Abstract
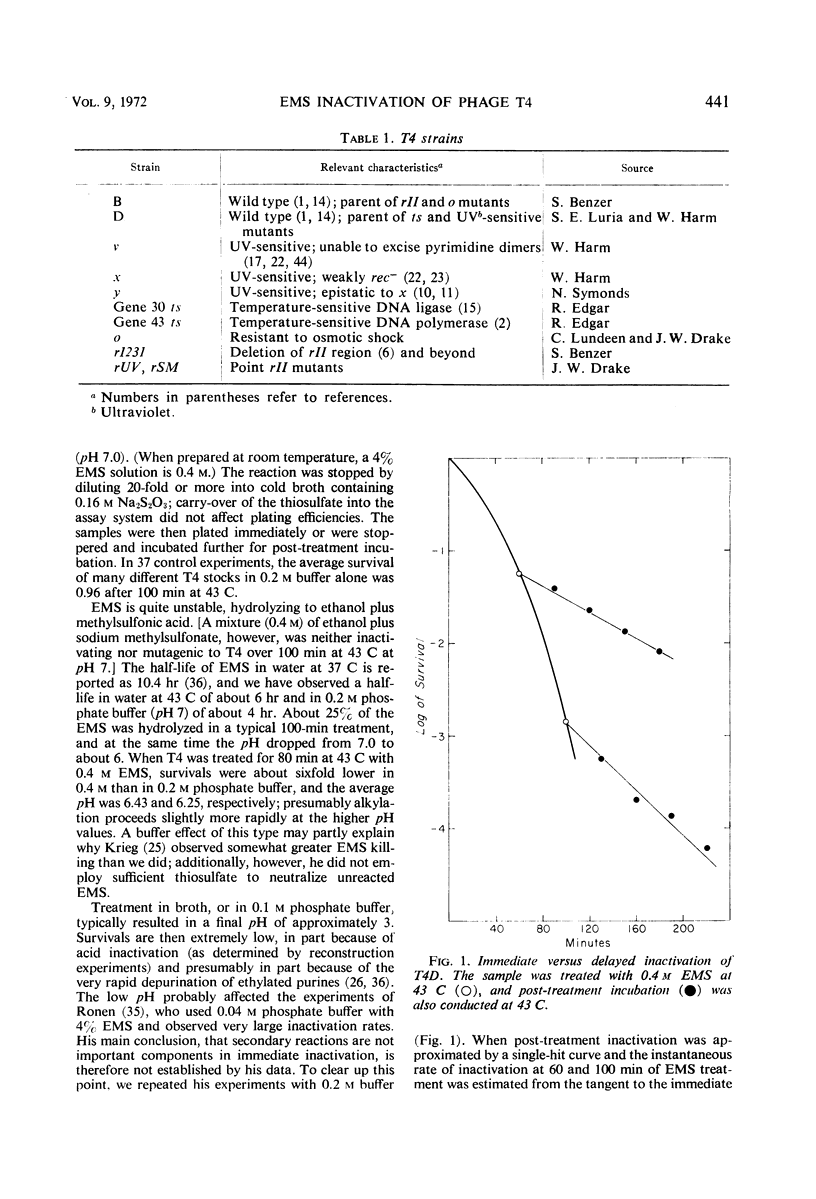
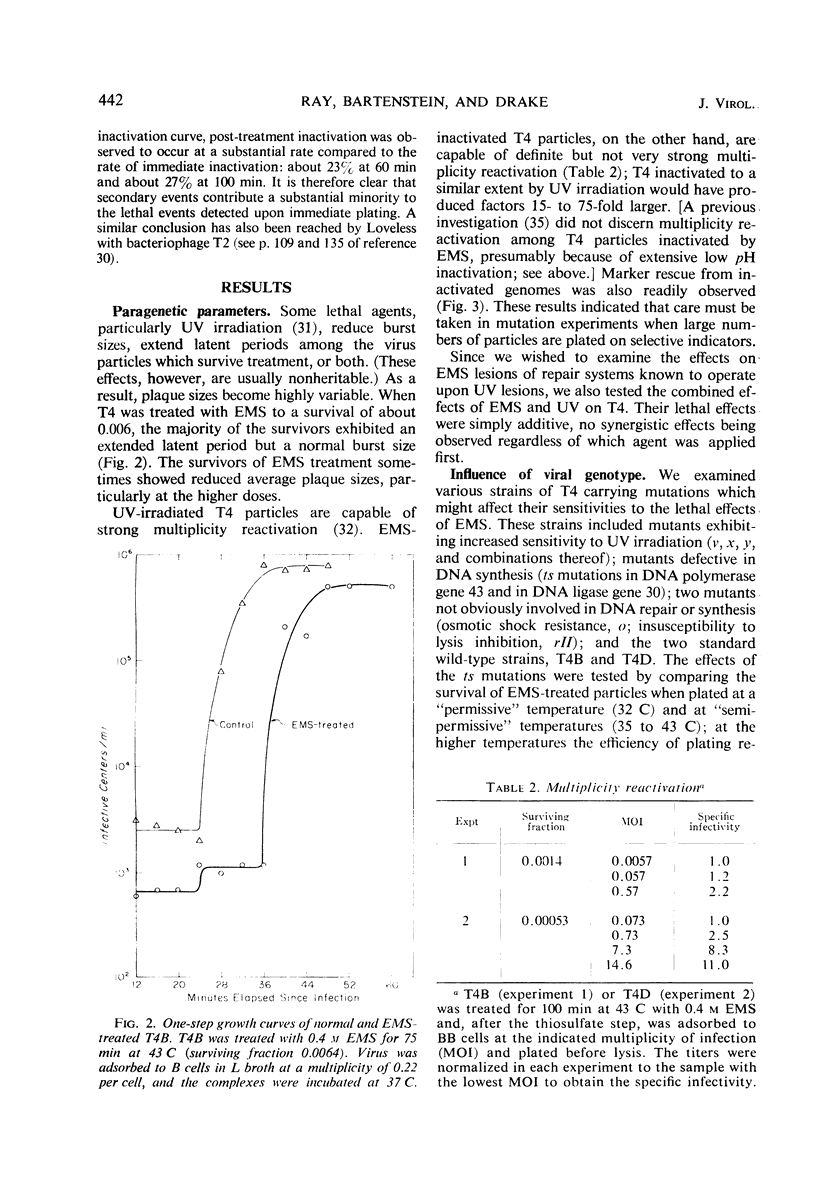
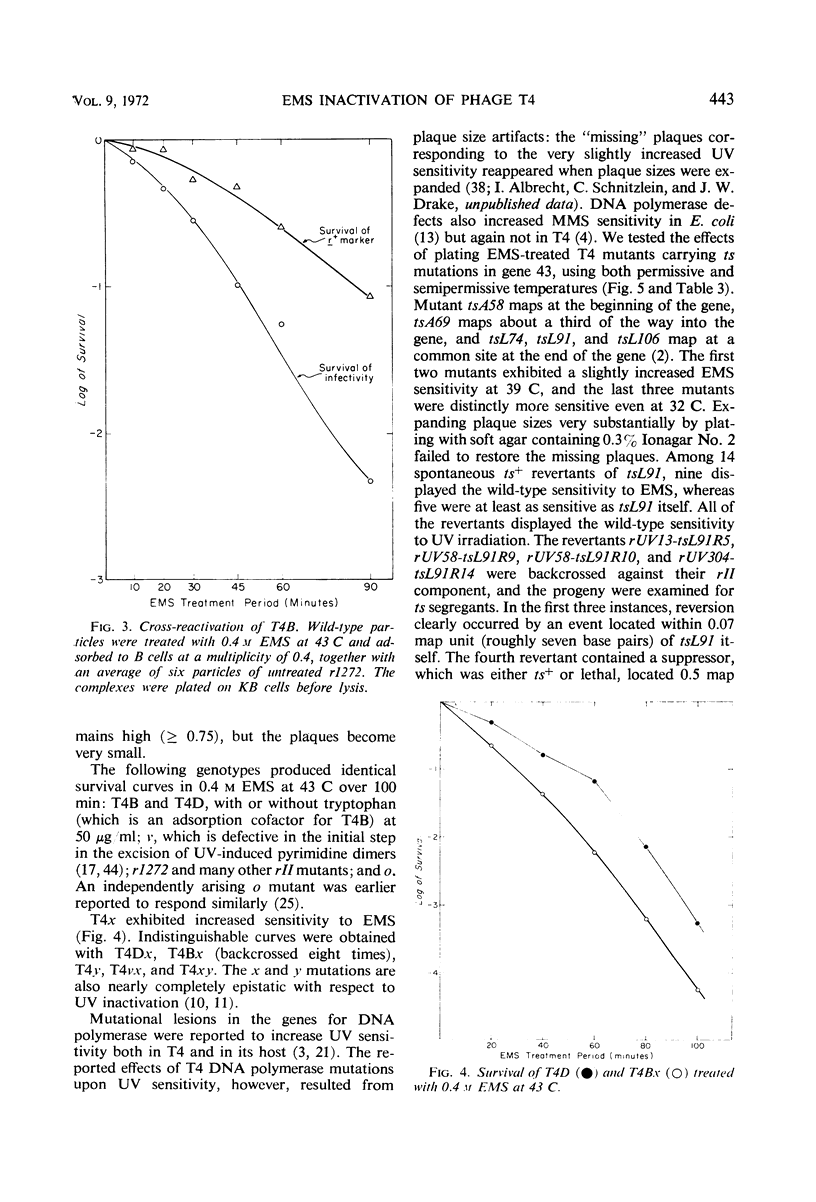
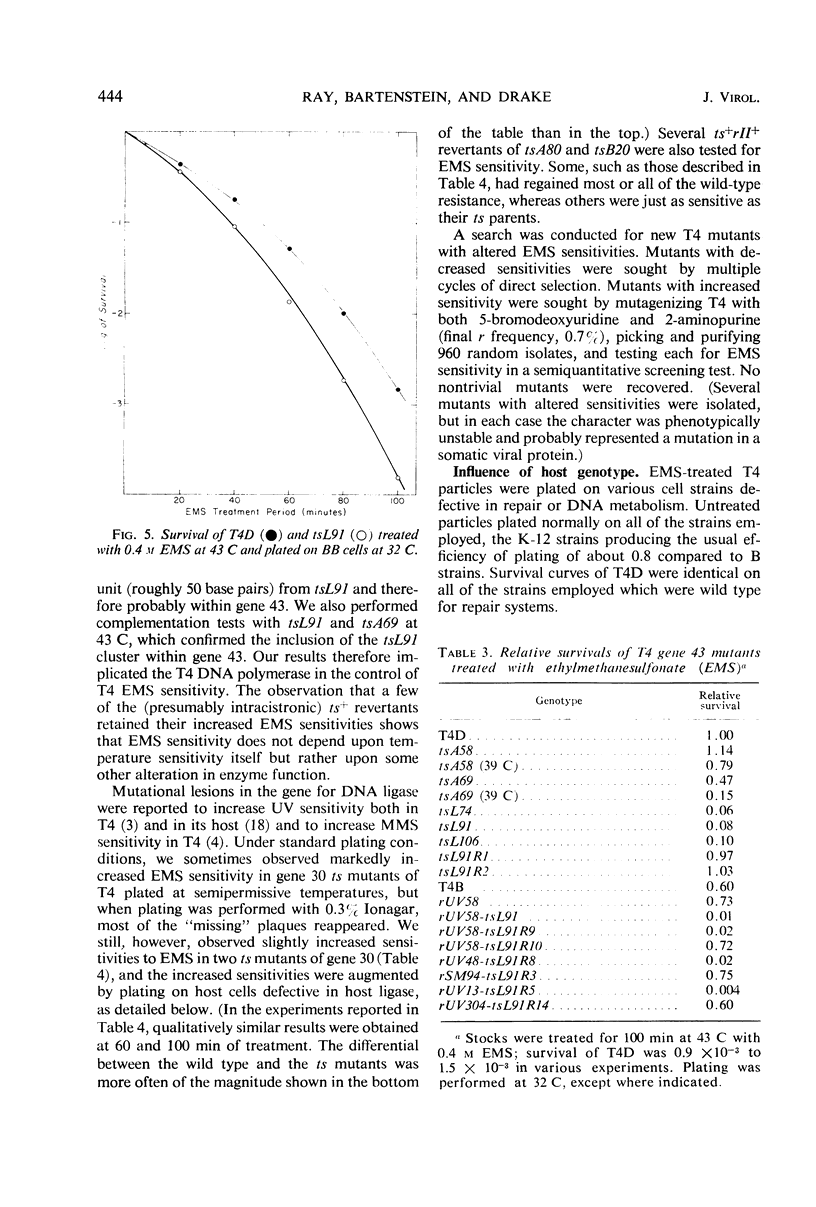
Inactivation of bacteriophage T4 by ethyl methanesulfonate (EMS) is a complex process which depends critically upon the conditions of treatment and upon both the viral and the host genotypes. EMS-inactivated particles are capable of multiplicity and cross-reactivation, indicating the need for caution in using EMS in certain types of mutation studies. The pyrimidine dimer excision systems of the phage and the host do not affect the EMS sensitivity of T4, but the T4x+y+ system does. Mutational defects in the deoxyribonucleic acid (DNA) ligase and the DNA polymerase systems both of the virus and of its host also affect viral EMS sensitivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. F., Albrecht I., Drake J. W. Properties of bacteriophage T4 mutants defective in DNA polymerase. Genetics. 1970 Jun;65(2):187–200. doi: 10.1093/genetics/65.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EFFECTS OF ALKYLATING AGENTS ON T2 AND T4 BACTERIOPHAGES. Biochem J. 1963 Oct;89:138–144. doi: 10.1042/bj0890138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W., Strom B., Bernstein H. Repair of alkylated bacteriophage T4 deoxyribonucleic acid by a mechanism involving polynucleotide ligase. J Virol. 1971 Mar;7(3):407–408. doi: 10.1128/jvi.7.3.407-408.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Bautz E., Freese E. ON THE MUTAGENIC EFFECT OF ALKYLATING AGENTS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1585–1594. doi: 10.1073/pnas.46.12.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S., Champe S. P. AMBIVALENT rII MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1961 Jul;47(7):1025–1038. doi: 10.1073/pnas.47.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M. Radiation-sensitive mutants of T4D. II. T4y: genetic characterization. Mutat Res. 1969 Nov-Dec;8(3):441–449. doi: 10.1016/0027-5107(69)90061-x. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Böhme H. Absence of repair of photodynamically induced damage in two mutants of Proteus mirabilis with increased sensitivity to monofunctional alkylating agents. Mutat Res. 1968 Jul-Aug;6(1):166–168. doi: 10.1016/0027-5107(68)90112-7. [DOI] [PubMed] [Google Scholar]
- Böhme H., Geissler E. Repair of lesions induced by photodynamic action and by ethyl methanesulfonate in E. coli. Mol Gen Genet. 1968;103(3):228–232. doi: 10.1007/BF00273691. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. II. The structural gene for polynucleotide ligase in bacteriophage T4. Proc Natl Acad Sci U S A. 1967 Aug;58(2):665–672. doi: 10.1073/pnas.58.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Hadi S. M., Goldthwait D. A. Endonuclease II of Escherichia coli. II. Enzyme properties and studies on the degradation of alkylated and native deoxyribonucleic acid. J Biol Chem. 1969 Nov 10;244(21):5879–5889. [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. Different sensitivities of T4D and lambda mutants to photodynamic action. Mol Gen Genet. 1970;109(3):264–268. doi: 10.1007/BF00267015. [DOI] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg G. W. Induction of DNA breakdown and death in Escherichia coli by phleomycin. Its association with dark-repair processes. Mol Gen Genet. 1969;104(1):1–11. doi: 10.1007/BF00277357. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- HILL R. F., FEINER R. R. FURTHER STUDIES OF ULTRAVIOLET-SENSITIVE MUTANTS OF ESCHERICHIA COLI STRAIN B. J Gen Microbiol. 1964 Apr;35:105–114. doi: 10.1099/00221287-35-1-105. [DOI] [PubMed] [Google Scholar]
- KRIEG D. R. Ethyl methanesulfonate-induced reversion of bacteriophage T4rII mutants. Genetics. 1963 Apr;48:561–580. doi: 10.1093/genetics/48.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A. The influence of radiomimetic substances on deoxyribonucleic acid synthesis and function studied in Escherichia coli/phage systems. III. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:497–508. doi: 10.1098/rspb.1959.0038. [DOI] [PubMed] [Google Scholar]
- Lindstrom D. M., Drake J. W. Mechanics of frameshift mutagenesis in bacteriophage T4: role of chromosome tips. Proc Natl Acad Sci U S A. 1970 Mar;65(3):617–624. doi: 10.1073/pnas.65.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppes R. Ethyl methanesulfonate: an effective mutagen in Chlamydomonas reinhardi. Mol Gen Genet. 1968;102(3):229–231. doi: 10.1007/BF00385978. [DOI] [PubMed] [Google Scholar]
- Luria S. E. A Growth-Delaying Effect of Ultraviolet Radiation. Proc Natl Acad Sci U S A. 1944 Dec 15;30(12):393–397. doi: 10.1073/pnas.30.12.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Dulbecco R. Genetic Recombinations Leading to Production of Active Bacteriophage from Ultraviolet Inactivated Bacteriophage Particles. Genetics. 1949 Mar;34(2):93–125. doi: 10.1093/genetics/34.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- Prakash L., Strauss B. Repair of alkylation damage: stability of methyl groups in Bacillus subtilis treated with methyl methanesulfonate. J Bacteriol. 1970 Jun;102(3):760–766. doi: 10.1128/jb.102.3.760-766.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen A. Inactivation of phage T4 by ethylmethane sulfonate. Biochem Biophys Res Commun. 1968 Oct 24;33(2):190–196. doi: 10.1016/0006-291x(68)90766-3. [DOI] [PubMed] [Google Scholar]
- Smithsm, Ymonds N., White P. The Kornberg polymerase and the repair of irradiated T4 bacteriophage. J Mol Biol. 1970 Dec 14;54(2):391–393. doi: 10.1016/0022-2836(70)90438-9. [DOI] [PubMed] [Google Scholar]
- Speyer J. F., Rosenberg D. The function of T4 DNA polymerase. Cold Spring Harb Symp Quant Biol. 1968;33:345–350. doi: 10.1101/sqb.1968.033.01.040. [DOI] [PubMed] [Google Scholar]
- Strauss B. S., Robbins M. DNA methylated in vitro by a monofunctional alkylating agent as a substrate for a specific nuclease from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Jun 18;161(1):68–75. doi: 10.1016/0005-2787(68)90295-5. [DOI] [PubMed] [Google Scholar]
- Strauss B., Coyle M., Robbins M. Alkylation damage and its repair. Cold Spring Harb Symp Quant Biol. 1968;33:277–287. doi: 10.1101/sqb.1968.033.01.032. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]



