Abstract
Objectives
To investigate whether the metabolically important visceral adipose tissue (VAT) relates differently to structural and functional brain changes in comparison with body weight measured as body mass index (BMI). Moreover, we aimed to investigate whether these effects change with age.
Design
Cross-sectional, exploratory.
Setting
University Clinic, Integrative Research and Treatment Centre.
Participants
We included 100 (mean BMI=26.0 kg/m², 42 women) out of 202 volunteers randomly invited by the city's registration office, subdivided into two age groups: young-to-mid-age (n=51, 20–45 years of age, mean BMI=24.9, 24 women) versus old (n=49, 65–70 years of age, mean BMI=27.0, 18 women).
Main outcome measures
VAT, BMI, subcutaneous abdominal adipose tissue, brain structure (grey matter density), functional brain architecture (eigenvector centrality, EC).
Results
We discovered a loss of cerebellar structure with increasing VAT in the younger participants, most significantly in regions involved in motor processing. This negative correlation disappeared in the elderly. Investigating functional brain architecture showed again inverse VAT–cerebellum correlations, whereas now regions involved in cognitive and emotional processing were significant. Although we detected similar results for EC using BMI, significant age interaction for both brain structure and functional architecture was only found using VAT.
Conclusions
Visceral adiposity is associated with cerebellar changes of both structure and function, whereas the regions involved contribute to motor, cognitive and emotional processes. Furthermore, these associations seem to be age dependent, with younger adults’ brains being adversely affected.
Keywords: Basic Sciences, Neurology
Article summary.
Article focus
The brain is the key organ for the regulation of energy homeostasis.
The distribution of excess energy within different fat depots rather than body weight per se determines healthy outcomes.
Yet it is unclear as to whether the brain relates to visceral fat storage, and how this depends on the individual's age.
Key messages
Visceral adipose tissue correlates negatively with cerebellar changes of both structure and function.
These associations are age-dependent, with younger adults’ brains being adversely affected.
Cerebellar dynamics suggest approaches to address health issues related to visceral fat storage.
Strengths and limitations of this study
(−) This study followed an exploratory approach within a cohort study.
(−) We did not have access to laboratory data as further covariates.
(−) We cannot entirely exclude drug side effects affecting the resting state data especially in older subjects.
(+) We assessed abdominal fat distribution directly using MRI.
(+) We found a regional overlap within the structural and the functional brain data.
Introduction
Excess body fat is one of the major health challenges worldwide. The way a human organism approaches energy abundance affects numerous health outcomes, in particular cardiovascular diseases, type 2 diabetes and several cancer diseases.1 As medical treatment of obesity has been largely unsuccessful, the substantial rise in the prevalence of obesity might actually cause life expectancy to level off or even decline until 2050.2
On the biomolecular level, rising body weight is a complex phenomenon that is still not fully understood.3 On the individual level, it might most suitably be treated as a gene–environment interaction.4 Besides the general shift towards greater body weight observed during the past 30 years, two more specific, body-weight-related aspects have drawn attention, namely (1) the age-related, weight-independent change of body composition with fat replacing lean mass5 6 and (2) the impact of abdominal fat distribution (AFD) with visceral adipose tissue (VAT) primarily affecting metabolic outcomes.3 7 8 Yet an integrative inquiry into both processes has so far not been made. Even though it has been assumed that central defects in energy balance may cause visceral adiposity to rise with age,9 it is not clear as to whether or how neuronal mechanisms might interact with age-related, weight-independent visceral adiposity.
The brain is a key organ for the regulation of energy homeostasis. Its respective output originates from the subconscious integration of homeostatic control mainly located in the hypothalamus, and ‘non-homeostatic’ circuits located in extra-hypothalamic structures.10 11 These processes ultimately ensure a body weight that is remarkably stable throughout the adult lifespan.11 12 Recent advances in imaging modalities revealed that in humans, body weight is associated with changes of regional brain structure, functional connectivity and goal-directed behaviour.13–16 These findings, however, are often non-homogeneous with respect to the correlations’ quality. Beside cohort- and method-related inconsistencies, one limitation may be the usage of a common adiposity index—almost exclusively the body mass index (BMI)—regardless of population-specific variables such as gender, age or degree of adiposity and leisure-time physical activity.6 Furthermore, studies that investigated the associations between body fat distribution as well as overall adiposity and structural or functional brain changes are rare. To the best of our knowledge, only a single very recent study examines the effects of both the BMI and the waist circumference on local grey matter volume.17 Yet, since anthropometry is unable to reliably estimate AFD,18 a key contributor to body weight-related phenomena has so far been hardly considered in human brain studies.
Thus, in order to better understand the complex relationship between body weight, ageing, gender, AFD and the brain, we applied both structural and task-independent functional brain MRI as well as MR-based abdominal fat quantification to 100 adults subdivided into two age groups (20–45 and 65–70 years of age). We asked whether the metabolically important VAT relates differently to structural and functional brain changes compared to overall adiposity measured as BMI, and whether these changes are age dependent. We chose MR-based fat quantification because it is thus far the only technique that allows direct, radiation-free assessment of intra-abdominal fat depots, and therewith abdominal fat distribution. This exploratory study describes an age-dependent, inverse correlation between intra-abdominal adiposity and regions of the cerebellum involved in motor, emotional and cognitive processing that was not detectable using the BMI.
Materials and methods
Study population
We investigated 100 adults out of Leipzig‘s LIFE-study (Germany). The LIFE-study is a prospective, longitudinal, population-based cohort study that intends to investigate molecular causes of environmental and lifestyle-associated diseases such as the metabolic syndrome, pancreatitis and dementia in 10 000 volunteers in the greater Leipzig region. Our study question arose before inspection of the data. For our analysis, we used datasets obtained between May 2011 and January 2012 (n=202). Within this cohort, we primarily included all participants defined as young-to-mid-age subjects, that is, between 20 and 45 years of age (n=51; mean BMI=24.9, range=17.2–34.6; 24 women). We compared this group to an older sample set (aged 65 and 70 years, n=49; mean BMI=26.9, range=20.3–35.9; 18 women) to investigate potential age-related effects. Written informed consent was obtained from every individual. Permission was obtained by the local ethics committee (263-2009-14122009). The study design was in accordance with the Declaration of Helsinki. At least 3 days prior to investigation, subjects received written and verbal instructions to refrain from substances such as coffee, tea and drugs in order to avoid brain data being affected by side effects induced by these substances.
Anthropometric measures
Body weight and height were measured prior to MR investigation to the nearest of 0.1 cm using a stadiometer and a digital balance.
MR methods
All MRI examinations were performed on a 3-Tesla Magnetom Verio scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head array coil. The body coil was used as the transmit–receive coil for abdominal MRI scans.
Brain: data acquisition
Anatomic T1-weighted images were acquired using a three-dimensional Magnetization-Prepared Rapid Gradient Echo (MPRAGE) sequence.19 The Alzheimer's Disease Neuroimaging Initiative (ADNI) standard protocol was used with the following parameters: TI 900 ms, TR 2300 ms, TE 2.98 ms, flip angle 9°, band width 240 Hz/pixel, image matrix 256×240, 176 partitions, FOV 256×240×176 mm3, sagittal orientation, 1 average.20 21 Voxel size 1×1×1 mm³, no interpolation.
Task-absent (‘resting state’) fMRI data using a gradient-echo echo-planar imaging (EPI) sequence were acquired under eyes-closed condition. The following parameters were used: 300 whole brain volumes, acquisition matrix=64×64, slice thickness=4 mm (0.8 mm gap), 30 slices, TR=2000 ms, TE=30 ms, flip angle=90° and bandwidth=1954 Hz/pixel. The total time for the resting-state fMRI session for each subject was approximately 10 min. A field map with the same resolution as the EPI sequence was collected to correct for geometric distortion due to B0 field inhomogeneities.22
Brain: data analysis
Structural T1-weighted images were processed with the VBM8 toolbox (dbm.neuro.uni-jena.de/vbm.html) using SPM8 (Wellcome Trust Centre for Neuroimaging, UCL, London, UK) and Matlab 7 (Mathworks, Sherborn, Massachusetts, USA). Preprocessing included bias-field correction, segmentation and normalisation to the standard Montreal Neurological Institute space including modulation to account for local compression and expansion during transformation in order to obtain grey matter density (GMD) images. Subsequently, images were smoothed with a Gaussian kernel of 8 mm full-width at half-maximum (FWHM). Voxel-wise statistical analyses were performed for both age groups separately and—in addition—using a full factorial design investigating the interaction between the factors age and VAT. In all analyses, subcutaneous adipose tissue (SAT), gender and total grey matter volume were used as additional covariates in the general linear model. In addition, we repeated the full factorial analysis using a classification of (1) deep white matter lesions according to Fazekas's rating scale23 24 and (2) the abdominal data quality as additional covariates. This, however, did not lead to different results. Furthermore, we repeated the analysis using the BMI as an additional covariate in order to investigate for the weight-independent visceral adiposity. This also did not lead to different results. In all analyses, clusters were obtained using a voxel-threshold of p<0.005. In order to control for false positives, a minimum cluster size threshold of k>500 was applied. Clusters were considered to be significant using a family-wise error (FWE) corrected p<0.05. Significant clusters were investigated using the SPM Anatomy toolbox.25 26
The analysis of the resting state data was performed with 99 subjects because functional data were not available for a single subject belonging to the young-to-mid-age group. Preprocessing of the task-independent fMRI data was performed using SPM8 including estimation and correction for motion and EPI deformation. Thereafter, the high-resolution anatomical image of the same subject was (intraindividually) co-registered with the functional images, and normalisation was performed using the unified segmentation approach.27 After normalisation, the resulting voxel size of the functional images was interpolated to an isotropic voxel size of 3×3×3 mm3. In the final step of the preprocessing, the functional images were smoothed using a Gaussian smoothing kernel of 8 mm FWHM. Eigenvector centrality (EC) mapping was performed using the LIPSIA software package.28 29 EC analysis was performed on the individual subject level for the entire brain using a similarity matrix based on Person's correlation coefficient. In order to use a similarity matrix with only positive numbers, a value of 1 was added to all matrix entries before computing the EC. Similar to the VBM analyses, statistical analyses were performed for both age groups separately and—in addition—using a full factorial design investigating the interaction of the factors age and VAT. In all analyses, SAT and gender were taken into account using additional covariates in the general linear model. In addition, we repeated the full factorial analysis including (1) a classification of deep white matter lesions according to Fazekas's rating scale,23 24 and (2) the abdominal data quality as additional covariates. This, however, did not lead to different results. Furthermore, we also repeated the full factorial analysis using the BMI as an additional covariate in order to investigate for the weight-independent visceral adiposity. This also did not lead to different results. In all analyses, clusters were obtained using a voxel threshold of p<0.005. In order to control for false positives, a minimum cluster size threshold of k>200 was applied. Clusters were considered to be significant using an FWE-corrected p<0.05.
Abdomen: data acquisition
MR imaging for the determination of AFD30 was performed by using an axial T1-weighted fast spin-echo technique with the following parameters: TE=18 ms/TR=520 ms, echo train length 7; slice thickness 5 mm, 5-mm gap between slice. Scanning matrix 320×306 (no partial Fourier); field of view 500 mm×375mm, final voxel size 1.6 mm×1.6 mm×5.0 mm, water saturation. Since field inhomogeneities are more likely at 3 T, we tested the usage of a dielectric pad to improve field homogeneity before the actual study started. This, however, turned out to be unnecessary based on the image quality provided by the scanner; besides, signal weaknesses were not significantly improved using such a pad. In order to avoid breathing artefacts, the participants were asked to hold their breath for a period of 18 s each, wherein five slices were recorded. From our experience, this duration was tolerated well if the individual was capable of holding breath at all. The table shift after each acquisition was 5 cm, and images were recorded in feed-to-head direction beginning 10 cm below the umbilicus and finishing in the liver region with a total acquisition time of approximately 10 min. Volunteers were imaged in the supine position with their arms folded upon their chest.
Abdomen: data analysis
The four lower stacks—that is, 20 consecutive slices—were included and therewith defined the abdominal region in our analysis. By that, we ensured that the umbilical region was included, but adipose tissue beyond the diaphragm was excluded in every participant. Since even single-slice investigations usually located on the umbilical region allow sufficient examination of abdominal adipose tissue distribution in both genders, we consider our approach as being appropriate. Yet, it does not allow drawing conclusions about whole-body fat; this; however, was not the scope of our study because metabolic effects are primarily related to abdominal fat distribution. Image analysis was conducted on a separate workstation by a single observer with 2 years of experience in MR-based fat quantification using an ImageJ (Rasband, 2004; V.1.45s11; available at http://rsbweb.nih.gov/ij/download.html) macro that quantified fat pixels semi-automatically and histogram-based (methodical paper in preparation). In short, to ensure highest possible anatomical accuracy, the segmentation algorithm followed a four-step approach that included automated operations such as basic morphology, Active Contours and interpolation, as well as manual circumscription of the visceral cavity on the stacks’ initial images; conventional automated thresholding binarised the image to separate fat from non-fat tissue. Adipose tissue volumes were calculated as the sum of fat pixels×slice thickness (ie, slice+gap) and corrected for body length to adjust the abdominal region for the individual‘s stature. VAT was defined as the adipose tissue within the abdominal cavity; SAT was defined as the adipose tissue between the skin and the musculoskeletal interlayer built by the hips, the rips and abdominal as well as intercostal muscles. Intermuscular adipose tissue was excluded from analysis. Signal weaknesses within the adipose tissue were left unadjusted in order to minimise human error. Total image analysis required around 4 min per dataset.
Results
Study population
In order to compare potential associations of body mass index (BMI) and VAT with brain structure (GMD) and functional brain connectivity (EC), and whether these are age-dependent, we examined 100 subjects (BMI range=17.2–35.9) subdivided into two age groups (table 1). GMD decreased significantly with age across the whole brain (data not shown). Note that although the BMI was not significantly lower in young-to-mid-age subjects, older participants showed greater VAT.
Table 1.
Characteristics of the study population*
| Cohort (n=100, 58 men) | Young (n=51, 26 men) | Old (n=49, 32 men) | p Value | |
|---|---|---|---|---|
| Age (years) | 51.7 ± 16.4 | 36.6 ± 6.9 | 67.5 ± 1.5 | <0.001 |
| BMI (kg/m²) | 26.0 ± 3.6 | 25.0 ± 3.3 | 27.0 ± 3.5 | n.s. |
| SAT (ml) | 3420.5 ± 1724.2 | 3353.8 ± 1410.7 | 4079.5 ± 1462.7 | <0.001 |
| VAT (ml) | 1887.0 ± 1258.2 | 1169.3 ± 784.0 | 2753.2 ± 1010.4 | <0.001 |
| AAT (ml) | 5654.8 ± 2186.3 | 4523.0 ± 1787.8 | 6832.7 ± 1937.1 | <0.001 |
| VAT/AAT | 0.33±0.13 | 0.25±0.11 | 0.40±0.10 | <0.001 |
*Data are mean±SD.
Age-dependent negative correlation between VAT and GMD in the cerebellum
We began by investigating whether BMI and VAT correlate with whole-brain GMD in the group of 20-year-old to 45-year-old subjects. We found that increasing body weight was associated with reduced GMD in the right dorsolateral prefrontal cortex (DLPFC) as well as regions of the left parietal cortex (data not shown). In contrast, VAT correlated negatively with GMD in both hemispheres of the cerebellum (figure 1, first row). Local maxima were assigned to lobule V and VI (Hem) using the SPM Anatomy toolbox. In order to investigate gender influences, we repeated the analysis for women and men separately. Although we detected significant associations in men only, a gender interaction was not significant. Hence, we were not able to find gender differences with respect to the negative correlation between VAT and GMD in the cerebellum in our subgroup of young-to-mid-age adults.
Figure 1.
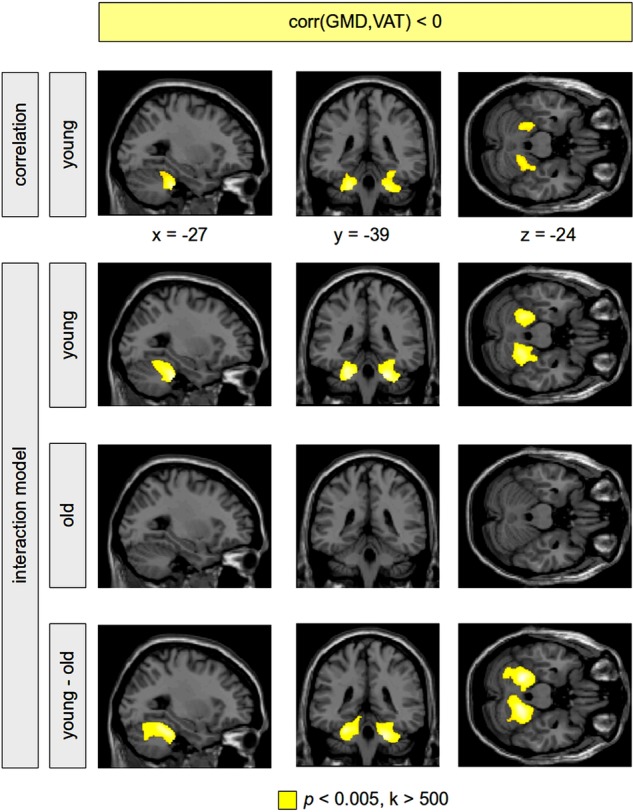
Negative correlation between grey matter density (GMD) and visceral adipose tissue (VAT). Analyses were performed using a correlational model including the sample of young-to-mid-age adults only (first row), and using a full factorial model including all subjects (rows 2–4). The correlation analysis within the younger subgroup showed a higher amount of VAT associated with a decrease of GMD in the cerebellum bilaterally (first row, colour-coded in yellow). Sex, age, subcutaneous adipose tissue and the total amount of grey matter volume were taken into account using additional covariates in the statistical analysis. Note that we did not find a significant positive correlation between GMD and VAT in the whole brain in the younger subgroup. Within the interaction model, the negative correlation between GMD and VAT was again present bilaterally in the cerebellum in the young-to-mid-age adults (second row); this correlation was not revealed for the older subjects (third row). The difference between both groups (the VAT–age interaction) turned out to be significant (fourth row). No significant positive correlation between GMD and VAT was found within both groups of young-to-mid-age as well as older subjects. Significant clusters are shown with a voxel threshold of p<0.005. In order to control for false positives, significant clusters are shown with a minimum cluster size of k>500. Using a family wise error corrected p<0.05, all clusters remained significant except for the cluster in the left cerebellum within the young-to-mid-age adults (first row), which was significant using the false discovery rate.
We next set out to investigate whether the negative correlation between VAT and the cerebellum's GMD which was observable in young-to-mid-age adults also occurred in older subjects. Therefore, we examined the 65-year-old to 70-year-old participants within a separate analysis. Here, however, we were not able to show a significant correlation between VAT and GMD in the cerebellum.
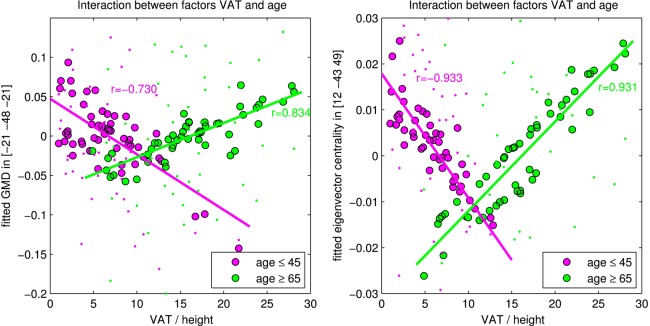
Finally, in order to investigate the difference between both age groups respecting the correlation between GMD and VAT, we performed a statistical analysis with all subjects using a full factorial model implementing VAT and age as two factors. This VAT–age interaction—that is, the correlation's difference between the two age groups—turned out to be significant in the cerebellum (figure 1, rows 2–4), indicating that the association of greater VAT and reduced GMD in the cerebellum disappeared in older subjects. Using the SPM Anatomy toolbox, local maxima were again assigned to lobule V and VI (Hem). For illustration, a dot-plot displays this interaction within the left cerebellum (figure 2, left).
Figure 2.
Interaction between the factors visceral adipose tissue (VAT) and age in the left cerebellum with changes of grey matter density (GMD, left plot) and eigenvector centrality (EC, right plot). The young-to-mid-age participants are shown with violet dots; older participants are shown in green colour. Circles with black edge show the fitted GMD/EC values with computing the interaction between VAT and age; the smaller dots show the normalised GMD/EC values including the error term of the general linear model. Thus, the dots show original GMD/EC values adjusted for confounds that were used in the model.
In both age groups we were not able to find significant positive correlations between BMI or VAT and GMD.
Age-dependent negative correlation between VAT and EC in the cerebellum
Next we investigated whether the degree of connectivity (ie, the EC) within the brain is associated with VAT in young-to-mid-age adults. A higher amount of VAT was accompanied with a decreased EC in the cerebellum (local maxima assigned to lobule VI (Hem) and VIIa Crus I and II (Hem)) as well as regions of the left posterior temporal and parietal lobe (figure 3, first row). Notably, with lobule VI we found a regional overlap within the structural and the functional data. Furthermore, we observed a positive correlation between VAT and EC in the cingulate cortex (data not shown).
Figure 3.
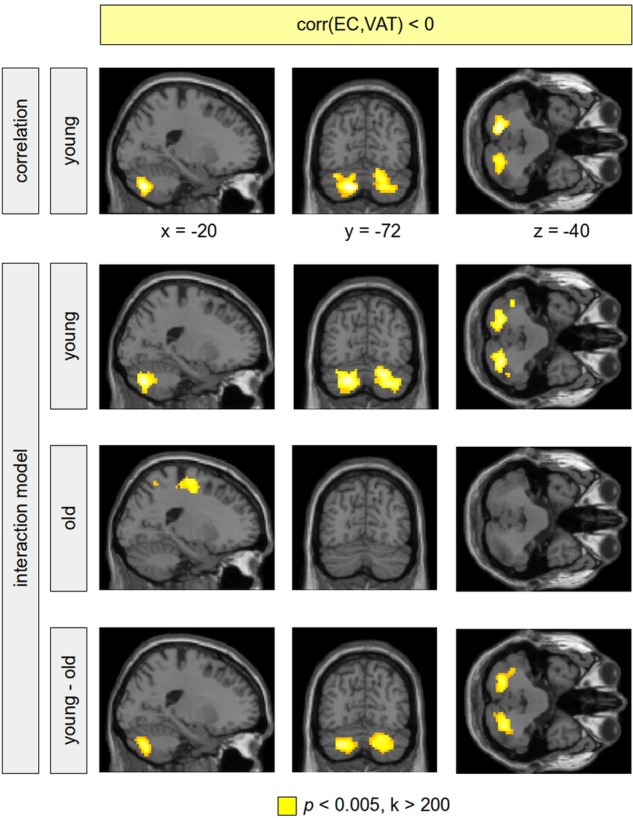
Negative correlation between eigenvector centrality (EC) and visceral adipose tissue (VAT). Analyses were performed using a correlational model including the sample of young-to-mid-age adults only (first row), and using a full factorial model including all subjects (rows 2–4). The correlation analysis within the younger subgroup showed a higher amount of VAT associated with a reduced connectivity of the cerebellum with other brain regions (first row, colour-coded in yellow). Sex and subcutaneous adipose tissue were modelled using additional covariates in the statistical design. Within the interaction model, the negative correlation between EC and VAT in the cerebellum was again present bilaterally in the cerebellum in the young-to-mid-age adults (second row); this correlation was not revealed for the older subjects (third row). The difference between both groups (the VAT–age interaction) turned out to be significant (fourth row). Significant clusters are shown with a voxel threshold of p<0.005. In order to control for false positives, significant clusters are shown with a minimum cluster size of k>200. All remaining clusters were significant using a family-wise error corrected p<0.05.
In addition, we investigated whether negative correlations between EC and VAT were present in the 65-year-old to 70-year-old subjects. In this group, VAT was negatively correlated with EC in regions of primary motor functions; this was not detectable in young-to-mid-age subjects (figure 4). Yet we did not find a significant correlation between VAT and EC in the cerebellum.
Figure 4.
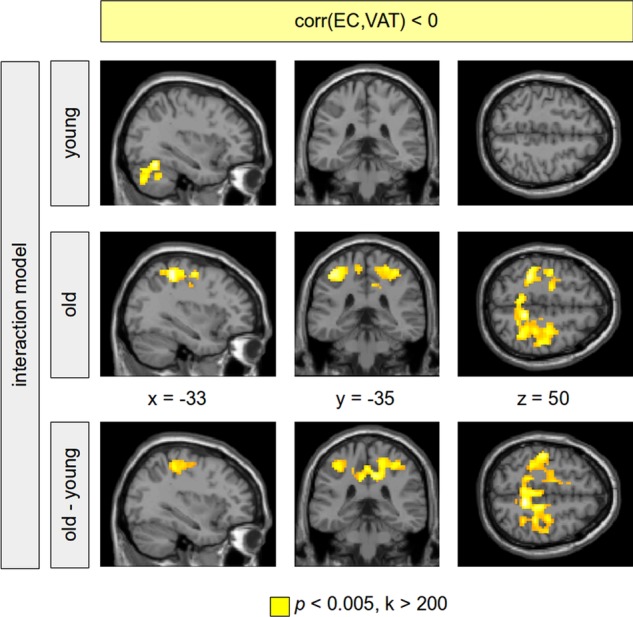
Negative correlation between eigenvector centrality (EC) and visceral adipose tissue (VAT). Results are shown provided by a full factorial model (VAT–age interaction) including all subjects. In the group of old subjects, a significant negative EC–VAT correlation was obtained predominantly in regions of primary motor functions (second row, colour-coded in yellow). This correlation was not observable in the group of young-to-mid-age adults (first row). The difference between both groups (the VAT–age interaction) was significant showing the age-related specificity of this VAT–age interaction. Clusters were reported using a voxel threshold of p<0.005. In order to control for false positives, significant clusters are shown with a minimum cluster size of k>200. Those clusters were significant using a family-wise error corrected p<0.05.
Finally, we investigated the difference between both age groups respecting the negative correlation between EC and VAT using all subjects within a full factorial design. The age groups differed significantly with respect to the negative correlation between VAT and EC in the cerebellum (figure 3, rows 2–4), suggesting that the association of increased VAT and reduced connectivity in the cerebellum is age-dependent. This result was even present when correcting for multiple comparisons. For illustration, a dot-plot displays this interaction within the left cerebellum (figure 2, right).
In order to investigate whether the BMI provides similar results compared to the VAT, we repeated the full factorial analysis using the BMI instead of VAT. Even though we found a negative correlation between BMI and EC within the cerebellum in the younger subjects (figure 5, top row), the difference between both age groups was significant only when using an uncorrected voxel threshold (figure 5, bottom row). Hence, VAT appears to be more sensitive to detect changes in functional connectivity than the BMI in our cohort of participants being overweight and slightly obese. This is reflected also by the peak T-values of the interaction contrast: using VAT, the peak T-values for the left and right cerebellum were 4.60 and 4.63, respectively; using BMI, the peak T-values for the left and right cerebellum were 3.36 and 3.09, respectively.
Figure 5.
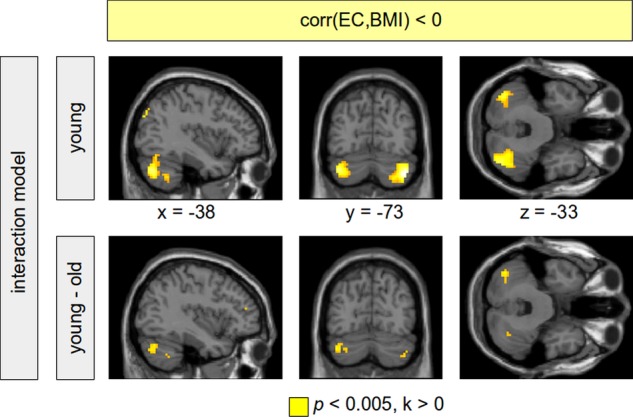
Negative correlation between eigenvector centrality (EC) and body mass index (BMI). Analyses were performed using a full factorial model (BMI–age interaction) including all subjects. In the group of young-to-mid-age adults, a higher BMI was associated with a decreased EC in the cerebellum bilaterally (top row, colour-coded in yellow). This correlation was not shown within the group of old subjects. The difference between both age groups, however, was not significant with correcting for multiple comparisons. Results are shown using a voxel threshold of p<0.005. Note that using visceral adipose tissue instead of BMI, a significant difference between both age groups can be shown using family-wise error correction (see figure 2).
Discussion
This exploratory study describes an age-dependent inverse correlation of GMD and functional brain connectivity with intra-abdominal adiposity in 100 adults using voxel-based morphometry, task-independent fMRI, anthropometry and abdominal MRI. We are able to show that visceral fat correlates negatively and BMI independently with the cerebellum's structure and functional connectivity in 20-year-old to 45-year-old subjects, and that this association is different in older people. To our knowledge, this is the first study that links cerebellar dynamics with abdominal fat distribution. Hence, this discussion will focus on bringing this finding into perspective with present knowledge about weight gain and the brain.
Although studies have attempted to estimate the amount of VAT using anthropometric measures such as the BMI or the waist circumference, significant degrees of residua usually disable the effective prediction of abdominal fat distribution. It is likely that with a cohort of participants who are overweight and slightly obese, processes related to alterations of VAT might interact below a threshold necessary for the BMI to detect changes. This is emphasised in figure 6, which illustrates two male participants out of our cohort who had identical BMI as well as similar age and body height, but considerable differences in both absolute and relative VAT volumes. One limitation of abdominal MRI so far, however, is the inability to provide information beyond fat area or volume, for instance adipocyte cell size and number, triglyceride content or inflammatory status. For that, adipose tissue biopsies would have been necessary. Further limitations of this study are (1) as a cross-sectional study we did not have access to retrospective BMI data that allowed the inclusion of former body weight development into the analysis; (2) we did not have access to laboratory data as further covariants; and (3) we cannot entirely exclude drug side effects affecting the resting state data especially in older subjects, although the participants were asked to refrain from taking medication that would confound the experiment as well as coffee and tea prior to the investigation.
Figure 6.
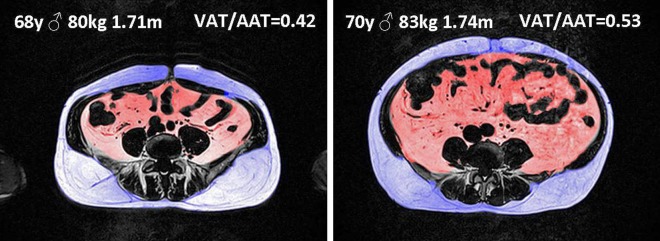
Example of abdominal fat segmentation. Abdominal fat distribution differs in two male participants of similar age (68 vs 70 years of age) and identical body mass index (BMI, 27.4 kg/m²) with visceral adipose tissue (VAT) 2.86 vs 4.16l, subcutaneous adipose tissue (SAT) 3.95 vs 3.73l, and VAT/(VAT+SAT) 0.42 vs 0.53 for the left versus the right subject, respectively. The surface values for the single umbilical slice shown revealed even greater differences in abdominal fat distribution (VAT/(VAT+SAT) 0.35 vs 0.57). Note that greater amount of lean mass in the left subject probably accounts for the identical BMI.
Our finding of reduced cerebellar GMD with increasing intra-abdominal fat is consistent with studies that mentioned comparable effects using the BMI13 16 31 and the waist circumference.17 Furthermore, the current understanding of cerebellar functions covers not only motor but also cognitive processes.32 Moreover, the cerebellum shows greater glucose metabolism as well as greater decrease of cerebral blood flow following satiation in obese subjects and seems to be involved in the neurobiology of obesity as well as structurally affected by leptin changes.33–36 Interestingly, the cerebellum expresses the Fto gene, which has been shown to be associated with both overall obesity and—very recently—VAT.37–39 Yet, to our knowledge, no study reported on impaired functional connectivity with increasing abdominal adiposity. Moreover, although several studies showed positive gender-dependent correlations between the BMI and regional brain structure,13 14 31 we and others17 were not able to find such associations. We attribute these controversial findings to cohort- and MRI-related methodical differences such as age, BMI range, as well as the data-processing conditions, acquisition protocols and head-array coils used.40–42
In the context of weight gain-associated brain changes, physical activity plays an important role in both directions. A relative lack of physical activity is considered as one key contributor for the increase of obesity prevalence.1 Abdominal adiposity in particular seems to be attributed to physical activity, whereas the effects on intra-abdominal adipose tissue are probably more pronounced compared to subcutaneous fat.43 44 Moreover, physical activity counters weight loss-induced metabolic adaptions that facilitate weight regain45 and reorganises the set point of nutritional balance.46 As cerebellar cortex and hippocampal dentate gyrus show enhanced synaptogenisis and neurogenisis in response to exercise training,47 48 one may assume that a lack of physical activity contributes to both visceral adiposity and cerebellar brain changes. This appears to be confirmed by our analysis’ local maxima being assigned to cerebellar regions likely involved in sensorimotor tasks (ie, lobule V and VI).49 We also found reduced functional connectivity between the cerebellar lobules VI and VII—latter likely involved in prefrontal–cerebellar loops and emotional processing—and the primary motor cortex in older subjects. Notably, lobule VI, which is supposed to be involved in numerous processes such as sensorimotor tasks, language, executive functions and emotional processing,49 was identified within both the structural and the functional analysis. Our findings thereby underscore an interdependency of physical activity, abdominal fat, cognitive function and cerebellar brain changes.
The adipocyte-derived hormone leptin may provide further explanations for the negative VAT–cerebellum correlation. Leptin is predominantly produced in white SAT, messages the switch between the starved and fed states and serves as an essential factor for the development of leptin-sensitive, hypothalamic feeding circuits.50 51 Its signal is conveyed via the long isoform of leptin receptors (Ob-Rb). This receptor's mRNA was shown to be present in the cerebellum.52 53 Moreover, Ob-Rb mRNA levels in both rodents and humans exceed all other tissues investigated, including the hypothalamus.54 55 Leptin itself exercises neuroprotective and neuritogenic effects onto cerebellar Purkinje cells and axons and its plasma concentrations correlate positively with cerebellar GMD.56 57 Furthermore, the cerebellum showed plasticity in response to leptin replacement.58 Interestingly, direct cerebello-hypothalamic projections from the fastigial and the interpositus cerebellar nucleus appear to modulate hypothalamic cell populations that are either glycaemia-sensitive or vagal-receptive.59 60 These findings raise the possibility that leptin influences the development of cerebellar structures that actively participate in (short-term) feeding regulation via cerebello-hypothalamic projections. Since leptin concentrations are mainly determined by SAT,61 which strengthened our model as a covariate, higher susceptibility to store energy within the VAT could be related to leptin levels relatively deficient to support the development of cerebellar projections that influence homeostatic mechanisms in the hypothalamus. On the other hand, cerebellar processes might actually be involved in the distribution of adipocytes into visceral or subcutaneous fat depots.
One of our key findings was a significant VAT–age interaction for cerebellar structure and connectivity. The human brain shrinks with age, and this shrinkage is differential and selective,62 whereas both cerebellar grey and white matter show accelerated structural decrease.63 Moreover, age-dependent changes in relaxation times as well as individual factors such as dehydration affect the MR acquisition of structural brain data and could account for the significant VAT–age interaction respecting older subjects.64–66 Furthermore, since fat replaces fat-free mass by degrees with rising age, brain structures have likely been affected prior to the age of 65. This study's exploratory and cross-sectional design does not enable to investigate these aspects. Nevertheless, our findings might indicate differential VAT–brain associations in young-to-mid-age compared to older subjects. Recently, a negative correlation between the cerebellum's GMD and neuron-specific enolase (NSE) was shown in a group of young overweight and obese subjects: an increase of serum NSE concentration—a marker of neuronal injury—was observed together with a decrease of GMD in cerebellar and hippocampal regions. Thus, in young subjects, overweight and obesity might contribute to an increased vulnerability in those brain regions.67 Another explanation might be an age-related hypothalamic neurodegeneration that affects energy balance by reducing energy expenditure and increasing food intake.9 These processes, however, do not necessarily have to impair the older brain. In contrast, as visceral adiposity-related vascular risk factors such as impaired insulin action and subclinical inflammation are usually treated in older subjects, these confounders rather than VAT itself could explain both the VAT–cerebellum association and the VAT–age interaction. Our data indicate age-dependent differences in the relationship between visceral adiposity and brain changes, in particular respecting the cerebellum's structure and connectivity.
In conclusion, we found a negative correlation between visceral adiposity and cerebellar structure as well as functional connectivity in 20-year-old to 45-year-old subjects which differed significantly compared to 65-year-old to 70-year-old individuals. As these associations did not show significance using the BMI, our findings indicate brain changes to differ depending on the observed fat depot and the cohort's BMI range. Furthermore, abdominal adiposity may be treated differently with respect to the human brain depending on the individual's age. Future studies investigating weight gain-associated brain changes ought to consider using direct measurements of adipose tissue or equations that account for variables such as age, gender, race and physical activity. Our data point to cerebellar regions involved in both motor and cognitive processes as being inversely associated with abdominal fat distribution in young adults.
Presented data are BMI, SAT, VAT and abdominal adipose tissue (AAT, ie, SAT+VAT) as well as the ratio of visceral over abdominal adipose tissue (VAT/AAT). Twenty consecutive slices (ie, 20cm) beginning 10 cm below the umbilicus defined the abdominal region. Significance was defined as p<0.001.
Note that although the BMI was not significantly lower in young-to-mid-age subjects, older participants showed greater adipose tissue volumes and greater contribution of VAT to AAT.
Supplementary Material
Acknowledgments
We thank both reviewers for their valuable suggestions that particularly improved the Results section and strengthened the comparison between using VAT and BMI for investigating the interaction between obesity and ageing. We would like to thank Christiane Hummitzsch, Nadine Orthofer and Annette Wiedemann for conducting the MR experiments. We further would like to thank the LIFE administration, particularly Dr Kerstin Wirkner, Susan Göthner and Christiane Backsmann for coordinating the investigations, as well as Daniel-Paolo Streitbürger for brain data preprocessing. Dr Steve Winston is kindly acknowledged for his language support. Finally, the authors thank Dr William Colmers and Daniel-Paolo Streitbürger for additional advice on the manuscript.
Footnotes
Contributors: MR, KM, MS and AV developed both idea and design of the study. MR wrote the project proposal, was the principal investigator and responsible for the MR investigations. He analysed the abdominal data, performed statistical analysis and was the lead writer. KM wrote the project proposal and was the promoting investigator. He was the primary analyst, performed the brain data as well as the statistical analysis, created the figures and wrote the manuscript's part on brain data acquisition as well as on analysis methods. The initial draft was prepared by MR and KM. It then circulated repeatedly among all authors for revision. MS is the scientific head of the IFB Adiposity Diseases, supervised the project and contributed to the data interpretation with respect to a medical perspective. AV is the head of the Department of Neurology at the Max Planck Institute for Cognitive and Brain Sciences, Leipzig, supervised the project and supported data interpretation with respect to a neurology perspective. KS coded the fat segmentation macro and contributed to the interpretation of the results. MLS interpreted the brain data and provided input into the data analysis. KA cared for the investigated participants respecting the MR examination and performed interobserver analysis using the fat segmentation macro. HS performed interobserver analysis using the fat segmentation macro and established the MR protocol. DS performed the analysis for incidental findings and white matter lesions. AP established the MR protocol and wrote the manuscript's part on abdominal fat data acquisition. YB supported data interpretation with respect to a genetics perspective and wrote the paragraph on genetics. All authors helped interpret data and revised successive drafts of the manuscript.
Funding: This work was supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1001. This work is supported by LIFE—Leipzig Research Center for Civilization Diseases, Universität Leipzig to MLS, KA and AV LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF) and by means of the Free State of Saxony within the framework of the excellence initiative. Furthermore, MLS is supported by the German Federal Ministry of Education and Research (BMBF; German Consortium for Frontotemporal Lobar Degeneration). YB is supported by IFB Adiposity Diseases (K50D).
Competing interests: MLS and KA declare that they have support from LIFE—Leipzig Research Center for Civilization Diseases—for the submitted work.
Ethics approval: Local Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Haslam DW, James WPT. Obesity. Lancet 2005;366:1197–209 [DOI] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 2005;352:1138–45 [DOI] [PubMed] [Google Scholar]
- 3.Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol 2008;6:1819–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Yanovski SZ, Carroll MD, et al. The epidemiology of obesity. Gastroenterology 2007;132:2087–102 [DOI] [PubMed] [Google Scholar]
- 5.Elia M. Obesity in the elderly. Obes Res 2001;9:244S––8S. [DOI] [PubMed] [Google Scholar]
- 6.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev 2001;2:141–7 [DOI] [PubMed] [Google Scholar]
- 7.Stegger JG, Schmidt EB, Obel T, et al. Body composition and body fat distribution in relation to later risk of acute myocardial infarction: a Danish follow-up study. Int J Obes 2011;35:1433–41 [DOI] [PubMed] [Google Scholar]
- 8.Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol-Endocrinol Metab 2010;299:E506–15 [DOI] [PubMed] [Google Scholar]
- 9.Xu AW, Kaelin CB, Morton GJ, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol 2005;3:2168–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature 2006;443:289–95 [DOI] [PubMed] [Google Scholar]
- 11.Shin AC, Zheng HY, Berthoud HR. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav 2009;97:572–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katzmarzyk PT, Perusse L, Malina RM, et al. Seven-year stability of indicators of obesity and adipose tissue distribution in the Canadian population. Am J Clin Nutr 1999;69:1123–9 [DOI] [PubMed] [Google Scholar]
- 13.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1428 healthy individuals. Obesity 2008;16:119–24 [DOI] [PubMed] [Google Scholar]
- 14.Horstmann A, Busse FP, Mathar D, et al. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front Hum Neurosc 2011;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33:1052–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walther K, Birdsill AC, Glisky EL, et al. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 2010;31:1052–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurth F, Levitt JG, Phillips OR, et al. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp 2012 Mar 15. doi: 10.1002/hbm.22021 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludescher B, Machann J, Eschweiler GW, et al. Correlation of fat distribution in whole body MRI with generally used anthropometric data. Investig Radiol 2009;44:712–19 [DOI] [PubMed] [Google Scholar]
- 19.Mugler JP, Brookeman JR. 3-Dimensional magnetization-prepared rapid gradient-echo imaging (3Dmp-rage). Magn Reson Med 1990;15:152–7 [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Jr., Bernstein MA, Fox NC, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Jr., Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement 2010;6:212–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 1995;34:65–73 [DOI] [PubMed] [Google Scholar]
- 23.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6 [DOI] [PubMed] [Google Scholar]
- 24.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–9 [DOI] [PubMed] [Google Scholar]
- 25.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005;25:1325–35 [DOI] [PubMed] [Google Scholar]
- 26.Eickhoff SB, Paus T, Caspers S, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 2007;36:511–21 [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–51 [DOI] [PubMed] [Google Scholar]
- 28.Lohmann G, Margulies DS, Horstmann A, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. Plos One 2010;5:e10232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohmann G, Muller K, Bosch V, et al. LIPSIA—a new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph 2001;25:449–57 [DOI] [PubMed] [Google Scholar]
- 30.Machann J, Thamer C, Schnoedt B, et al. Standardized assessment of whole body adipose tissue topography by MRI. J Magn Reson Imaging 2005;21:455–62 [DOI] [PubMed] [Google Scholar]
- 31.Pannacciulli N, Del PA, Chen K, et al. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 2006;31:1419–25 [DOI] [PubMed] [Google Scholar]
- 32.Bloedel JR, Bracha V. Duality of cerebellar motor and cognitive functions. In: Schmahmann JD. ed Duality of cerebellar motor and cognitive functions. 1st edn. London, UK: Academic Press Ltd, 1997:. 614. [DOI] [PubMed] [Google Scholar]
- 33.Gautier JF, Chen K, Salbe AD, et al. Differential brain responses to satiation in obese and lean men. Diabetes 2000;49:838–46 [DOI] [PubMed] [Google Scholar]
- 34.London ED, Berman SM, Chakrapani S, et al. Short-term plasticity of gray matter associated with leptin deficiency and replacement. J Clin Endocrinol Metab 2011;96:E1212–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GJ, Volkow ND, Felder C, et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 2002;13:1151–5 [DOI] [PubMed] [Google Scholar]
- 36.Wang GJ, Yang J, Volkow ND, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci USA 2006;103:15641–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Liu Y, White CC, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet 2012;8:e1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249 796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gronenschild EH, Habets P, Jacobs HI, et al. The effects of FreeSurfer Version, Workstation Type, and Macintosh Operating System Version on anatomical volume and cortical thickness measurements. PLoS One 2012;7:e38234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tardif CL, Collins DL, Pike GB. Regional impact of field strength on voxel-based morphometry results. Hum Brain Mapp 2010;31:943–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaza E, Klose U, Lotze M. Comparison of a 32-channel with a 12-channel head coil: are there relevant improvements for functional imaging? J Magn Reson Imaging 2011;34:173–83 [DOI] [PubMed] [Google Scholar]
- 43.McCarthy HD, Ellis SM, Cole TJ. Central overweight and obesity in British youth aged 11–16 years: cross sectional surveys of waist circumference. BMJ 2003;326:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gollisch KS, Brandauer J, Jessen N, et al. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab 2009;297:E495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maclean PS, Higgins JA, Wyatt HR, et al. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 2009;297:R793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:pii:e1000465 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Black JE, Isaacs KR, Anderson BJ, et al. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 1990;87:5568–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van PH, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999;2:266–70 [DOI] [PubMed] [Google Scholar]
- 49.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 2009;44:489–501 [DOI] [PubMed] [Google Scholar]
- 50.Pinto S, Roseberry AG, Liu H, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004;304:110–15 [DOI] [PubMed] [Google Scholar]
- 51.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004;304:108–10 [DOI] [PubMed] [Google Scholar]
- 52.Mercer JG, Moar KM, Hoggard N. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology 1998;139:29–34 [DOI] [PubMed] [Google Scholar]
- 53.Savioz A, Charnay Y, Huguenin C, et al. Expression of leptin receptor mRNA (long form splice variant) in the human cerebellum. Neuroreport 1997;8:3123–6 [DOI] [PubMed] [Google Scholar]
- 54.Bjorbaek C, Elmquist JK, Michl P, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 1998;139:3485–91 [DOI] [PubMed] [Google Scholar]
- 55.Burguera B, Couce ME, Long J, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology 2000;71:187–95 [DOI] [PubMed] [Google Scholar]
- 56.Oldreive CE, Harvey J, Doherty GH. Neurotrophic effects of leptin on cerebellar Purkinje but not granule neurons in vitro. Neurosci Lett 2008;438:17–21 [DOI] [PubMed] [Google Scholar]
- 57.Pannacciulli N, Le DS, Chen K, et al. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett 2007;412:248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baicy K, London ED, Monterosso J, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA 2007;104:18276–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu JN, Zhang YP, Song YN, et al. Cerebellar interpositus nuclear and gastric vagal afferent inputs reach and converge onto glycemia-sensitive neurons of the ventromedial hypothalamic nucleus in rats. Neurosci Res 2004;48:405–17 [DOI] [PubMed] [Google Scholar]
- 60.Li B, Guo CL, Tang J, et al. Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neurosignals 2009;17:132–43 [DOI] [PubMed] [Google Scholar]
- 61.Park KG, Park KS, Kim MJ, et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract 2004;63:135–42 [DOI] [PubMed] [Google Scholar]
- 62.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 2006;30:730–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 2005;26:1261–70 [DOI] [PubMed] [Google Scholar]
- 64.Cho S, Jones D, Reddick WE, et al. Establishing norms for age-related changes in proton T1 of human brain tissue in vivo. Magn Reson Imaging 1997;15:1133–43 [DOI] [PubMed] [Google Scholar]
- 65.Duning T, Kloska S, Steinstrater O, et al. Dehydration confounds the assessment of brain atrophy. Neurology 2005;64:548–50 [DOI] [PubMed] [Google Scholar]
- 66.Streitburger DP, Moller HE, Tittgemeyer M, et al. Investigating structural brain changes of dehydration using voxel-based morphometry. Plos One 2012;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mueller K, Sacher J, Arelin K, et al. Overweight and obesity are associated with neuronal injury in the human cerebellum and hippocampus in young adults—a combined MRI, serum marker and gene expression study. Transl Psychiatry 2012;2:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.