Abstract
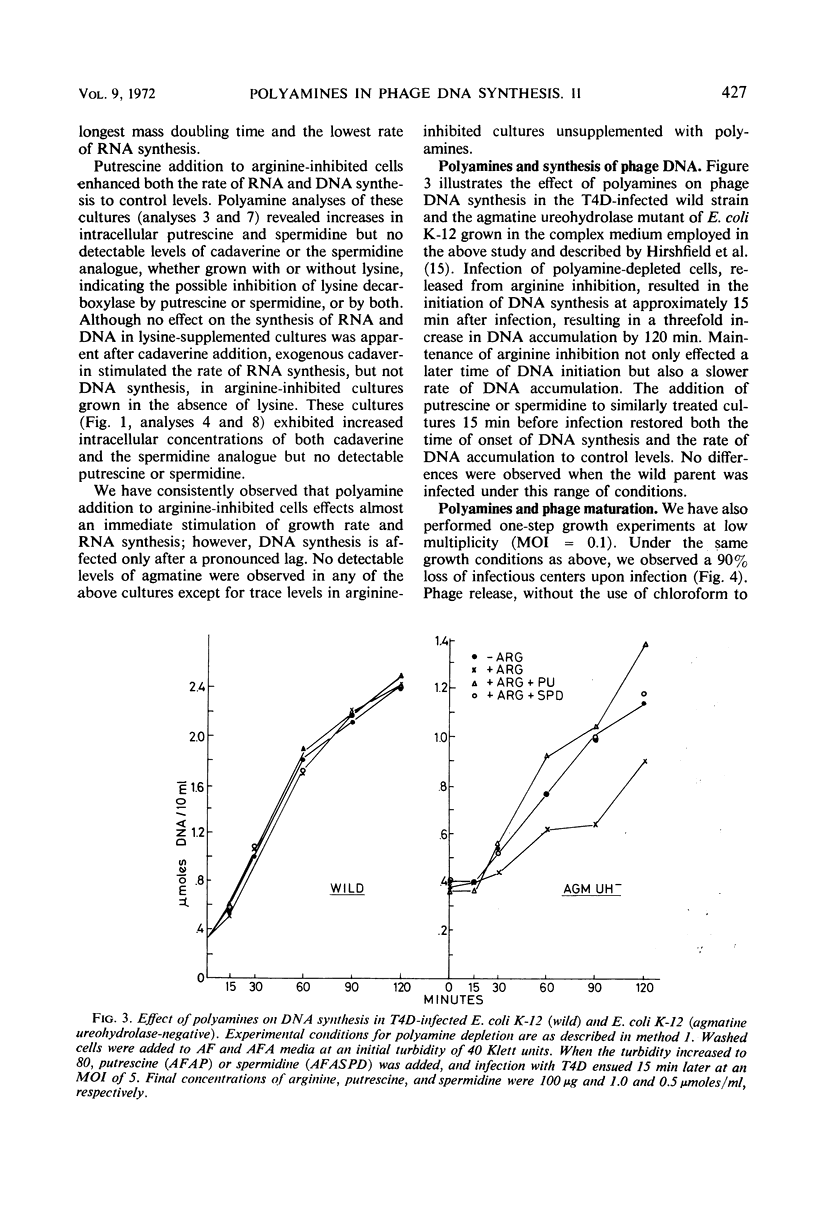
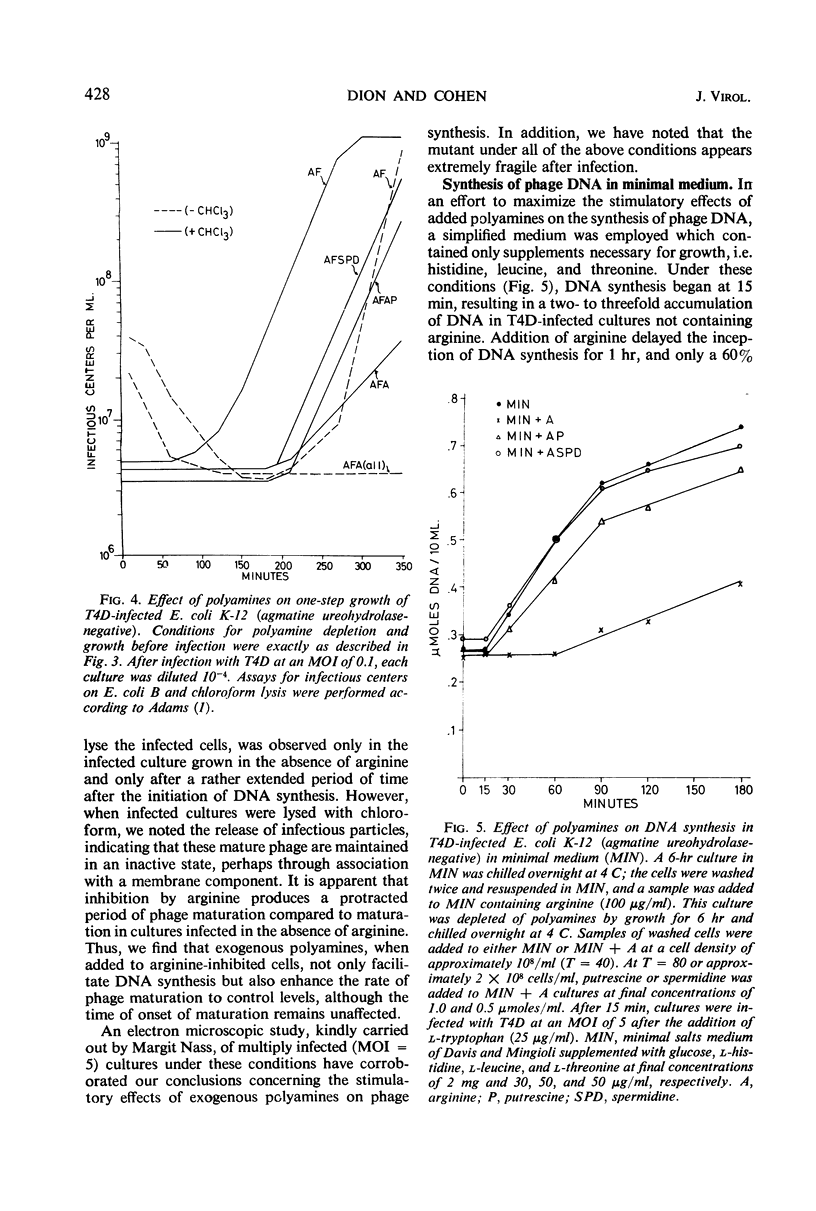
Polyamine depletion produced by exogenous arginine in Escherichia coliK-12 cultures defective in agmatine ureohydrolase activity resulted in a marked inhibition of the rates of growth and nucleic acid synthesis. Addition of putrescine or spermidine to such depleted cultures restored the control rate of growth and nucleic acid accumulation. The omission of lysine resulted in a further decrease in the rates of growth and nucleic acid synthesis in polyamine-depleted cells. The addition of exogenous cadaverine increased the rates of growth and ribonucleic acid synthesis to those observed in lysine-supplemented cultures, suggesting that lysine or a derivative of lysine serves a function similar to cadaverine. Addition of lysine to polyamine-depleted cultures at neutral pH results in the synthesis of cadaverine and a new spermidine analogue, both containing lysine carbon. This new metabolite has been isolated and identified as N-3-aminopropyl-1, 5-diaminopentane. T4D infection of the polyamine-depleted mutant resulted in a very low rate of DNA synthesis and phage maturation. The addition of putrescine or spermidine 15 min before infection restored phage DNA synthesis and phage maturation to control rates, i.e., rates observed in infected cells grown in the absence of arginine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK M. L. THE EFFECTS OF POLYAMINES ON THE REPLICATION OF T4RII MUTANTS IN ESCHERICHIA COLI K-12 (LAMBDA). Virology. 1965 Jun;26:221–227. doi: 10.1016/0042-6822(65)90049-8. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S. V., Kosuge T. Spermidine and spermine--polyamine components of turnip yellow mosaic virus. Virology. 1970 Apr;40(4):930–938. doi: 10.1016/0042-6822(70)90139-x. [DOI] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. I. Lack of dependence of polyamine synthesis on bacteriophage deoxyribonucleic acid synthesis. J Virol. 1972 Mar;9(3):419–422. doi: 10.1128/jvi.9.3.419-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- GUIRARD B. M., SNELL E. E. EFFECT OF POLYAMINE STRUCTURE ON GROWTH STIMULATION AND SPERMINE AND SPERMIDINE CONTENT OF LACTIC ACID BACTERIA. J Bacteriol. 1964 Jul;88:72–80. doi: 10.1128/jb.88.1.72-80.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBST E. J., GLINOS E. B., AMUNDSEN L. H. An analysis of the putrescine requirement of Hemophilus parainfluenzae. J Biol Chem. 1955 May;214(1):175–184. [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM K. H. ISOLATION AND PROPERTIES OF A PUTRESCINE-DEGRADING MUTANT OF ESCHERICHIA COLI. J Bacteriol. 1963 Aug;86:320–323. doi: 10.1128/jb.86.2.320-323.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol. 1967 Nov;94(5):1516–1519. doi: 10.1128/jb.94.5.1516-1519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TUCKER R. G. EFFECT OF METAL IONS AND POLYAMINES ON THE DEVELOPMENT OF BACTERIOPHAGE PHI-R. J Gen Microbiol. 1963 Aug;32:287–294. doi: 10.1099/00221287-32-2-287. [DOI] [PubMed] [Google Scholar]




