Abstract
The objective here was to summarize the evidence for, and quantify the link between, serum markers of lipid metabolism and risk of obesity-related cancers. PubMed and Embase were searched using predefined inclusion criteria to conduct meta-analyses on the association between serum levels of TG, TC, HDL, ApoA-I, and risk of 11 obesity-related cancers. Pooled relative risks (RRs) and 95% confidence intervals were estimated using random-effects analyses. 28 studies were included. Associations between abnormal lipid components and risk of obesity-related cancers when using clinical cutpoints (TC ≥ 6.50; TG ≥ 1.71; HDL ≤ 1.03; ApoA-I ≤ 1.05 mmol/L) were apparent in all models. RRs were 1.18 (95% CI: 1.08–1.29) for TC, 1.20 (1.07–1.35) for TG, 1.15 (1.01–1.32) for HDL, and 1.42 (1.17–1.74) for ApoA-I. High levels of TC and TG, as well as low levels of HDL and ApoA-I, were consistently associated with increased risk of obesity-related cancers. The modest RRs suggest serum lipids to be associated with the risk of cancer, but indicate it is likely that other markers of the metabolism and/or lifestyle factors may also be involved. Future intervention studies involving lifestyle modification would provide insight into the potential biological role of lipid metabolism in tumorigenesis.
1. Introduction
Obesity is a major worldwide problem, over 30% of adults in Western populations are obese, and there is growing evidence of the associated health risks associated [1–5]. The link between obesity and cancer risk has been studied extensively, but the results of individual studies do not suggest a consistent association [1, 6, 7]. Common cancers studied in the context of obesity include colorectal, breast, prostate, endometrial, pancreatic, liver, ovarian, kidney, gallbladder, leukaemia, and oesophageal cancers [4, 7–17].
The underlying mechanisms of action are not clear [1, 18–20]. A solid understanding could translate directly to patient benefit through implementation of therapeutic strategies to reduce cancer risk and mortality [19]. Assuming that the lipid metabolism plays a role in the biological processes driving the development of cancer, this could be easily modified by existing methods such as exercise, medication, or diet. Increased physical activity levels improve cardiovascular and overall mortality in healthy populations [21–24]. Also, physical activity after cancer diagnosis is associated with improvements in cancer outcomes [25, 26] and metabolic markers such as cholesterol [27, 28]. As such, improvements in lipid levels through uptake of physical activity may also translate into improvements in cancer-specific survival. Experimental evidence largely suggests that statins, a commonly used drug to lower cholesterol levels, reduce cancer risk, though further trials are needed [29]. However, a large meta-analysis by the Cholesterol Treatment Trialists' Collaboration showed no statistically significant associations between statin use and cancer risk [30]. Nevertheless, due to heterogeneity of plasma lipid profiles in overweight and obese people, there may be some inconsistency in the associations for serum lipids and cancer risk [31]. Moreover, study populations were often small, insufficient information was collected (e.g., lack of BMI measurement, few lipid components measured), and timing of blood sampling in relation to diagnosis varied widely [32, 33]. In addition, it is thought that tissue types are influenced differently by lipid components and to varying degrees [2, 32].
With these meta-analyses, we aimed to summarize and quantify the evidence for the link between markers of lipid metabolism and risk of obesity-related cancers. We examined the associations between four components of the lipid profile measured in serum (total cholesterol (TC), triglycerides (TGs), high-density lipoprotein (HDL), and apolipoprotein A-I (ApoA)) and risk of cancers previously shown to be linked with obesity.
2. Methods
2.1. Literature Search Strategy
We used computerised literature search databases (PubMed search followed by an Embase search) to identify full text and abstracts published to date. Searches were conducted both with and without MeSH terms (“neoplasms/epidemiology,” “cancer,” “hyperlipidemias,” “lipoproteins, HDL,” “hypertriglyceridemia,” “lipoproteins, apo A”). Except for English language, human subjects, adults, and publications within the last 10 years no additional restrictions were added to the search. We also included “grey literature,” such as letters and abstracts presented in relevant conference meetings. All references of the selected articles were checked, including hand searches.
2.2. Inclusion Criteria
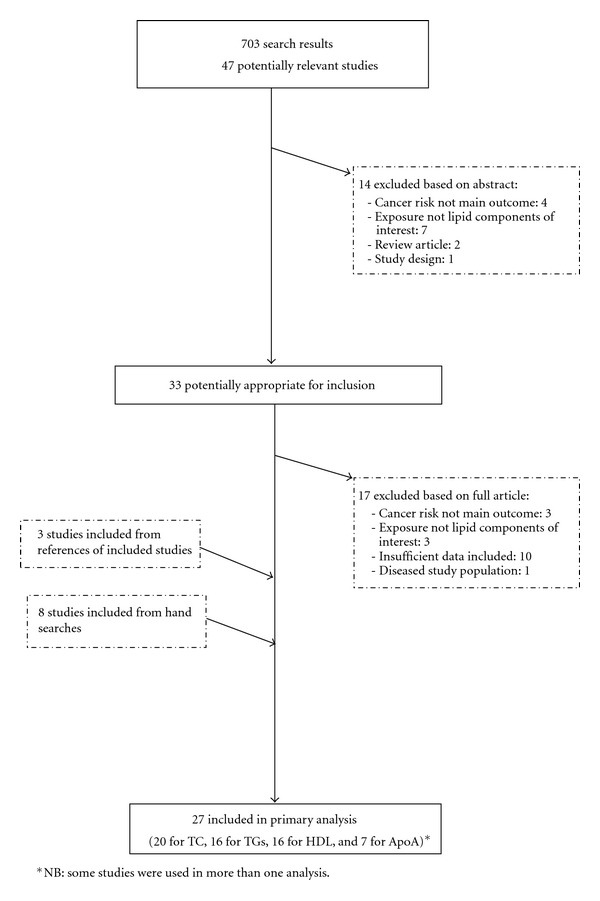
The final collection of selected studies was chosen based on the following criteria: the publication pertained to an epidemiological study (cohort or case-control studies), which measured the serum concentration of at least one of the selected lipid components (TC, TGs, HDL, and ApoA-I) prior to cancer diagnosis; the analytical methods were well described, with sufficient data available; the cancers included must have previously been linked to an increased risk associated with obesity. Those included were colorectal, breast, prostate, endometrial, pancreatic, liver, ovarian, kidney, gallbladder, leukaemia, and oesophageal cancer [7]. To include studies with large enough power, only those with at least 20 cancer cases were included. Initially, titles and abstracts of articles were reviewed in order to ascertain whether they potentially fit the inclusion criteria. If there was doubt over whether an article met the relevant criteria, it was subjected to a thorough assessment. After this first selection, all articles underwent detailed evaluations of the methods and results. Figure 1 illustrates the study exclusion process.
Figure 1.
2.3. Data Extraction
The following details were recorded for each study: author, year of publication, country where the study was undertaken, serum lipid component levels (mmol/l), study type (case-control or cohort), cancer type, number of cases and total subjects for each level of the lipid component(s) measured. To allow for ease of comparison, all values in conventional units (mg/dL) were converted into SI units (mmol/l) using conversion factors [34].
2.4. Statistical Methods
The effect of each lipid component on cancer risk was evaluated by calculating the random effects summary relative risk to allow for possible heterogeneity between study results. The analyses were conducted for dichotomized values of TC, TGs, HDL, and apoA-I). The following clinical cutpoints were used TC ≥ 6.50; TG ≥ 1.71; HDL ≤ 1.03; ApoA-I ≤ 1.05 mmol/L, all of which mirrored the NCEP and WHO guidelines as closely as practicable [35–39]. Some studies presented with dichotomised serum lipid levels, but for those that did not, participants from each study were divided into two groups based on their serum lipid level (“high” and “low”), and this mirrored the NCEP and WHO guidelines as closely as practicable. A first meta-analysis used all cancers from all studies. Potential heterogeneity of the study results was assessed with weighted forest plots, which display the relative risk estimates of cancer risk for each lipid component. Heterogeneity of the study results was also statistically evaluated using the Q-statistic as well as the I 2 statistic [40]. Sufficient data allowed for an individual analysis (using the methods described above) of prostate, colorectal, and breast cancers. Finally, we performed a metaregression to evaluate the effect of study design (i.e prospective cohort versus case-control studies). No other potential confounders were included in the metaregression due to the nature in which the data was presented through the papers. For example, some papers acknowledge having data for fasting status of the individuals at time of sampling, but did not provide the actual data by individuals. Potential publication bias and effect modification (by country and year) were assessed using Begg's Test and Egger's funnel plot. All analyses were performed using STATA (version 11.2).
3. Results
The initial searches produced a total of 701 articles. 33 studies were selected for further evaluation based on information from abstracts. Of these, 17 were excluded and a further 12 were added from hand searches and references of included studies, giving a total of 28 studies used in the primary data analysis. Eight studies were conducted in Asia, 12 in Europe, and eight in the USA (Table 1). All studies reported on at least one of the four lipid components under investigation. The nine included cancers were studied in association with cancer risk; all cancer diagnoses were histologically confirmed. Of those articles examined for inclusion, the major reasons for exclusion were missing information on methods and statistical analysis (n = 10), while a further three were excluded because serum lipid components were not the exposure of interest, and three were removed because incident cancer risk was not the outcome variable in the analysis.
Table 1.
Details of included studies.
| Author (y) | Country | Cases/controls | Study Type | Cancer(s) included | Mean age (SE)/age range | Timing of measurement | Measurement method | Cholesterol measure | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| Inoue et al. (2009) [63] | Japan | 1263/26461 | Cohort | Colon/rectum and breast | 56.0 (8.2) | Fasting and nonfasting | NS | TGs, HDL | Adjusted for age, study area, smoking status, weekly ethanol intake, and total serum cholesterol |
| Iso et al. (2009) [64] | Japan | 1093/32275 | Cohort | Colorectal, prostate, leukemia, breast and cervical | 54.0 | Fasting and nonfasting | Enzymatic | TC | Age, BMI, pack year of smoking, ethanol intake, hypertension, diabetes, hyperlipidemia medication use, total vegetable intake, coffee intake, and public health centre |
| Kitahara et al. (2011) [65] | Korea | 44935/ 1144784 |
Cohort | Oesophagus, colon/rectum, pancreas, prostate, kidney, and gallbladder | 30–95 | Fasting | NS | TC | Cigarette smoking, alcohol consumption, BMI, physical activity, hypertension, and fasting serum glucose |
| Mainous et al. (2005) [66] | USA | 203/3075 | Cohort | Cancer | 30+ | 12 hr fasting | NS | HDL | Age, gender, smoking status, and BMI |
| Melvin et al. (2012) [67] | Sweden | 6871/ 227603 |
Cohort | Breast and ovarian | 25+ | Fasting and nonfasting | Enzymatic and immunoturbidimetric | TC, TGs, HDL, ApoA | Glucose, TGs, TC, age, parity fasting status, and SES |
| Chang et al. (2007) [68] | Turkey | 150/71 | Case | Breast | 49.2 (11.8) | 12 hr fasting | Enzymatic | TC, TGs, HDL, ApoA | HDL, apoA-I, apoB, ApoA-I/ApoB ratio, and VLDL |
| Furberg et al. (2004) [69] | Norway | 1287/ 27912 |
Cohort | Breast | 43.6 (0.1) | Nonfasting | Enzymatic | HDL | Age, country of residence, parity, height, TC, recreational and occupation activity. Some models also included blood pressure, BMI, TGs, age at first birth, time since last meal, dietary energy, and fat intake |
| Ha et al. (2009) [70] | Korea | 714/169660 | Cohort | Breast | 55.9 (5.0) | Fasting | NS | TC | Age and BMI |
| Hayashi et al. (2012) [71] | Japan | 377/528 | Case | >60 | Fasting | NS | TGs | Age, PSA level, prostatic volume, BMI, and TGs level | |
| Kaye et al. (2002) [72] | USA | 158/725 | Case | Breast | 50–79 | NS | NS | TC | NS |
| Kim et al. (2009) [73] | Korea | 690/1380 | Case | Breast | 48.5 (7.6) | 8 hr fasting | Enzymatic | TGs, HDL | HDL, age, family history of breast cancer, age at menarche, age at the first full-term pregnancy, and total cholesterol |
| Lindemann et al. (2009) [74] | Norway | 100/31273 | Cohort | Endometrial | 56.1 | Nonfasting | Enzymatic | TC, TGs, HDL | Age, other lipids, and BMI |
| Cust et al. (2007) [75] | France | 284/546 | Case | Endometrial | 56.9 | Fasting and nonfasting | Enzymatic | TC, TGs, HDL | Age, laboratory batch, and case-control status |
| Seth et al. (2012) [76] | Sweden | 1144/ 224288 |
Cohort | Endometrial | 25+ | Fasting and nonfasting | Enzymatic and immunoturbidimetric | TC, TGs, HDL, ApoA | Glucose, TC, TGs, age, parity, fasting status, and SES |
| Magura et al. (2008) [77] | USA | 312/319 | Case | Prostate | 50–74 | NS | NS | TC, TGs, HDL | Age, family history of prostate cancer, BMI, type 2 diabetes, smoking, and multivitamin use |
| Mondul et al. (2010) [78] | USA | 438/6378 | Cohort | Prostate | 35+ | NS | Enzymatic | TC | Age, race, BMI, education level, smoking status, intake of meat, dairy, tomato products and alcohol, family history of prostate cancer, PSA screening, and use of diabetes medications |
| Mondul et al. (2011) [79] | USA | 2041/27052 | Cohort | Prostate | 50–69 | NS | Enzymatic | TC, HDL | Age, serum α-tocopherol, family history of PCA, education level, and urban residence |
| Platz et al. (2009) [80] | USA | 1251/4335 | Cohort | Prostate | 63.1 | Nonfasting | Enzymatic | TC | Age, race, first-degree family history of prostate cancer, BMI, self-reported diabetes mellitus, regular aspirin use, and history of myocardial infarction |
| Van Hemelrijck et al. (2011) [81] | Sweden | 2008/65719 | Cohort | Prostate | 35+ | Fasting and nonfasting | Enzymatic and immunoturbidimetric | HDL, ApoA | Age, glucose, TGs, and TC, fasting status, and SES. |
| Van Hemelrijck et al. (2011) [82] | Sweden | 5112/ 195548 |
Cohort | Prostate | 45–75 | Fasting and nonfasting | Enzymatic and immunoturbidimetric | TC, TGs | Glucose and/or TGs and/or TC, SES, fasting status, and time between measurements and entry |
| Bravi et al. (2005) [83] | Italy | 1294/1451 | Case | Prostate | 46–74 | NS | NS | TC | Age, centre, education, BMI, physical activity, tobacco smoking, alcohol consumption, and family history of prostate cancer |
| Tsushima et al. (2005) [84] | USA | 1004/21255 | Cohort | Colorectal | NS | Nonfasting | NS | TGs | Age, elapsed time since last calorific intake and 50 g glucose load, BMI, heart rate, cigarette smoking history, alcohol intake, and 24 h intake of total calories |
| Van Duijnhoven et al. (2011) [85] | W. Europe | 939/939 | Cohort | Colorectal | 35–70 | Fasting and nonfasting | Enzymatic | TC, TGs, HDL, ApoA | Age, gender, BMI, other lipids, height, weight, smoking habits, physical activity, education, consumption of fruit, vegetables, meat, fish, and alcohol, intake of fibre, energy from fat, and energy from nonfat |
| Chung et al. (2006) [86] | Korea | 105/105 | Case | Colorectal | 58.6 (8.3) | 12 hr fasting | Enzymatic | TC, TGs | Age, gender, BMI, glucose, triglycerides, and total cholesterol |
| Van Hemelrijck et al. (2012) [87] | Sweden | 156/82391 | Cohort | Kidney | 20+ | Fasting and nonfasting | Enzymatic and immunoturbidimetric | TC, TGs, HDL, ApoA | Age, gender, glucose, TGs and TC, creatinine levels, fasting status, and SES |
| Andreotti et al. (2008) [88] | USA | 264/1839 | Case | Biliary | 34–75 | Overnight fasting | NS | TC, TGs, HDL, ApoA | Age, gender, BMI, waist-to-hip ratio, cigarette smoking, alcohol drinking, hypertension, diabetes, and gallstone status |
| Asano et al. (2008) [89] | Japan | 97/2507 | Cohort | Gastric | 59.2 (0.3) | Fasting and nonfasting | Enzymatic | TC | Age and gender |
NS: not specified; BMI: body mass index; TC: total cholesterol; TGs: triglycerides; HDL: high-density lipoprotein; ApoA: apolipoprotein A-I; ApoB: apolipoprotein B; VLDL: very low-density lipoprotein.
For each lipid component studied we looked at the risk of developing cancer in those with abnormal versus normal levels. The random-effects analysis, comparing overall cancer risk and total cholesterol level, showed a pooled effects relative risk of 1.18 (95% CI 1.08–1.29) (Figure 2). The Q-statistic and I 2-statistic suggested heterogeneity (Q = 250.02; df = 18; P = <0.000; I 2 = 92.8%), which warranted the use of a random-effects model. The association between TGs and overall cancer risk resulted in a pooled relative risk of 1.20 (95% CI 1.07–1.35). The pooled relative risk was 1.15 (95% CI 1.01–1.32) when studying the association between HDL and risk of obesity-related cancers. The pooled effects relative risk of overall cancer was 1.42 (95% CI 1.17–1.74) for ApoA-I (Figure 2).
Figure 2.
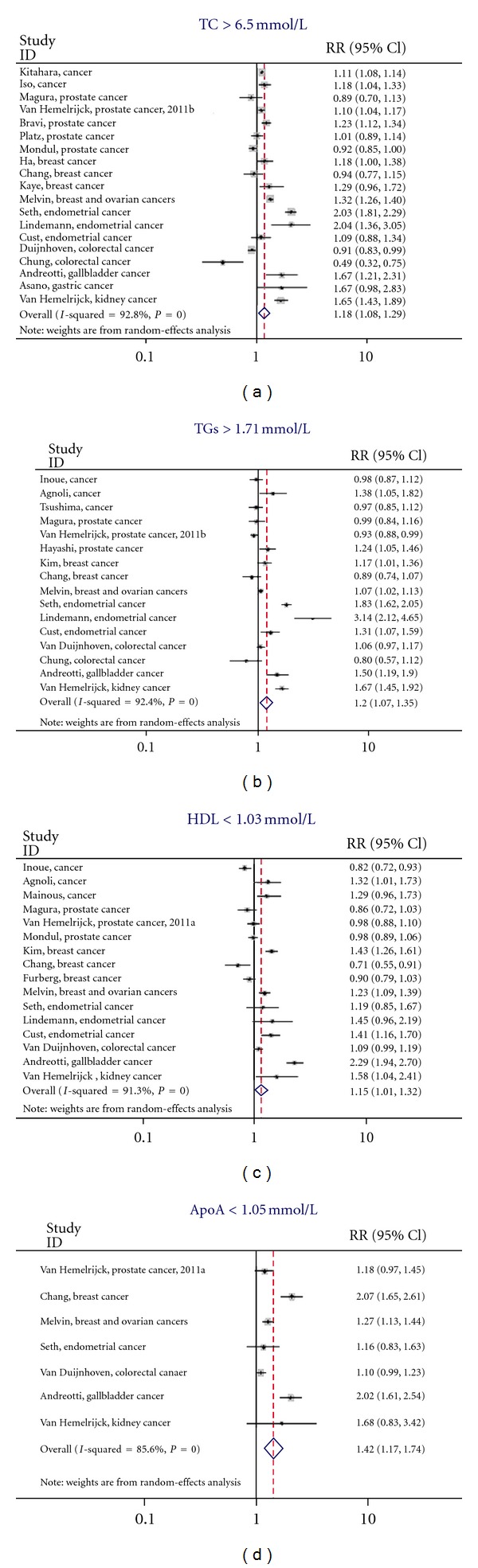
Individual forest for lipid components, the I-squared statistic is also illustrated in each plot—total cholesterol; triglycerides; high-desity Lipoprotein; apolipoprotein A-I.
We also conducted a stratified analysis by study type and found that the pooled RRs for case-control studies were slightly different (i.e., RR (95% CI) = 0.98 (0.81–1.18)) and (RR (95% CI) = 1.20 (1.04–1.38) for case-control and cohort studies on TC, resp.). We investigated this further with a metaregression, but did not find a statistically significant effect (i.e., P value of 0.193 when studying TC). Begg's test did not indicate significant publication bias (P = 0.506), which is evident from the funnel plot, as there is a relatively symmetric distribution observed among studies with small sample size (Figure 3).
Figure 3.
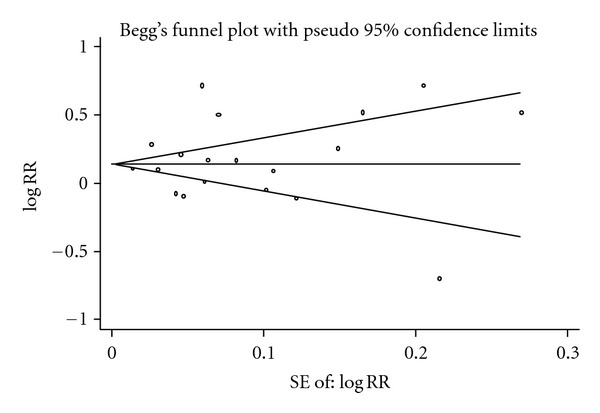
Funnel plot of Begg's test (with 95% CI) to quantify presence of publication bias.
Finally, we also performed a meta-analysis specifically for TC and risk of prostate, breast, and colorectal cancer. The pooled relative risk for prostate cancer was 1.04 (95% CI: 0.87–1.24), whereas it was 1.08 (95% CI: 0.89–1.31) and 1.20 (95% CI: 0.44–3.26) for breast and colorectal cancers, respectively.
4. Discussion
These meta-analyses summarize the current evidence for a link between serum markers of lipid metabolism and risk of obesity-related cancers. All pooled models showed evidence for an association between abnormal lipid components and risk of obesity-related cancers when using clinical cutpoints.
The precise aetiology of the link between obesity and risk of cancer has yet to be determined, but there has been growing evidence for a role of lipid metabolism in tumour development [18]. Apart from the studies listed on the link between serum lipids and cancer risk (Table 1), there is also preclinical evidence. For instance, it is thought that androgens stimulate prostate tumor growth via activation of pathways that regulate lipogenic gene expression, resulting in lipid accumulation [41]. Hyperlipidemia has also been shown to be involved in colorectal tumour development and initiation and progression of breast and prostate cancers [42–44]. Moreover, there is experimental evidence that fatty acid synthase (FAS), the enzyme that synthesizes fatty acids de novo, is involved in tumorigenesis [45–47]. For example, prostate cancers overexpressing FAS display aggressive behavior, with the highest expression in patients with bony metastatic disease [47, 48]. In addition, nutritional studies showed that diets high in fat are linked to accelerated tumour growth and metastasis [42, 49]. Furthermore, cholesterol-lowering drugs such as statins have been shown to reduce the formation and spread of metastatic cancer cells [50, 51]. Finally, the immune system is thought to play a role in the link between HDL, ApoA-I, and tumorigenesis [52]. These lipid components decrease free proinflammatory cytokines such as tumour necrosis factor-α (TNF-α) which consequently reduces tissue damage, infiltration of macrophages and neutrophils, and attenuates tumour formation [53]. Therefore, low levels of HDL and Apo A-I may contribute to an inflammatory process linked to tumour biology.
Recent years have seen a multitude of reviews and meta-analyses comparing obesity and specific cancer risks; results varied widely depending on the type of cancer investigated, with relative risks ranging from 1.02 to 4.10 (breast cancer [11, 54–56], endometrial cancer [57], pancreatic [58–60], liver [17], prostate cancer [61], and colon and rectal cancer [15, 16]). As a result, our findings for an association between serum lipid components and risk of cancer also varied by type of cancer, as can be seen from our results for HDL (Figure 2), show that there may be stronger correlation between serum HDL levels and cancer risk, dependent on cancer type. Those results focusing on a single cancer showed more consistent results, suggesting that even among obesity-related cancers there may be a different association with serum lipid levels.
4.1. Strengths and Limitations of This Study
The greatest strength of this study is that we examined four different components of the lipid profile in relation to risk of developing cancer in the context of obesity. We also made all possible efforts to include all relevant available publications, including searching the two main online databases (PubMed and Embase). Additionally, our clearly defined objective criteria for exposure, outcome, and other study characteristics were specified a priori. There was no evidence of publication bias in these analyses.
A number of the studies subdivided levels of lipid components, but this was not performed consistently across the studies. Studies which had not dichotomised serum lipid levels from the outset were divided into two groups based on their serum lipid level (“high” and “low”) to mirror the NCEP and WHO guidelines as closely as practicable [38, 62]. This crude categorization may have compromised the accuracy and resulted in miscategorising of individuals, but given the rather small differences in cutoffs we do not believe that this has had a major impact on our analyses.
Heterogeneity among studies may also arise from different method of assessment of serum lipids. By performing random-effects analyses, we have taken into account between-study variation. Within-person variation is a likely interference with results as the one measurement taken may not be representative for a person's average, or previous lipid levels. However, this variation will be present in all studies using a single measurement. In addition, adjustments made for confounding factors (e.g., gender or age) were not consistent across included studies and some sample sizes were relatively small or excluded one gender. Again, random effects analyses take into account this heterogeneity and in addition we included a metaregression analysis for study type.
In addition, the studies did not provide age-specific data, so it was not possible to conduct age-specific meta-analyses which presents us with a limitation. Persons younger than middle aged more rarely have abnormal lipid profiles and are also considerably less likely to be diagnosed with the cancers of interest than in those people aged over 50. Thus, this leaves our study population with a relatively low probability of having both sufficient exposure and number of cases in the lower age range. We do not believe that this will have had a major effect on our results, although it is worth considering that this may have diluted the strength of our findings somewhat.
Due to the information provided in the included studies, we had no means to adjust our analyses for cancer screening practices. Undoubtedly, these practices vary around the world and thus the differences could lead to the introduction of detection bias.
Finally, the analyses of the three individual cancers (prostate, breasts and colorectal cancers) did not produce statistically significant relative risks, which most likely follows from a lack of power due to the limited number of studies available for inclusion. Future research, with larger sample sizes, repeated measurements, and consistent adjustments for confounding could provide information to inform a more reliable estimate of links between serum lipid components and cancer risk.
4.2. Conclusions
Abnormal levels of all lipid components studied were statistically significantly associated with an increased risk of obesity-related cancers, with the strongest association for serum ApoA-I. Despite a suggestion for a link between the lipid metabolism and risk of cancer, the magnitude of the pooled relative risk was relatively small. This may be because the studied lipid components are markers of obesity or because they are markers of other lifestyle factors potentially associated with tumorigenesis. Since lipid components are easily modified through lifestyle interventions such as diet or exercise, research into serum lipid components and cancer risk presents a prime opportunity for intervention studies to help provide the desired insight into their biological role.
Conflict of Interests
The authors declare that they have no potential conflict of interests to disclose.
Acknowledgments
Funding was received from Cancer Research UK and the Swedish Cancer Society. This research was also supported by the Experimental Cancer Medicine Centre at King's College London and also by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. In addition, M. Loda's work ML's work was supported by the NIH grants RO1CA131945, P01 CA08902, P50 CA90381, and the Prostate Cancer Foundation. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health, the NIH or the Prostate Cancer Foundation.
Abbreviations
- (TC):
Total cholesterol
- (TGs):
Triglycerides
- (HDL):
High-density lipoprotein
- (ApoA-I):
Apolipoprotein A-I
- (BMI):
Body mass index.
References
- 1.Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13(2):155–165. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 2.Jee SH, Kim HJ, Lee J. Obesity, insulin resistance and cancer risk. Yonsei Medical Journal. 2005;46(4):449–455. doi: 10.3349/ymj.2005.46.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung RT. Obesity as a disease. British Medical Bulletin. 1997;53(2):307–321. doi: 10.1093/oxfordjournals.bmb.a011615. [DOI] [PubMed] [Google Scholar]
- 4.Pender JR, Pories WJ. Epidemiology of obesity in the United States. Gastroenterology Clinics of North America. 2005;34(1):1–7. doi: 10.1016/j.gtc.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clinics in Dermatology. 2004;22(4):296–302. doi: 10.1016/j.clindermatol.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. European Journal of Cancer Prevention. 2002;11(2):S94–S100. [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 8.Malnick SDH, Knobler H. The medical complications of obesity. QJM. 2006;99(9):565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 9.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan) Archives of Internal Medicine. 2010;170(9):791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Itallie TB. Obesity: adverse effects on health and longevity. American Journal of Clinical Nutrition. 1979;32(12):2723–2733. doi: 10.1093/ajcn/32.12.2723. [DOI] [PubMed] [Google Scholar]
- 11.Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Research and Treatment. 2010;123(3):641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 12.Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma—epidemiological evidence. Alimentary Pharmacology and Therapeutics. 2010;31(10):1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 13.Fuemmeler BF, Pendzich MK, Tercyak KP. Weight, dietary behavior, and physical activity in childhood and adolescence: implications for adult cancer risk. Obesity Facts. 2009;2(3):179–186. doi: 10.1159/000220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Disease. 2009;11(6):547–563. doi: 10.1111/j.1463-1318.2009.01766.x. [DOI] [PubMed] [Google Scholar]
- 15.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World Journal of Gastroenterology. 2007;13(31):4199–4206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. American Journal of Clinical Nutrition. 2007;86(3):556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 17.Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary & Pancreatic Diseases International. 2005;4(2):173–177. [PubMed] [Google Scholar]
- 18.Leroith D, Novosyadlyy R, Gallagher EJ, et al. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Experimental and Clinical Endocrinology and Diabetes. 2008;116(1):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 19.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nature Reviews Cancer. 2011;11(12):886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 20.Meikle PJ, Christopher MJ. Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Current Opinion in Lipidology. 2011;22(3):210–215. doi: 10.1097/MOL.0b013e3283453dbe. [DOI] [PubMed] [Google Scholar]
- 21.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107(19):2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 22.Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. Journal of the American Medical Association. 2006;296(2):171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Gregg EW, Cauley JA, Stone K, et al. Relationship of changes in physical activity and mortality among older women. Journal of the American Medical Association. 2003;289(18):2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 24.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-Month results of a randomized trial. Annals of Internal Medicine. 2006;144(7):485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 25.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Archives of Internal Medicine. 2009;169(22):2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McTiernan A, Irwin M, VonGruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. Journal of Clinical Oncology. 2010;28(26):4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligibel JA, Giobbie-Hurder A, Olenczuk D, et al. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes & Control. 2009;20(8):1523–1528. doi: 10.1007/s10552-009-9358-3. [DOI] [PubMed] [Google Scholar]
- 28.Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of Clinical Oncology. 2008;26(6):907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 29.Gonyeau MJ, Yuen DW. A clinical review of statins and cancer: helpful or harmful? Pharmacotherapy. 2010;30(2):177–194. doi: 10.1592/phco.30.2.177. [DOI] [PubMed] [Google Scholar]
- 30.Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PloS One. 2012;7(1) doi: 10.1371/journal.pone.0029849.e29849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooradian AD, Haas MJ, Wehmeier KR, Wong NCW. Obesity-related changes in high-density lipoprotein metabolism. Obesity. 2008;16(6):1152–1160. doi: 10.1038/oby.2008.202. [DOI] [PubMed] [Google Scholar]
- 32.Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and On-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. Journal of the American College of Cardiology. 2010;55(25):2846–2854. doi: 10.1016/j.jacc.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 33.Kok DE, van Roermund JG, Aben KK, den Heijer M, Swinkels DW, Kampman E, et al. Blood lipid levels and prostate cancer risk, a cohort study. Prostate Cancer and Prostatic Diseases. 2011;14(4):340–345. doi: 10.1038/pcan.2011.30. [DOI] [PubMed] [Google Scholar]
- 34.McAuley D. Conventional Units—International Units. 1993, http://www.globalrph.com/conv_si.htm.
- 35.Jungner I, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I in relation to serum cholesterol and triglycerides in 43 000 Swedish males and females. International Journal of Clinical & Laboratory Research. 1992;21(2-4):247–255. doi: 10.1007/BF02591655. [DOI] [PubMed] [Google Scholar]
- 36.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. American Journal of Nephrology. 2009;30(3):171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millan J, Pinto X, Munoz A, Zuniga M, Rubies-Prat J, Pallardo LF, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vascular Health and Risk Management. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 38.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 39.Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiology Biomarkers and Prevention. 2009;18(11):2814–2821. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. Chapter 15. London, UK: BMJ Publishing; 2008. [Google Scholar]
- 41.Swinnen JV, Heemers H, Van De Sande T, et al. Androgens, lipogenesis and prostate cancer. Journal of Steroid Biochemistry and Molecular Biology. 2004;92(4):273–279. doi: 10.1016/j.jsbmb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Mehta N, Hordines J, Volpe C, Doerr R, Cohen SA. Cellular effects of hypercholesterolemia in modulation of cancer growth and metastasis: a review of the evidence. Surgical Oncology. 1997;6(3):179–185. doi: 10.1016/s0960-7404(97)00027-3. [DOI] [PubMed] [Google Scholar]
- 43.Arliss RM, Biermann CA. Do soy isoflavones lower cholesterol, inhibit atherosclerosis, and play a role in cancer prevention? Holistic Nursing Practice. 2002;16(5):40–48. doi: 10.1097/00004650-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Mutoh M, Akasu T, Takahashi M, et al. Possible involvement of hyperlipidemia in increasing risk of colorectal tumor development in human familial adenomatous polyposis. Japanese Journal of Clinical Oncology. 2006;36(3):166–171. doi: 10.1093/jjco/hyi233. [DOI] [PubMed] [Google Scholar]
- 45.Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. Journal of Pathology. 2011;223(2):283–294. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas- mediated cytotoxicity. Journal of the National Cancer Institute. 1997;89(11):783–789. doi: 10.1093/jnci/89.11.783. [DOI] [PubMed] [Google Scholar]
- 47.Migita T, Ruiz S, Fornari A, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. Journal of the National Cancer Institute. 2009;101(7):519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Molecular Cancer Research. 2003;1(10):707–715. [PubMed] [Google Scholar]
- 49.Kimura Y, Sumiyoshi M. High-fat, high-sucrose, and high-cholesterol diets accelerate tumor growth and metastasis in tumor-bearing mice. Nutrition and Cancer. 2007;59(2):207–216. doi: 10.1080/01635580701499537. [DOI] [PubMed] [Google Scholar]
- 50.Brown M, Hart C, Tawadros T, Ramani V, Sangar V, Lau M, et al. The differential effects of statins on the metastatic behaviour of prostate cancer. British Journal of Cancer. 2012;106(10):1689–1696. doi: 10.1038/bjc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasuda Y, Shimizu M, Shirakami Y, et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Science. 2010;101(7):1701–1707. doi: 10.1111/j.1349-7006.2010.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs EJ, Gapstur SM. Cholesterol and cancer: answers and new questions. Cancer Epidemiology Biomarkers and Prevention. 2009;18(11):2805–2806. doi: 10.1158/1055-9965.EPI-09-1027. [DOI] [PubMed] [Google Scholar]
- 53.Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-α content and enhancing prostaglandin release. Circulation Research. 2003;92(3):330–337. doi: 10.1161/01.res.0000054201.60308.1a. [DOI] [PubMed] [Google Scholar]
- 54.Pichard C, Plu-Bureau G, Neves-e Castro M, Gompel A. Insulin resistance, obesity and breast cancer risk. Maturitas. 2008;60(1):19–30. doi: 10.1016/j.maturitas.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Adderley-Kelly B, Williams-Stephens E. The relationship between obesity and breast cancer. The ABNF Journal. 2003;14(3):61–65. [PubMed] [Google Scholar]
- 56.Barnett JB. The relationship between obesity and breast cancer risk and mortality. Nutrition Reviews. 2003;61(2):73–76. doi: 10.1301/nr.2003.febr.73-76. [DOI] [PubMed] [Google Scholar]
- 57.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiology Biomarkers and Prevention. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 58.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Molecular Carcinogenesis. 2012;51(1):53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiao L, Berrington de Gonzalez A, Hartge P, Pfeiffer RM, Park Y, Freedman DM, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes & Control. 2010;21(8):1305–1314. doi: 10.1007/s10552-010-9558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Gonzalez AB, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. British Journal of Cancer. 2003;89(3):519–523. doi: 10.1038/sj.bjc.6601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moyad MA. Is obesity a risk factor for prostate cancer, and does it even matter? A hypothesis and different perspective. Urology. 2002;59(4):41–50. doi: 10.1016/s0090-4295(01)01175-x. [DOI] [PubMed] [Google Scholar]
- 62.Report of A WHO/IDF Consulation. Geneva, Switzerland: World Health Organisation; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. [Google Scholar]
- 63.Inoue M, Noda M, Kurahashi N, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. European Journal of Cancer Prevention. 2009;18(3):240–247. doi: 10.1097/CEJ.0b013e3283240460. [DOI] [PubMed] [Google Scholar]
- 64.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. International Journal of Cancer. 2009;125(11):2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 65.Kitahara CM, De González AB, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. Journal of Clinical Oncology. 2011;29(12):1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mainous AG, III, Wells BJ, Koopman RJ, Everett CJ, Gill JM. Iron, lipids, and risk of cancer in the Framingham offspring cohort. American Journal of Epidemiology. 2005;161(12):1115–1122. doi: 10.1093/aje/kwi131. [DOI] [PubMed] [Google Scholar]
- 67.Melvin JC, Seth D, Holmberg L, et al. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(8):1381–1384. doi: 10.1158/1055-9965.EPI-12-0188. [DOI] [PubMed] [Google Scholar]
- 68.Chang SJ, Hou MF, Tsai SM, et al. The association between lipid profiles and breast cancer among Taiwanese women. Clinical Chemistry and Laboratory Medicine. 2007;45(9):1219–1223. doi: 10.1515/CCLM.2007.263. [DOI] [PubMed] [Google Scholar]
- 69.Furberg AS, Veierød MB, Wilsgaard T, Berstein L, Thune I. Serum high density lipoprotein cholesterol, metabolic profile, and breast cancer risk. Journal of the National Cancer Institute. 2004;96(15):1152–1160. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 70.Ha M, Sung J, Song YM. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes & Control. 2009;20(7):1055–1060. doi: 10.1007/s10552-009-9301-7. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi N, Matsushima M, Yamamoto T, Sasaki H, Takahashi H, Egawa S. The impact of hypertriglyceridemia on prostate cancer development in patients aged ≥60 years. BJU International. 2012;109(4):515–519. doi: 10.1111/j.1464-410X.2011.10358.x. [DOI] [PubMed] [Google Scholar]
- 72.Kaye JA, Meier CR, Walker AM, Jick H. Statin use, hyperlipidaemia, and the risk of breast cancer. British Journal of Cancer. 2002;86(9):1436–1439. doi: 10.1038/sj.bjc.6600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim Y, Park SK, Han W, et al. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiology Biomarkers & Prevention. 2009;18(2):508–515. doi: 10.1158/1055-9965.EPI-08-0133. [DOI] [PubMed] [Google Scholar]
- 74.Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Serum lipids and endometrial cancer risk: results from the HUNT-II study. International Journal of Cancer. 2009;124(12):2938–2941. doi: 10.1002/ijc.24285. [DOI] [PubMed] [Google Scholar]
- 75.Cust AE, Kaaks R, Friedenreich C, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocrine-Related Cancer. 2007;14(3):755–767. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 76.Seth D, Garmo H, Wigertz A, et al. Lipid profiles and the risk of endometrial cancer in the Swedish AMORIS study. International Journal of Molecular Epidemiology and Genetics. 2012;3(2):122–133. [PMC free article] [PubMed] [Google Scholar]
- 77.Magura L, Blanchard R, Hope B, Beal JR, Schwartz GG, Sahmoun AE. Hypercholesterolemia and prostate cancer: a hospital-based case-control study. Cancer Causes & Control. 2008;19(10):1259–1266. doi: 10.1007/s10552-008-9197-7. [DOI] [PubMed] [Google Scholar]
- 78.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes and Control. 2010;21(1):61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes & Control. 2011;22(11):1545–1552. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Platz EA, Till C, Goodman PJ, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiology Biomarkers & Prevention. 2009;18(11):2807–2813. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Hemelrijck M, Walldius G, Jungner I, et al. Low levels of apolipoprotein A-I and HDL are associated with risk of prostate cancer in the Swedish AMORIS study. Cancer Causes & Control. 2011;22(7):1011–1019. doi: 10.1007/s10552-011-9774-z. [DOI] [PubMed] [Google Scholar]
- 82.Van Hemelrijck M, Garmo H, Holmberg L, et al. Prostate cancer risk in the Swedish AMORIS study. Cancer. 2011;117(10):2086–2095. doi: 10.1002/cncr.25758. [DOI] [PubMed] [Google Scholar]
- 83.Bravi F, Scotti L, Bosetti C, et al. Self-reported history of hypercholesterolaemia and gallstones and the risk of prostate cancer. Annals of Oncology. 2006;17(6):1014–1017. doi: 10.1093/annonc/mdl080. [DOI] [PubMed] [Google Scholar]
- 84.Tsushima M, Nomura AMY, Lee J, Stemmermann GN. Prospective study of the association of serum triglyceride and glucose with colorectal cancer. Digestive Diseases and Sciences. 2005;50(3):499–505. doi: 10.1007/s10620-005-2464-5. [DOI] [PubMed] [Google Scholar]
- 85.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011;60(8):1094–1102. doi: 10.1136/gut.2010.225011. [DOI] [PubMed] [Google Scholar]
- 86.Chung YW, Han DS, Park YK, et al. Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case-control study in Korea. Digestive and Liver Disease. 2006;38(9):668–672. doi: 10.1016/j.dld.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Van Hemelrijck M, Garmo H, Hammar N, et al. The interplay between lipid profiles, glucose, BMI and risk of kidney cancer in the Swedish AMORIS study. International Journal of Cancer. 2012;130(9):2118–2128. doi: 10.1002/ijc.26212. [DOI] [PubMed] [Google Scholar]
- 88.Andreotti G, Chen J, Gao Y-T, et al. Serum lipid levels and the risk of biliary tract cancers and biliary stones: A population-based study in China. International Journal of Cancer. 2008;122(10):2322–2329. doi: 10.1002/ijc.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asano K, Kubo M, Yonemoto K, et al. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: the Hisayama study. International Journal of Cancer. 2008;122(4):909–914. doi: 10.1002/ijc.23191. [DOI] [PubMed] [Google Scholar]


