3):393–400. doi: 10.1038/sj.ejcn.1602329. [DOI] [PubMed] [Google Scholar]
- Royal College of Obstetricians and Gynecologists. Exercise in pregnancy. Statement. 2006:4. Retrieved from: http://www.rcog.org.uk/womens-health/clinical-guidance/exercise-pregnancy.
- Ruige JB, Dekker JM, Blum WF, Stehouwer CD, Nijpels G, Mooy J, Heine RJ. Leptin and variables of body adiposity, energy balance, and insulin resistance in a population-based study: The Hoorn study. Diabetes Care. 1999;22(7):1097–1104. doi: 10.2337/diacare.22.7.1097. [DOI] [PubMed] [Google Scholar]
- Rutkowska E, Lepecka-Klusek C. The role of physical activity in preparing women for pregnancy and delivery in Poland. Health Care Women International. 2002;23:919–923. doi: 10.1080/07399330290112416. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. American Journal of Epidemiology. 2004;160(8):758–765. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- Savitz D, Herring A, Mezei G, Evenson K, Terry J, Jr, Kavet R. Physical activity and magnetic field exposure in pregnancy. Epidemiology. 2006;17:222–225. doi: 10.1097/01.ede.0000197294.05564.08. [DOI] [PubMed] [Google Scholar]
- Schmidt MD, Freedson PS, Pekow P, Roberts D, Sternfeld B, Chasan-Taber L. Validation of the Kaiser Physical Activity Survey in pregnant women. Medicine & Science in Sports & Exercise. 2006;38(1):42–50. doi: 10.1249/01.mss.0000181301.07516.d6. [DOI] [PubMed] [Google Scholar]
- Schramm WF, Stockbauer JW, Hoffman HJ. Exercise, employment, other daily activities, and adverse pregnancy outcomes. American Journal of Epidemiology. 1996;143(3):211–218. doi: 10.1093/oxfordjournals.aje.a008731. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, Paul RH. Risk factors for preeclampsia in healthy nulliparous women: A prospective multicenter study. The National Institute of Child Health and Human Development network of maternal-fetal medicine units. American Journal of Obstetrics and Gynecology. 1995;172(2):642–648. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Evenson KR, Dole N. Pregnancy-related weight gain: A link to obesity? Nutrition Reviews. 2004;62:S105–S111. doi: 10.1111/j.1753-4887.2004.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Siega-Riz A, Viswanathan M, Moos M, Deierlein A, Mumford S, Knaack J, Lohr K. A systematic review of outcomes of maternal weight gain according to the institute of medicine recommendations: Birth weight, fetal growth, and postpartum weight retention. American Journal of Obstetrics and Gynecology. 2009;201(4):339.e1–339.14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. Journal of the American Medical Association. 1997;278(13):1078–1083. [PubMed] [Google Scholar]
- Spinapolice RX, Feld S, Harrigan JT. Effective prevention of gestational hypertension in nulliparous women at high risk as identified by the rollover test. American Journal of Obstetrics and Gynecology. 1983;146:166–168. doi: 10.1016/0002-9378(83)91047-5. [DOI] [PubMed] [Google Scholar]
- Stein A, Rivera J, Pivarnik J. Measuring energy expenditure in habitually active and sedentary pregnant women. Medicine & Science in Sports & Exercise. 2003;35(8):1441–1446. doi: 10.1249/01.MSS.0000079107.04349.9A. [DOI] [PubMed] [Google Scholar]
- Sternfeld B. Physical activity and pregnancy outcome: Review and recommendations. Sports Medicine. 1997;23:33–47. doi: 10.2165/00007256-199723010-00004. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Quesenberry CP, Eskenazi B, Newman LA. Exercise during pregnancy and pregnancy outcome. Medicine & Science in Sports & Exercise. 1995;27(5):634–640. [PubMed] [Google Scholar]
- Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: A meta-analysis of intervention trials. BJOG: An International Journal of Obstetrics and Gynaecology. 2011;118(3):278–284. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- Stuebe A, Oken E, Gillman M. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. American Journal of Obstetrics and Gynecology. 2009;201(1):58.e1–58.e8. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons Downs D, Feinberg M, Hillemeier MH, Weisman CS, Chase GA, Chuang CH, Francis LA. Design of the Central Pennsylvania Women’s Health Study (CePAWHS) strong healthy women intervention: Improving preconceptional health. Maternal and Child Health Journal. 2009;13(1):18–28. doi: 10.1007/s10995-008-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons Downs D, Hausenblas HA. Pregnant women’s exercise intention and behavior: A prospective examination of the theory of planned behavior. Women’s Health Issues. 2003;13:222–228. [PubMed] [Google Scholar]
- Symons Downs D, Hausenblas HA. Exercising during pregnancy and postpartum: An elicitation study using the framework of the theory of planned behavior. Journal of Midwifery and Women’s Health. 2004;49:138–144. doi: 10.1016/j.jmwh.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Symons Downs D, Hausenblas HA. Pregnant women’s third trimester exercise intention and behavior: Application of the theory of planned behavior. Psychology and Health. 2007;22:545–559. [PubMed] [Google Scholar]
- Symons Downs D, LeMasurier G, DiNallo J. Baby steps: Pedometer-determined and self-reported leisure-time exercise behaviors of pregnant women. Journal of Physical Activity and Health. 2009;6:63–72. doi: 10.1123/jpah.6.1.63. [DOI] [PubMed] [Google Scholar]
- Symons Downs D, Ulbrecht JS. Understanding exercise beliefs and behaviors in women with gestational diabetes. Diabetes Care. 2006;29:236–240. doi: 10.2337/diacare.29.02.06.dc05-1262. [DOI] [PubMed] [Google Scholar]
- Thornton PL, Kieffer EC, Salabarria-Pena Y, Odoms-Young A, Willis SK, Kim H, Salinas MA. Weight, diet, and physical activity-related beliefs and practices among pregnant and postpartum Latino women: The role of social support. Maternal and Child Health Journal. 2006;10:95–104. doi: 10.1007/s10995-005-0025-3. [DOI] [PubMed] [Google Scholar]
- Tobias D, Zhang C, van Dam R, Bowers K, Hu F. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: A meta-analysis. Diabetes Care. 2011;34(1):223–229. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, & Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: 2008. Retrieved from: http://www.health.gov/PAguidelines/Report/ [Google Scholar]
- van der Ploeg HP, van Poppel MNM, Chey T, Bauman A, Brown W. The role of pre-pregnancy physical activity and sedentary behaviour in the development of gestational diabetes mellitus. Journal of Science and Medicine in Sport. 2011;14(2):149–152. doi: 10.1016/j.jsams.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Vladutiu CJ, Evenson KR, Marshall SW. Physical activity and injuries during pregnancy. Journal of Physical Activity and Health. 2010;7:761–769. doi: 10.1123/jpah.7.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollebregt KC, Wolf H, Boer K, van der Wal MF, Vrijkotte TG, Bonsel GJ. Does physical activity in leisure time early in pregnancy reduce the incidence of reeclampsia or gestational hypertension? Acta Obstetrica et Gynecologica Scandinavica. 2010;89(2):261–267. doi: 10.3109/00016340903433982. [DOI] [PubMed] [Google Scholar]
- Weisman CA, Hillemeier M, Symons Downs D, Chuang C, Dyer A. Preconception predictors of weight gain during pregnancy: Prospective findings from the Central Pennsylvania Women’s Health Study. Women’s Health Issues. 2010;20:126–132. doi: 10.1016/j.whi.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman CA, Hillemeier MM, Symons Downs D, Feinberg M, Chuang CH, Botti J, Dyer AM. Improving women’s preconceptional health: Long-term effects of the Strong Healthy Women behavior change intervention in the Central Pennsylvania Women’s Health Study. Women’s Health Issues. 2011;21:265–271. doi: 10.1016/j.whi.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. Gestational diabetes and the risk of offspring obesity. Pediatrics. 1998;101(2):E9. doi: 10.1542/peds.101.2.e9. [DOI] [PubMed] [Google Scholar]
- Wolfe LA, Mottola MF, MacKinnon C. Joint SOGC/CSEP clinical practice guideline: Exercise in pregnancy and the postpartum period. Canadian Journal of Applied Physiology. 2003;28:330–341. [PubMed] [Google Scholar]
- Yeo S, Steele NM, Chang MC, Leclaire SM, Ronis DL, Hayashi R. Effect of exercise on blood pressure in pregnant women with a high risk of gestational hypertensive disorders. Journal of Reproductive Medicine. 2000;45(4):293–298. [PubMed] [Google Scholar]
- Yeo S, Davidge S, Ronis DL, Antonakos CL, Hayashi R, O’Leary S. A comparison of walking versus stretching exercises to reduce the incidence preeclampsia: A randomized clinical trial. Hypertension in Pregnancy. 2008;27(2):113–130. doi: 10.1080/10641950701826778. [DOI] [PubMed] [Google Scholar]
- Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Archives of Internal Medicine. 2006;166(5):543–548. doi: 10.1001/archinte.166.5.543. [DOI] [PubMed] [Google Scholar]