Abstract
Objective: To investigate the effect of glutamine (Gln) on pro-inflammatory cytokines (TNF-α, IL-2 and IL-10) and the balance between pro-inflammatory cytokines and anti-inflammatory cytokines in severe acute pancreatitis (SAP) rats receiving nutritional support in different ways. Methods: Male SD rats (n=80) were randomly assigned into 5 groups: sham group, SAP+ parenteral nutrition (PN) group, SAP+ enteral nutrition (EN) group, SAP+EN+Gln group and SAP+PN+Gln group. At the same time, rats in 5 groups were sacrificed at 4 and 7 days after nutritional support. ELISA was employed to detect the pro-inflammatory cytokines including TNF-α, IL-2 and IL-10. Results: The serum TNF-α in the EN+Gln group after 7-day treatment was significantly lower than that in the EN, PN and PN+Gln groups at corresponding time point (P<0.05). The serum IL-2 in the EN+Gln group after 7-day treatment was markedly higher than that in the EN, PN and PN+Gln groups at corresponding time point (P<0.01). After 7-day treatment, the serum IL-2 in the EN+Gln and EN groups were markedly higher than that after 4-day treatment (P<0.01), but the serum IL-2 in the PN group was significantly lower than that after 4-day treatment (P<0.01). The serum IL-10 after 7-day treatment was markedly lower than that after 4-day treatment in all groups (P<0.01), and PN group had the lowest serum IL-10. Serum IL-10 in the EN+Gln group was significantly higher than that in the PN and PN+Gln groups at both time points (P<0.01). The serum IL-10 in the EN group was significantly higher than that in the PN group after 4-day treatment (P<0.01), but the serum IL-10 in the EN group was comparable to that in the PN group after 7-day treatment. The serum IL-10/TNF-α in the EN+Gln group was only slightly higher than that in the control group at both time points. The serum IL-10/TNF-α in the EN group was significantly lower than that in the EN+Gln group at both time points (P<0.05). The serum IL-10/TNF-α in the PN group was markedly lower than that in the EN group and EN+Gln group (P<0.05 and P<0.01, respectively).Conclusion: EN in combination with Gln are superior to EN alone, PN alone and PN in combination with Gln in regulating inflammation in SAP rats, and the EN has more potent capability to regulate the balance between pro-inflammation and anti-inflammation than PN.
Keywords: Pancreatitis, glutamine, inflammatory cytokines, rat
Introduction
Severe acute pancreatitis (SAP) is an acute abdominal disease with high risk. The onset of SAP is abrupt, its pathogenesis is complicated, the disease condition changes rapidly, it has some complications and its prognosis is poor. The traditional abnormal activation of enzymes and the theory of autodigestion can not completely elucidate the pathogenesis of SAP and its complicated progression. In early 1990s, with the proposal of systemic inflammatory response syndrome (SIRS), some investigators found that the occurrence of SAP is related to the over-activation of macrophages and neutrophils which may secret a variety of cytokines resulting in SIRS. This theory provides strategy and idea for the clinical study on SAP [1]. As shown in the theory of leukocyte over-activation for AP, the mononuclear macrophages are activated during the abnormal pancreatin activation, and these cells may secret a large amount of pro-inflammatory cytokines and reactive oxygen species which then result in over-activation of granular cells and endothelial cells and subsequent release of numerous inflammatory mediators. On one hand, this may cause microcirculatory disturbance in pancreas to deteriorate the pancreatic injury; on the other hand, this may result in damage to distant organs. Over-activation of leukocytes has been regarded as a major cause of deterioration of AP, multiple organ failure (MOF) and even death [2]. Cytokines may exert effect at a low level, which is efficient, pleiotropic and overlapping. Thus, to investigate the complicated network of some cytokines in vivo is important. The present study aimed to investigate the effect of Glutamine (Gln) on the serum levels of TNF-α, IL-2 and IL-10 in SAP rats receiving nutritional support in different ways and to explore the role of Gln in regulating the balance between pro-inflammation and anti-inflammation in SAP rats. Our findings may provide the mechanisms underlying the effect of Gln on cytokines in SAP rats receiving nutritional support in different ways.
Materials and methods
Materials
Sodium taurocholate (purity: 95%; Sigma), pentothal sodiun (Shanghai Xinya Pharmaceutical Co., Ltd.), TUNEL Kit (Roche. Germany), polyclonal antibody against Bax, streptomycin-avidin-biotin complex for immunohistochemistry kit (Maixin Bitech Co., Ltd); EN solution included 95% hydrolyzed whey protein [GRANDE, USA] and medium-chain fatty acid [Shanghai Shiliao Co., Ltd), mixed vitamins, minerals (Institute of Nutrition in Academy of Military Medical Science). The ratio of energy produced by carbohydrates, protein and fat is 49:17:34. PN solution included 500g/L glucose, 85g/L compound amino acids, 200 g/L fat emulsion, addamel and water-soluble vitamins (insulin : glucose = 1:4.0). The PN solution and EN solution can provide100 kca1 per 100 ml. The ratio of energy produced by carbohydrates, protein and fat is 55:17:28. Alanyl - glutamine dipeptide (Shanghai Xuxin Chemical Company; Purity: 98%), Dipeptiven (L-alanyl-L-glutamine; Huarui Pharmaceutical Co., Ltd.), ELISA kit for TNF-α (Bethy, USA), ELISA kit for IL-2 (Bethy, USA), ELISA kit for IL-10 (Bethy, USA), MULTISKAN MK3 microplate reader (Thermo, USA).
Animals and grouping
Specific pathogen free Sprague-Dawley (SD) male rats (n=80) aged 6-7 weeks and weighing 200±10 g were purchased from the Experimental Animal Center. These animals were allowed to accommodate to the environment for 1 week during which they were given ad libitum access to water and fodd. These rats were randomly assigned into 5 groups: sham group, SAP+ parenteral nutrition (PN) group, SAP + enteral nutrition (EN) group, SAP+EN+Gln group and SAP+PN+Gln group. Rats in different groups were sacrificed at 4 and 7 days after Gln treatment. In the sham group, laparotomy was performed, and the pancreas was taken out and washed with normal saline followed by wound closure, and rats were intragastrically given EN solution. In the SAP+EN group and SAP+EN+Gln group, normal saline was used to flush intestine at 6 h after regaining consciousness and rats received intragastrical injection of EN solution at 12 h after regaining consciousness. The rate was increased from 1.0 ml/h at beginning to 2.0 ml/h (or 50 ml/d) at 36 h followed by maintained injection. In the PN group, the rate was increased from 1.0 ml/h to 2.0 ml/h (or 50 ml/d) within 36 h. In the g; SAP+EN+Gln group, Gln was added to the EN solution; in the SAP+PN+Gln group, Dipeptiven was added to the PN solution. At 1-7 days after introduction of SAP, rats in SAP+PN group and SAP+PN+Gln group were treated with PN solution with and without Gln, respectively, in which the nitrogen (2.5 gN), the calories (1046.5kJ) and the heat/nitrogen (124.7:1) were identical in both group. In the SAP+EN+Gln group, rats were also treated with 98% alanyl - glutamine dipeptide (0.4 g/kg body weight) in which the glutamine was 78±4.2 g. In the SAP+PN+Gln group, Dipeptiven was added to the PN solution at a ratio of 2 ml/kg body weight (0.4 g of L-alanyl-L-glutamine / kg body weight / d; ≈77.2±6.4 g of Gln). The Gln content was identical in the SAP+EN+Gln group and SAP+PN+Gln group. At 4 and 7 days after treatment, blood was collected from the orbit followed by detection of inflammatory cytokines.
Preparation of animal model and treatments
Before introduction of SAP, rats received food deprivation for 12 h. Then, rats were intraperitoneally anesthetized with 2.5% pentothal sodium at 0.1 ml/100 g. After shaving and sterilization, laparotomy was performed, and 3.8% sodium taurocholate was alowly injected at the tail of the pancreas to induce SAP. In the SAP+PN group and SAP+PN+Gln group, PN solution was administered via the external jugular vein. In the SAP+EN group and SAP+EN+Gln group, EN solution was administered via the stomach and duodenum.
Pathological examination of pancreas
At 4 and 7 days after treatment, rats were sacrificed and the pancreas was collected for HE staining followed by observation under a light microscope.
Detection of inflammatory cytokines
Serum levels of TNF-α, IL-2 and IL-10 were measured with ELISA kits according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was done with SPSS version 11.5 for Windows. Data were expressed as mean ± standard deviation (x̅ ±s). A value of P<0.05 was considered statistically significant.
Results
Pathological changes in pancreas
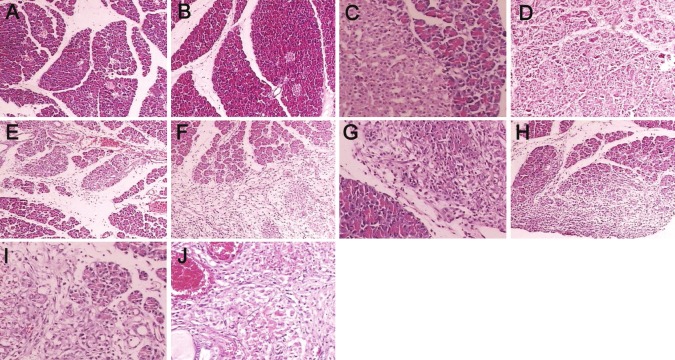
HE staining showed the acini and structure of pancreas were clear and regularly arranged, cells had normal morphology, and hemorrhage and necrosis were absent. In the SAP+PN group, spotty hemorrhage was found on the pancreatic surface and fat surrounding the pancreas, bloody ascites was also noted, saponification spots were observed in the pancreas and omentum at 4 days after treatment. Under light microscope, scattered necrosis of acini was found accompanied by evident infiltration of inflammatory cells. At 7 days after treatment, macroscopic patchy necrosis was found in the pancreas, saponification spots were observed in the tissues surrounding the pancreas and omentum, accompanied by a large amount of bloody ascites. The parenchymal necrosis and infiltration of inflammatory cells in the pancreas were more severe than those at 4 days after treatment. In the SAP+EN+Gln group, SAP+PN+Gln group and SAP+EN group, the pathological changes at both time points were significantly attenuated when compared with SAP+PN group, and the improvement in pathological changes was the most obvious in the SAP+EN+Gln group (Figure 1).
Figure 1.
Pathological changes in pancreas of the Sham group, SAP+EN group, SAP+EN+Gln group, SAP+PN+Gln group, SAP+PN group. A. Sham group (4d, HE×100); B. Sham group (7 d, HE×100); C. SAP+EN group (4 d, HE×100); D. SAP+EN group (7 d, HE×100); E. SAP+EN+Gln group (4 d, HE×100); F. SAP+EN+Gln group (7 d, HE×100); G. SAP+PN+Gln group (4 d, HE×100); H. SAP+PN+Gln group (7 d, HE×100); I. SAP+PN group (4d, HE×100); J. SAP+PN group (7 d, HE×100).
Serum TNF-α
Rats with SAP had significantly increased serum TNF-α when compared with those in sham group, and the serum TNF-α was the highest in the SAP+PN group (P<0.01). The serum TNF-α in the EN+Gln was markedly lower than that in the EN group and PN group (P<0.01). The serum TNF-α in the PN+Gln group was markedly reduced when compared with PN group (P<0.01). In addition, for SAP rats in different groups, the serum TNF-α decreased over time, and rats in the EN+Gln group had the lowest serum TNF-α (P<0.01) (Table 1).
Table 1.
serum TNF-α in different groups (pg/ml, x̅ ±s)
| Group (n=8) | 4d | 7d |
|---|---|---|
| Control | 20.62±4.79 | 19.59±4.55 |
| PN group | 424.87±28.63a | 276.16±18.61a,d |
| PN+Gln group | 230.81±38.1 a,b,c | 143.2±24.23a,b,c,d |
| EN group | 237.33±37.48a,b,c | 137.9±35.88a,b,c,d |
| EN+Gln group | 169.81±48.1a,c | 87.20±14.2a,c,d |
P<0.01 vs control group;
P<0.01 vs EN+Gln group;
P<0.01 vs PN group;
P<0.01 vs 4d.
Serum IL-2
The serum IL-2 in rats with SAP was significantly lower than that in the control group (P<0.05). In addition, the serum IL-2 in the PN group was markedly reduced when compared with EN group, PN+Gln group and EN+Gln group ((P<0.05 or P<0.01). The serum IL-2 in the EN+Gln group was markedly higher than that in the EN group (P<0.01). After 7-day treatment, the serum IL-2 in the EN+Gln group and EN group was significantly higher than that after 4-day treatment (P<0.01) but the serum IL-2 in the PN group after 7-day treatment was markedly lower than that after 4-day treatment (P<0.01) (Table 2).
Table 2.
Serum IL-2 in different groups (pg/ml, x̅ ±s)
| Group (n=8) | 4d | 7d |
|---|---|---|
| Control | 269.08±48.71 | 274.91±53.2 |
| PN group | 151.22±21.57a,b | 136.10±19.4a,b,d |
| PN+Gln group | 184.21±23.4 a,c* | 224.13±23.08 a*,b*,c,d |
| EN group | 195.96±47.39a,c* | 221.43±53.55 a*,b*,c,d |
| EN+Gln group | 222.09±26.0a,c | 263.11±32.50c,d |
P<0.01 vs control group;
P<0.05;
P<0.01vs EN+Gln group;
P<0.05;
P<0.01 vs PN group
P<0.05;
P<0.01 vs 4 d.
Serum IL-10
The serum IL-10 in rats with SAP was significantly lower than that in the control group (P<0.01). The serum IL-10 in the EN group and PN group was markedly reduced when compared with the EN+Gln group (P<0.01 or 0.05). In addition, the serum IL-10 in SAP rats after 7-day treatment was significantly lower that that after 4-day treatment (P<0.01), and rats in the PN group had the lowest serum IL-10. In addition, the serum IL-10 in the EN+Gln group was markedly higher than that in the PN group at both time points (P<0.01), and the serum IL-10 in the EN group was significantly higher than that in the PN group after 4-day treatment (P<0.01). However, after 7-day treatment, the serum IL-10 was comparable between EN group and PN group (Table 3).
Table 3.
Serum IL-10 in different groups (pg/ml, x̅ ±s)
| Group (n=8) | 4d | 7d |
|---|---|---|
| Control | 23.70±6.25 | 21.80±5.75 |
| PN group | 169.5±12.02a | 98.3±6.97a,d |
| PN+Gln group | 194.35±28.31a,c | 103.42±8.71a,c,d |
| EN group | 198.37±23.89a,b,c | 105.14±12.66a,b,d |
| EN+Gln group | 221.87±18.22a,c | 115.37±9.47a,c,d |
P<0.01 vs control group;
P<0.05 vs EN+Gln group;
P<0.01 vs PN group;
P<0.01 vs 4 d.
Serum IL-10/TNF-α
The serum IL-10/TNF-α in the EN+Gln group was slightly higher than that in the control group at both time points, but the serum IL-10/TNF-α in remaining groups was markedly lower than that in the control group (P<0.05). At both time points, the serum IL-10/TNF-α in the EN group was significantly lower than that in the EN+Gln group (P<0.05), and the serum IL-10/TNF-α in the PN group was markedly lower than that in the EN group, PN+Gln group and EN+Gln group (P<0.05 or P<0.01) (Table 4).
Table 4.
serum IL-10/TNF-α in different group ( x̅ ±s)
| Group (n=8) | 4d | 7d |
|---|---|---|
| Control | 1.19±0.42 | 1.16±0.41 |
| PN group | 0.40±0.02a,b | 0.36±0.02a,b |
| PN+Gln group | 0.90±0.41b*,c* | 0.85±0.12 a*,b*,c |
| EN group | 0.86±0.18b*,c* | 0.82±0.27 a*,b*,c |
| EN+Gln group | 1.47±0.76c | 1.37±0.24c |
P<0.01 vs control group;
P<0.05;
P<0.01 vs EN+Gln group;
P<0.05;
P<0.01 vs PN group
P<0.05.
Discussion
The pathogenesis of AP is still poorly understood. Traditionally, AP is attributed to the autodigestion by pancreatin. However, treatment to inhibit the pancreatin usually fails to achieve favorable outcome. In recent years, increasing attention has been paid to the relationship between cytokines and AP. Studies in depth have shown that the over-activation of proinflammatory cytokines and/or attenuation of anti-inflammatory cytokines during the course of AP may cause SIRS, MODS or even death [3]. To date, the role of cytokines in the pathogenesis of AP has been a hot topic in the studies on AP [4]. Cytokines are a group of small molecule polypeptides which are secreted by some activated immune cells and stroma cells. These cytokines can bind to the receptors on target cells to exert effect involving in the immune regulation and inflammatory response. In the AP, the injured pancreas may act as a antigen or inflammatory stimulus to activate the infiltrated macrophages and neutrophils in the pancreas, leading to the release of a large amount of cytokines and subsequent of inflammatory cascade. In early 1990, it was found that the inflammatory cascade is triggered by inflammatory cytokines, which may finally result in the transformation of focal pancreatitis into SIRS and MODS.
In the hypothesis of SIRS and compensator anti-inflammatory response syndrome (CARS) [5], SIRS is present when the pro-inflammatory cytokines are dominant, CARS is present when the anti-inflammatory cytokines are dominant and the balance between anti-inflammation and pro-inflammation suggests the homeostasis which is helpful for the recovery from inflammation. In early stage of SAP, a variety of pro-inflammatory cytokines are released, and SIRS and MODS are present clinically. Two weeks later, the over-response of the immune system is compromised, and then the pancreatic and systemic infection secondary to immunosuppression is present. There is evidence showing that the serum TNF-α, IL-l, IL-6 and IL-8 in SAP patients are significantly higher than those in patients with mild AP, but SAP patients had markedly lowered serum IL-10. This suggests that high level proinflammatory cytokines and the imbalance between anti-inflammatory cytokines and pro-inflammatory cytokines are crucial factors in the occurrence of SAP.
Results in animal experiments and clinical studies have revealed that the TNF-α increases during the AP. The abnormal increase in TNF-α is related to the complications of pancreatitis. Animal experiments reveal that antagonist of TNF-α may block the pancreatic necrosis and improve the biochemical parameters to increase the survival rate. In addition, TNF-α may cause over-activation of granular cells and stimulate the release of IL-6 by endothelial cells. IL-6 is a major cause of sustained necrosis of the pancreas and the deterioration of disease condition. Moreover, IL-6 may induce the release of TNF-α in a positive feedback pattern leading to a vicious cycle. Thus, TNF-α acts as a pro-inflammatory cytokine playing important roles in the pathogenesis of AP [6]. There is evidence showing that TNF can be used as an early indicator to evaluate the severity of pancreatitis. At early stage of SAP, the TNF increases and involves in the occurrence and development of pancreatitis, and the TNF level is related to the disease condition and mortality. Recent studies also show that TNF-α expression during the SAP is attributed to the primary response as a result of direct stimulation of inflammatory cells by pancreatin.
Our results showed the TNF-α in SAP rats was significantly higher than that in the control group, suggesting the pro-inflammation. In addition, the TNF-α in the EN+Gln group was significantly lower than that in the EN group and PN group, and the TNF-α in the EN group was markedly higher than that in the PN group. These findings indicate that the anti-inflammation in SAP is attenuated, and the additional Gln in EN may exert anti-inflammatory effect to a certain extent.
IL-2 is mainly produced by the activated Th cells. The bioeffects of Il-2 are to induce the proliferation and differentiation of T cells and B cells to produce cytokines or immunoglobulins. In addition, IL-2 can enhance the killing effect of NK cells and LAK cells on cancer cells or virus-infected cells and induce these cells to produce cytokines including TNF-α involving in the immune regulation. The Il-2 may activate mononuclear phagocytes to enhance the secretion and phagocytosis of these cells. In the AP, Th cells reduce, and inhibitory T cells increase relatively. Under this condition, the response of Th cells is compromised leading to the reduction in IL-2. The reduction in IL-2 may attenuate the immune regulation leading to the compromised cellular immunity [7].
In the present study, results revealed that the IL-2 in SAP rats was markedly reduced when compared with control group. This suggests that these SAP rats present with compromised immune function. In addition, the IL-2 in the EN+Gln group was significantly higher than that in the EN group and PN group; the IL-2 in the EN group and Gln groups at 7-day treatment was markedly higher than that after 4-day treatment; the IL-2 in the PN group after 7-day treatment was markedly reduced when compared with that after 4-day treatment. These indicate that the anti-inflammatory capability after addition of Gln is more potent than that in the EN group and PN group, and the balance between pro-inflammation and anti-inflammation is improved after addition of Gln.
In recent years, studies have confirmed that IL-10 is a polyphonic cytokines and has extensive bioeffects [8]. In addition, evidence from basic studies confirm that IL-10 can inhibit the synthesis of cytokines, reduce the severity of AP and has been regarded as a major cytokine to inhibit the synthesis of pro-inflammatory cytokines and colony stimulating factor.
As an endogenous anti-inflammatory cytokine produced in the inflammatory reaction, IL-10 plays important roles in the regulation of balance among cytokines, maintenance of hemostasis and attenuation of inflammation. Il-10 is one of important anti-inflammatory cytokines. Previous studies have shown that the IL-10 is reduced at early stage of mild AP [9], but the serum IL-10 increases in SAP. There is evidence showing that IL-10 is closely related to the AP. Endogenous or exogenous IL-10 may inhibit the activities of monocytes and macrophages, inhibit the release of pro-inflammatory cells (such as TNF-α and IL-6), regulate the systemic immune response and inflammatory reaction and exert protective effect on the pancreas [10].
The mechanisms underlying the protective effect of IL-10 are complicated and can be classified as follows: (1) IL-10 can inhibit the cellular immunity and suppress the synthesis of cytokines including IL-1, IL-6, TNF and GM-CSF; (2) IL-2 can reduce the expression of specific adhesion molecules to compromise the migration of monocytes and multinucleated cells and inhibit the aggregation of neutrophils; (3) IL-10 may inhibit the synthesis of interferon in NF-kB producing cells; (4) IL-10 may inhibit the endotoxin mediated coagulation, block the release of reactive oxygen species and NO by activated macrophages; (5) IL-10 can improve the microcirculatory disturbance and increase the blood supply to the pancreas; (6) IL-10 can induce the bcl-2 expression to inhibit the apoptosis of pancreatic acini; (7) IL-7 may increase the differentiation of B cells to produce a large amount of IgG and IgA [11].
Our results showed the IL-10 in the SAP rats was significantly higher than that in the control group, and the IL-10 in the Gln groups was markedly higher than that in the EN group and PN group. In addition, the IL-10/TNF-α in the EN+Gln group was close to that in the control group, but the IL-10/TNF-α in the PN group and EN group was lower than that in the control group, and rats in the PN group had the lowest IL-10/TNF-α. These findings suggest that EN in combination with application of Gln is helpful for the improvement of anti-inflammation in SAP rats, the regulation of balance between inflammation and anti-inflammation and the recovery from SAP.
Taken together, our results demonstrate that EN in combination with application of Gln is superior to EN alone and PN alone in regulating the inflammation in SAP rats. In addition, the capability of EN to balance anti-inflammation and pro-inflammation is more potent than that of PN. Our findings provide theoretic evidence for clinical treatment of SAP. Of note, the treatment of SAP requires multi-disciplinary comprehensive therapy. There are controversies in the time of treatment and drugs for early treatment of SAP with EN. In addition, some problems on clinical application of Gln are required to be resolved such as the ways in which Gln is administered, time of administration and the combined use with antibiotics. Moreover, standardized guideline is required for the clinical application of Gln in the treatment of SAP.
References
- 1.Shi C, Zhao X, Lagergren A, Sigvardsson M, Wang X, Andersson R. Immune status and inflammatory response differ locally and systemically in severe acute pancreatitis. Scand J Gastroenterol. 2006;41:472–480. doi: 10.1080/00365520500318965. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Wang B, Wu J, Wang G. Beneficial effects of growth hormone on bacterial translocation during the course of acute necrotizing pancreatitis in rats. Pancreas. 2001;23:148–156. doi: 10.1097/00006676-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189–194. doi: 10.1007/s00534-002-0720-z. [DOI] [PubMed] [Google Scholar]
- 4.Denning TL, Takaishi H, Crowe SE, Boldogh I, Jevnikar A, Ernst PB. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med. 2002;33:1641–1650. doi: 10.1016/s0891-5849(02)01141-3. [DOI] [PubMed] [Google Scholar]
- 5.Jones SA, Butler RN, Sanderson IR, Wilson JW. The effect of specific caspase inhibitors on TNF-alpha and butyrate-induced apoptosis of intestinal epithelial cells. Exp Cell Res. 2004;292:29–39. doi: 10.1016/j.yexcr.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Chen B, Sun H, Deng Z, Andersson R, Zhang Q. The protective effects of Lipoxin A4 during the early phase of severe acute pancreatitis in rats. Scand J Gastroenterol. 2011;46:211–219. doi: 10.3109/00365521.2010.525715. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. J Surg Res. 2006;135:18–26. doi: 10.1016/j.jss.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Duarte-Rojo A, Suazo-Barahona J, Ramirez- Iglesias MT, Uscanga LF, Robles-Diaz G. Time frames for analysis of inflammatory mediators in acute pancreatitis: improving admission triage. Dig Dis Sci. 2009;54:2282–2287. doi: 10.1007/s10620-008-0615-1. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Cui N, Miao B, Zhao E. Immune dysregulation in patients with severe acute pancreatitis. Inflammation. 2011;34:36–42. doi: 10.1007/s10753-010-9205-4. [DOI] [PubMed] [Google Scholar]
- 10.Zou WG, Wang DS, Lang MF, Jin DY, Xu DH, Zheng ZC, Wu ZH, Liu XY. Human interleukin 10 gene therapy decreases the severity and mortality of lethal pancreatitis in rats. J Surg Res. 2002;103:121–126. doi: 10.1006/jsre.2001.6327. [DOI] [PubMed] [Google Scholar]
- 11.Kamei K, Yasuda T, Ueda T, Qiang F, Shiozaki H, Ohyanagi H, Takeyama Y. Significant expression of interleukin 15 in rat experimental severe acute pancreatitis. Eur Surg Res. 2010;44:159–169. doi: 10.1159/000283241. [DOI] [PubMed] [Google Scholar]