Abstract
The auditory system is the most susceptible to damages from blast waves. Blast injuries always lead to varying degrees of hearing impairment. Although a disorder of the cochlear blood flow (CoBF) has been considered to be related to many pathological processes of the auditory system and to contribute to various types of hearing loss, changes in the CoBF induced by blast waves and the relationship between such changes and hearing impairment are undefined. To observe the changes in the cochlear microcirculation after exposure to an explosion blast, investigate the relationship between changes in the CoBF and hearing impairment and subsequently explore the mechanism responsible for the changes in the CoBF, we detected the perfusion of the cochlear microcirculation and hearing threshold shift after exposure to an explosion blast. Then, an N-nitro-L-arginine-methyl ester (L-NAME, NO synthase inhibitor) solution and artificial perilymph were applied to the round window (RW) of the cochlea before the blast exposure, followed by an evaluation of the CoBF and hearing function. The results indicated that the changes in the CoBF were correlated to the strength of the blast wave. The cochlear blood flow significantly increased when the peak value of the blast overpressure was greater than approximately 45 kPa, and there was no significant change in the cochlear blood flow when the peak value of the blast overpressure was less than approximately 35 kPa. Following local administration of the NO synthase inhibitor L-NAME, the increase in the CoBF induced by the blast was inhibited, and this reduction was significantly associated with the hearing threshold.
Keywords: Cochlea, blood flow, hearing function, blast injury, guinea pigs
Introduction
Blast injuries can occur in normal life, during military acts and in the workplace. They can cause injuries to various organ systems [1]. These injuries differ, ranging from relatively minor to lethal, in accordance with the magnitude of the blast wave [2,3]. As an air-containing organ, the auditory system is considered to be most susceptible to blast wave-associated damage. Such damages can be full range, affecting the region from the tympanic membrane to the inner ear, and lead to temporary and permanent losses of hearing sensitivity [4]. These structural damages cause conductive hearing loss, sensorineural hearing loss or both [1-3].
In addition to mechanical damage, vascular, metabolic, and chemical changes will produce temporary or permanent hearing impairment. Although CoBF is considered to provide important support for auditory function and is suspected to play an important role in many pathological processes [5-7], the difficulty of measuring CoBF directly in vivo has limited the investigation of the correlation between the change in the blood flow and hearing dysfunction in auditory blast injuries. The effect of treatments that aim to promote blood flow in the cochlea is also uncertain [8,9]. Therefore, the role of disordered CoBF in hearing impairment induced by a blast still remains ambiguous. Many previous studies have focused on a high level of noise and impulse noise. However, due to differences in the experimental conditions, criteria and research methods, the results have been varied [10-14]. A better understanding of the CoBF changes will be helpful for the prevention and management of hearing disorders resulting from blast damage.
Materials and methods
Subjects
A total of 45 guinea pigs of both sexes were used, weighing 250-350 g and with normal Preyer’s reflexes. The animals were housed under standard laboratory conditions with free access to food and water. Examinations of the tympanic membrane revealed no evidence of pathology. All operative procedures were performed in a state of deep surgical anaesthesia with pentobarbital sodium (30 mg/kg) administered intraperitoneally.
Experimental protocols were approved by the Management Committee of Experimental Animals of Third Military Medical University, China.
Surgery and preparation
The animals were initially anaesthetised with pentobarbital sodium (3 mg/kg intraperitoneal injection). This injection was repeated every 60 min using half of the initial dose. The left bulla was exposed and opened via a retroauricular approach. A round hole (2 mm diameter) was drilled in the upper portion of the bulla to observe the position of the laser Doppler probe, and a small hole (1 mm diameter) was drilled under the observation hole for the insertion of a stainless steel tube (1 mm outer diameter and 0.5 mm inner diameter) that was used to bolster the probe. The tip of the tube was placed on the bony surface of the basal turn of the cochlea. The probe was fixed, and the holes were sealed with dental self-curing resin. The incision was sutured, and the outer tip of the tube protruded beyond the skin. The systemic blood pressure (BP) of all animals was recorded by a pressure transducer (MLT0380/D Reusable BP transducer, AD Instruments Pty Ltd, Australia) through a catheter that was inserted into the left femoral artery before surgery, and the data were collected using a quad bridge (ML118, AD Instruments Pty Ltd). The BP data were recorded and analysed using Chart Software (v5.5.6, Copyright: 1994-2008, AD Instruments Pty Ltd, Austrilia), and after being divided into 5 s segments, each individual segment was averaged and converted to the percentage change.
Histopathology of cochlea
At the end of each experiment, the animals were killed under pentobarbital anaesthesia (30 mg/kg, intraperitoneal) followed by cervical dislocation. The temporal bone was removed, and the bulla was opened immediately. A small hole was created in the apex of the bone shell by a needle, and the round window was opened by the same method. The fixative solution (4% paraformaldehyde, buffered at pH 7.3) was perfused three times into the cochlea through the apical hole using a pipette. Then, the cochleae were immersed in the same fixative solution at 4°C overnight. After fixation, the excess bone around the cochlea was removed, and the cochlea was decalcified in 10% EDTA solution (buffered at pH 7.3) for 5 days. Next, the specimens were embedded in paraffin and sectioned in the horizontal plane at a thickness of 5 μm. The sections were stained with haematoxylin-eosin and studied by light microscopy.
Detonation and measurement of blast wave
To generate the blast wave, a detonator (paper shell, 600 mg cyclotrimethylenetrinitramine, RDX inside) was fixed in an open space at a height of 0.4 meter and detonated by electrodes. The electric pressure transducers were fixed at the same level and distance to record the pressure of the blast waves. A dynamic data acquisition meter (TESTELECTRONIC Inc, Chengdu, China) was used to record the signal from the transducers. The signal was recorded and analysed using DAP Software (v3.01, copyright: 2005.6.6).
Measurement of CoBF by laser doppler
The blood flow of the basal turn of the cochlea was registered with a laser-Doppler blood flow meter (ML191, AD Instruments Pty Ltd, Australia). The needle-shaped probe (0.4 mm diameter) was inserted in the bolster tube until its tip reached the surface of the basal turn of the cochlea. The visible red laser light, with a wavelength of 830 nm, was supplied through an optic fibre. The blood flow meter was connected to a Power Lab System (AD Instruments), and the percentage of back scatter (BSC) and the laser Doppler flowmetry output signal were recorded using Chart Software (v5.5.6, Copyright: 1994-2008, AD Instruments). Both the LDF and BSC outputs were factory calibrated to produce a certain voltage output per calibrated unit. The BSC output represents the relative strength of the returned signal.
Evaluation of hearing function
To assess the hearing function, the auditory brain stem response (ABR) was measured. An evoked potential instrument (MK-NMD, MINGKAN Technologies, Chongqing, China) was used to generate acoustic stimuli and subsequently record the evoked potentials. An alternating broadband clicking sound (repetition rate of 11/s) was given to test the ear and used as the stimulus sound, and white noise sound was used as a contralateral masking noise. To record the bioelectrical potentials, subdermal stainless-steel needle electrodes were inserted at the vertex (active), dorsolateral to the measured ear (reference) and the nasal root (ground). The thresholds were determined from a set of responses at varying intensities with 5 dB SPL intervals, and the electrical signals were averaged from 512 to 1024 repetitions. The thresholds at each frequency were verified twice.
Blast exposure and evaluation of CoBF and hearing function
To ensure that the animals were exposed to a suitable intensity of blast overpressure, the animals were placed at distances from 0.5 meter to 0.9 meter at 0.1 meter intervals (3 animals for each distance). The blast overpressures were measured at the same distances and times. The normal LDF was recorded for 5 minutes a half hour before the exposure to the explosion blast, and the same recording process was performed immediately and 1 hour, 3 hours, 1 day and 2 days after the exposure. The ABR was recorded immediately and 1 hours, 3 hours, 1 day and 1 week after the exposure to the explosion blast. The CoBF and ABR of an additional 3 animals were measured at same time points, serving as the blank group.
Measurement of CoBF and evaluation of hearing function after L-NAME infusion and blast exposure
For this experiment, 27 guinea pigs were divided into 3 groups. L-NAME (Beyotime Institute of Biotechnology, China) was dissolved in a 1% artificial perilymph solution (137 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM NaH2PO4, 12 mM NaHCO3, and 10 mM glucose; pH adjusted to 7.4 at 37°C) [15]. Group A consisted of 9 animals that received a 2 μl infusion of a 1% L-NAME solution into the RW by a micropipette (75N 5 μl SYR, HAMILTON CO). Group B consisted of the same number of animals that received the same infusion. Group B consisted of 9 animals that received the same dose of artificial perilymph infused into the RW. Both groups A and C were exposed to a blast generated by the same detonator at the distances of 0.5 meter, 0.6 meter and 0.7 meter (3 animals for each distance) 15 minutes after the RW infusion. The CoBF and ABR were measured in all animals of the 3 groups 10 minutes before the blast exposure; the CoBF was recorded immediately, 1 hour, 2 hours, and 3 hours after the blast exposure, and the ABR was measured 2.5 hours after the blast exposure in groups A, B and C.
Statistical analysis
Peak values and durations of the overpressure, BP, data of CoBF, and TS of all groups in each distance were averaged and analyzed using a 1-way analysis of variance (ANOVA). Data of CoBF and TS between group A and group C were averaged and analyzed using independent samples T-test. Correlation between the maximum values of the CoBF baseline and peak values of the blast overpressure was analyzed using bivariate correlation analysis. All analysis processes were conducted using SPSS statistics 17.0. The distribution are reported as mean±S.E.M.
Results
The systemic blood pressure, which was recorded until the end of each experiment, showed values within the normal range, and the percentage changes were minimal in all animals.
Pressure changes of the blast waves at different distance
As an open field explosion, free-field blast waves were produced with a characteristic pressure-time history. The intensity of the blast wave decreased with the increase in the distance from the epicentre, and the positive phase duration had the opposite relationship.
Table 1 shows the peak value and duration of the overpressure at each distance. The nearest point had the highest peak overpressure value of 63.64±5.78 kPa and the shortest duration time of 0.348±0.022 s. The farthest distance had the weakest overpressure intensity of 23.34± 1.52 kPa and the longest duration of 0.467±0.008 s.
Table 1.
Peak value and duration of overpressurea
| Distance (m)b | nc | Peak value (kPa) | Duration (s) |
|---|---|---|---|
| 0.5 | 3 | 64.52±4.60d | 0.348±0.022e |
| 0.6 | 3 | 46.06± 4.32d | 0.381±0.018e |
| 0.7 | 3 | 34.62± 4.75d | 0.411±0.019e |
| 0.8 | 3 | 27.07± 6.78d | 0.440±0.011e |
| 0.9 | 3 | 23.30± 2.90d | 0.467±0.008e |
Results are expressed as mean ± S.E.M.
The heads of the transducers were placed at the same distances as the external auditory canal
The tests of the blast wave were repeated three times for each distance.
Significantly different between the peak values of the blast waves at each distance (P<0.05).
Significantly different between the durations of the positive phase at each distance (P<0.05).
Histopathological changes of the cochlea
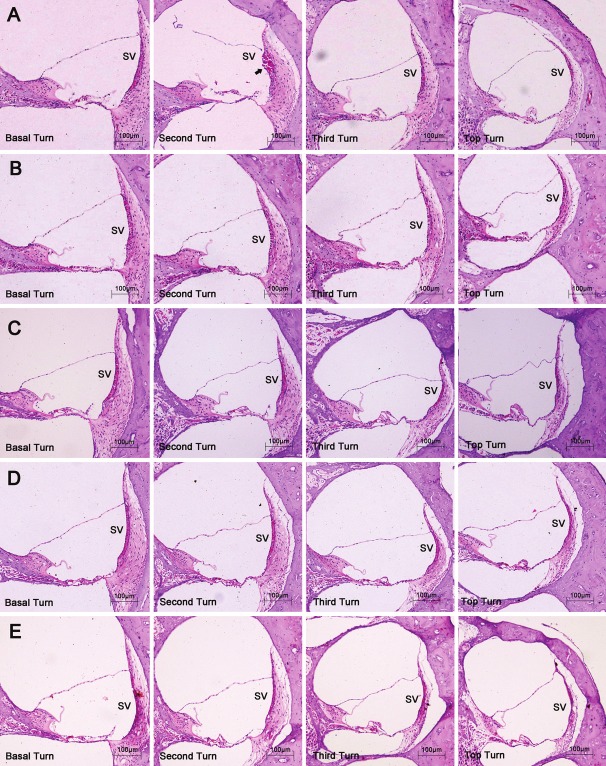
The pathological changes of the cochlea were not significant under the light microscope. Disorder of the organ of Corti was common for all distances. Although the irregular arrangement of sensory cells could be observed for the close distances (0.5 meter) and was relatively distinct in the basal and second turns, the complete detachment of the sensory epithelium was not observed. Structural damage to the later wall of the cochlea was rare, with only one case of rupture of the stria vascularis found in the second turn at the distance of 0.5 meter. Figure 1 shows the pathological changes of the inner ear for each distance.
Figure 1.
The pathological changes of the inner ear. Haematoxylin-eosin, original magnification ×100. SV, stria vascularis. A, B, C, D and E: show the pathological changes of the four turns of the cochlea at each distance, respectively. The arrow indicates the rupture of the stria vascularis in the second turn.
Blood flow measured by LDF
After exposure to a blast wave, the cochlear blood flow (CoBF) showed appropriate distance and time-dependent patterns. At close distances (0.5 meter and 0.6 meter), the baseline CoBF increased immediately after the blast exposure, reached a peak value (0.5 meter, 227.76% of the initial level; 0.6 m, 142.79% of the initial level) at 2 to 3 hour (0.5 meter, 3 hours; 0.6 meter, 2 hours) and dropped gradually after reaching the maximum, recovering within 24 hours. As the distance increased, the variations in the CoBF were gentler, and there was no significant difference (p>0.05) when the distance was greater than 0.7 meter (Figure 2). Bivariate correlation analysis between the maximum value of the CoBF baseline and peak value of the blast overpressure indicated that the correlation was significant at the 0.01 level (2-tailed) (Figure 3).
Figure 2.
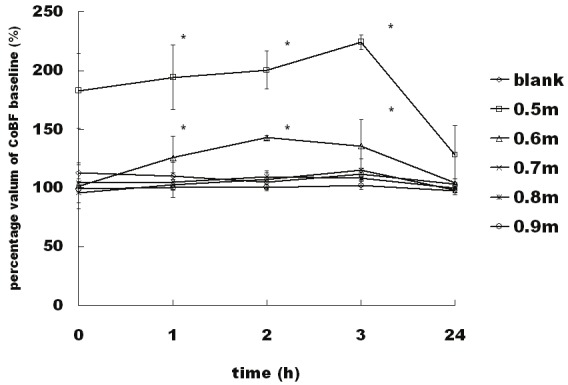
Time course of the cochlear blood flow (CoBF) measured by LDF at different distances. The y axis depicts variations in the percentage of CoBF compared with the initial baseline levels, and the x axis depicts the time. The values are means ± S.E.M.
Figure 3.
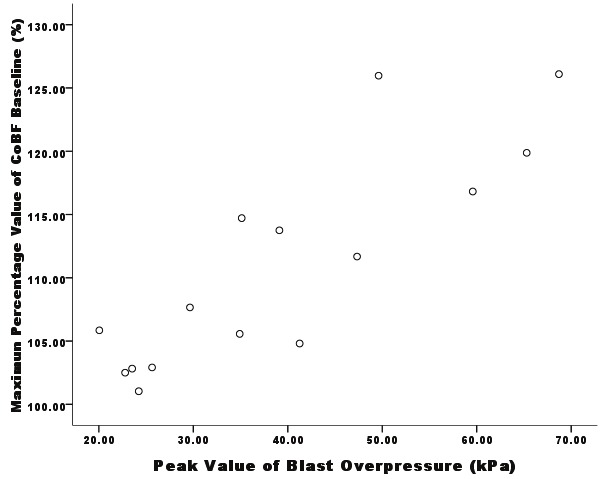
Correlation between the maximum values of the CoBF baseline and peak values of the blast overpressure. The correlation is significant at the 0.01 level (2-tailed) (analysed by SPSS 17.0.0).
Hearing threshold shift after blast
Hearing threshold shifts (TS) were significant with 87.5 dB (0.5 meter) to 47.5 dB (0.9 meter) (P<0.01), immediately after the blast wave exposure for all distances, followed by a gradual recovery course. Although the hearing threshold shift also showed a time-dependent pattern, the recovery courses were not so quick compared with the CoBF changes. The TS remained at 55 dB (0.5 meter) to 31.25 dB (0.9 meter) even 1 week after the blast exposure. At every time point of the test, the TS values were significantly different between each distance (Figure 4).
Figure 4.
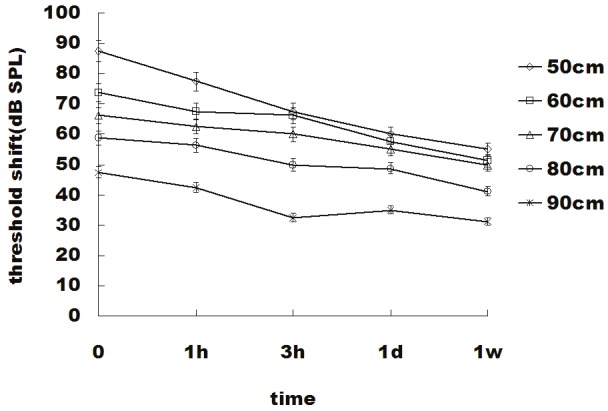
Time course of the hearing threshold shift. The y axis depicts the hearing threshold shift in dB SPL, and the x axis depicts the time. The values are means ± S.E.M. The difference between each distance is significant (P<0.05).
Observation of CoBF and hearing threshold shift after L-NAME infusions and blast exposure
The BP was simultaneously recorded using the same Chart Software after anaesthetisation until the end of the experiment. The results showed values within the normal range, and the percentage changes were minimal in all animals from the 3 groups.
The changes in the CoBF presented different patterns in the 3 groups during the experiment. Following the L-NAME RW administration, the CoBF decreased and reached its lowest baseline value (percentage value of change: 55.3±2.8%) (Figure 5) at 2 hours in the animals that were administered L-NAME (group B). This decrease lasted for 3 hours, followed by recovery to the normal level. Varying degrees of the decrease in the CoBF were also detected in the animals that were exposed to the blast at 3 distances after the L-NAME RW administration (group A). Contrasting changes were observed in the animals that were administered artificial perilymph (group C), with the CoBF increasing after the blast exposure (P<0.05) at all 3 distances; these changes lasted for 3 hours. At most of the time points, the differences between groups A and C were significant (Figure 6).
Figure 5.
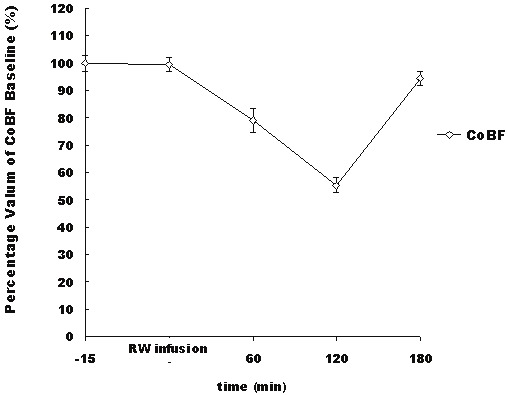
Percentage changes in the CoBF following the administration of 2 μl of 1% L-NAME in the RW. The CoBF decreased after the RW infusion, reached its lowest point at 2 hour, and then recovered after 3 hour. The data points are means±S.E.
Figure 6.
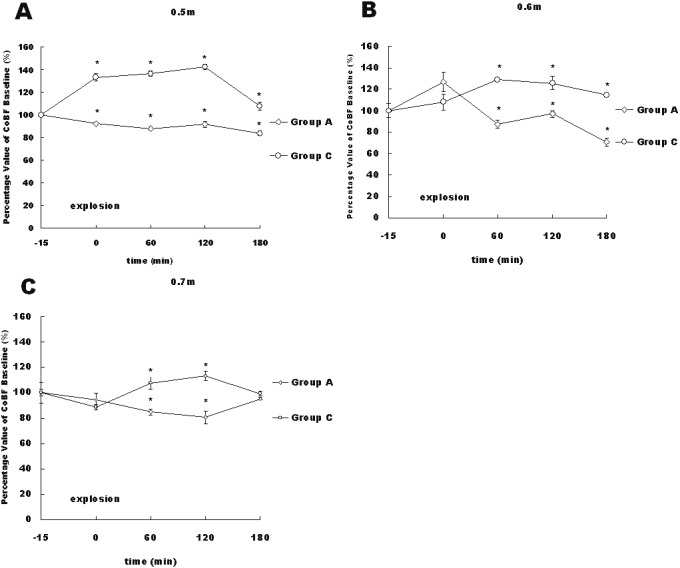
Percentage changes in the CoBF at 3 distances after the explosion of 600 mg of RDX following the administration of 2 μl of 1% L-NAME (group A) or artificial perilymph (group C) in the RW. The data points are means±S.E. The time course from RW infusion until 3 hour after the explosion is displayed. The results indicated significant differences at 1, 2 and 3 hours after the explosion between groups A and B at distances of 0.5 meter and 0.6 meter and at 1 and 2 hours after the explosion between groups A and C at the distance of 0.7 meter (*, P<0.05).
The hearing threshold shifts (TS) were significant for all distances between group A (75.0±7.1 dB at 0.5 meter, 70.0±9.1 dB at 0.6 meter, 56.3±4.8 dB at 0.7 meter) and group C (57.5±6.5 dB at 0.5 meter, 58.8±4.8 dB at 0.6 meter, 42.5±8.7 dB at 0.7 meter). Meanwhile, the TS values were minimal (1.3±2.3 dB) between before and 3 hours after the L-NAME solution RW infusion in group B (P>0.05).
Discussion
Blast pressure can produce a wide range of traumas, especially in air-containing structures. The auditory system has been considered the most susceptible organ and can endure serious injuries after a blast explosion [16]. The severity of the resulting hearing dysfunction is related to the distance from the epicentre in a simple open-space explosion. However, the trauma can be fatal if the injuries occur near a violent explosion, and the management of such trauma would focus on life support measures and non-auditory organ systems. Therefore, a moderate intensity of blast is necessary to evaluate injuries of the auditory system individually. In this study, we generated a relatively mild blast with a small power explosive (600 mg RDX) and placed the animals at moderate distances (0.5 meter to 0.9 m) corresponding with the medium intensity peak value of the blast overpressure (63.64±5.78 kPa to 23.34±1.52 kPa). There were no obvious signs of injury to the life support systems in all animals when exposed to this blast range, but tympanic membrane ruptures and damage to the middle ear were observed, as was distortion to the structure of the organ of Corti. However, as the main theme of this study, no distinct damage of the microvasculature structure was detected. The absence of that type of damage suggested that changes in the COBF were more likely to be functional.
Similar to its role in other tissues and organs, the microvasculature is a key component of the cochlea. Although the CoBF is estimated to require only on the order of 1/10000 of the total cardiac output in guinea pigs and 1/1000000 in humans [17], a normal blood supply to the cochlea is critically important for maintaining the inner ear potential and sustaining the production of endolymph. Moreover, sensory hair cells are vulnerable to ischaemia [18,19]. Although disorders of the CoBF have been considered to be involved in many pathophysiological processes of the inner ear [20-22], the difficulty of direct detection has limited the investigation of CoBF pathological changes, especially after blast injury. Early studies suggested that noise can reduce the cochlear blood flow, decrease the red blood cell density and increase the aggregation of red blood cells [10,11,23]. However, contrasting results have indicated an elevation in the CoBF after exposure to high-intensity noise [24] and blast waves [14]. Other researchers have suggested that changes in the cochlear microcirculation are intensity-related [25]. Such inconsistent results are likely due to the diversity in the experiment conditions and test methods. Although the definitions of impulsive noise and blasts are arbitrary, the distinction in the intensity, transmission pattern and mechanical features between them is apparent. Blast waves always have more intensive variation in the resulting pressure and involve the high speed movement of air over a very short time. Consequently, the pathological changes in the cochlear microcirculation induced by a blast may differ from the changes induced by high-intensity noise or impulse noise. In this study, our findings revealed an obvious relationship between the variations in the CoBF and the strength of the blast. Although increases in the CoBF were observed when the peak value of the blast overpressure was greater than 45.68±6.21 kPa, the CoBF was stabilised when the peak value of the blast overpressure was less than 35.05±4.11 kPa. This observation suggested the existence of a threshold for the blast overpressure peak value to cause CoBF changes. Unlike the hearing threshold shift, the time course of the CoBF changes indicated a rapidly recovering process. The baselines of the CoBF recovered to the normal level within 3 to 24 hours. Taking into consideration the histopathology changes in the cochlea, such results indicated that the structure of the cochlear microvasculature was not as vulnerable to the blast overpressure compared with other structures of the middle and inner ear, indicating strong autoregulatory ability. This autoregulation more likely occurs locally, in agreement with previous studies [17,26-28].
Although disorders of the CoBF have been considered to be associated with hearing loss in many diseases, the impairment of hearing function induced by a blast involves a few pathologic factors. Most previous studies of blast injuries have focused on the mechanisms involved in the damage to structures of the auditory system and the subsequent hearing loss induced by these damages [29-33]. The role of the CoBF disorder induced by a blast is ambiguous. In this study, we used a NO synthase inhibitor, L-NAME, to inhibit the changes in the CoBF induced by the blast and evaluated the effect of this inhibition on hearing function. As an important regulator of CoBF, nitric oxide (NO) is a potent vasodilator [34-36]. It can cause smooth muscle and pericyte relaxation by activating cGMP and affecting its downstream targets [37] and can also directly inhibit voltage-gated calcium channels, causing smooth muscle cells to relax [38]. This dilation effect can be inhibited by N-nitro-L-argininem-ethyl ester (L-NAME), a NO synthase inhibitor [39]. Such an inhibitory effect is dose-dependent, and this effect varies with the regional blood flow in the cochlea via different pathways and administration strategies [40]. In this study, we investigated the changes in the CoBF and evaluated the hearing function after the administration of an L-NAME solution in the RW. A decrease in the CoBF was observed immediately after the administration and lasted for 3 hour, with the lowest baseline value reaching 55.3±2.8% of the initial level at 2 hour after the administration. In the meantime, this local application did not impact the hearing function or systemic blood pressure, suggesting that there is a compensatory range of CoBF with respect to hearing impairment under normal situations. This L-NAME application also inhibited the increase in the CoBF after the blast exposure. In contrast to a normal situation, such inhibition corresponded with a more obvious TS compared with the application of artificial perilymph. These findings suggested that the increase in the blood flow has a positive role in the protection of hearing function, indicating that NO plays an important role in the regulation of the CoBF. Although the CoBF has a relatively wide compensatory range for hearing impairment under normal conditions, the hearing function appears to be more vulnerable to decreases in the CoBF. This observation also implies the importance of blood flow-supporting measures in auditory blast trauma. These findings will be helpful to better understand the CoBF changes and hearing dysfunction caused by auditory blast injuries and provide potential new methods of the prevention and management of these injuries in the clinic.
Acknowledgements
This study was supported by “Eleventh five” medical foundation of China Army (08Z027) and the National Science Alliance Foundation (NSAF, 10776038). We would like to thank Professor Xinan Lai and Professor Bingcang Li of the Research Institute of Surgery, State Key Laboratory of Trauma, Burns and Combined Injury for their valuable advices at many stages of this work.
References
- 1.Matsumoto Y, Hatano B, Matsushita Y, Nawashiro H, Shima K. [The characteristics of blast traumatic brain injury] . No Shinkei Geka. 2010;38:695–702. [PubMed] [Google Scholar]
- 2.Phillips YY, Zajtchuk JT. Blast injuries of the ear in military operations. Ann Otol Rhinol Laryngol Suppl. 1989;140:3–4. doi: 10.1177/00034894890980s501. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart P, Cronin D, Williams K, Ouellet S. Investigation of head response to blast loading. J Trauma. 2011;70:E29–36. doi: 10.1097/TA.0b013e3181de3f4b. [DOI] [PubMed] [Google Scholar]
- 4.Chandler DW, Edmond CV. Effects of blast overpressure on the ear: case reports. J Am Acad Audiol. 1997;8:81–88. [PubMed] [Google Scholar]
- 5.Chen YS, Tseng FY, Liu TC, Lin-Shiau SY, Hsu CJ. Involvement of nitric oxide generation in noise-induced temporary threshold shift in guinea pigs. Hear Res. 2005;203:94–100. doi: 10.1016/j.heares.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Mazurek B, Haupt H, Georgiewa P, Klapp BF, Reisshauer A. A model of peripherally developing hearing loss and tinnitus based on the role of hypoxia and ischemia. Med Hypotheses. 2006;67:892–899. doi: 10.1016/j.mehy.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol. 2003;8:207–221. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- 8.Lamm K, Arnold W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO(2) and auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear Res. 2000;141:199–219. doi: 10.1016/s0378-5955(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 9.Sprem N, Branica S, Dawidowsky K. Vasodilator and vitamins in therapy of sensorineural hearing loss following war-related blast injury: retrospective study. Croat Med J. 2001;42:646–649. [PubMed] [Google Scholar]
- 10.Attanasio G, Buongiorno G, Piccoli F, Mafera B, Cordier A, Barbara M, Filipo R. Laser Doppler measurement of cochlear blood flow changes during conditioning noise exposure. Acta Otolaryngol. 2001;121:465–469. [PubMed] [Google Scholar]
- 11.Arpornchayanon W, Canis M, Suckfuell M, Ihler F, Olzowy B, Strieth S. Modeling the measurements of cochlear microcirculation and hearing function after loud noise. Otolaryngol Head Neck Surg. 2011;145:463–469. doi: 10.1177/0194599811407829. [DOI] [PubMed] [Google Scholar]
- 12.Goldwin B, Khan MJ, Shivapuja B, Seidman MD, Quirk WS. Sarthran preserves cochlear microcirculation and reduces temporary threshold shifts after noise exposure. Otolaryngol Head Neck Surg. 1998;118:576–583. doi: 10.1177/019459989811800503. [DOI] [PubMed] [Google Scholar]
- 13.Lamm K, Arnold W. Noise-induced cochlear hypoxia is intensity dependent, correlates with hearing loss and precedes reduction of cochlear blood flow. Audiol Neurootol. 1996;1:148–160. doi: 10.1159/000259195. [DOI] [PubMed] [Google Scholar]
- 14.Hu B. [Cochlear microcirculation in living guinea pigs following explosion] . Zhonghua Er Bi Yan Hou Ke Za Zhi. 1991;26:6–9, 61. [PubMed] [Google Scholar]
- 15.Bobbin RP, Jastreboff PJ, Fallon M, Littman T. Nimodipine, an L-channel Ca2+ antagonist, reverses the negative summating potential recorded from the guinea pig cochlea. Hear Res. 1990;46:277–287. doi: 10.1016/0378-5955(90)90009-e. [DOI] [PubMed] [Google Scholar]
- 16.Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehabil Res Dev. 2009;46:797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. Disorders of cochlear blood flow. Brain Res Brain Res Rev. 2003;43:17–28. doi: 10.1016/s0165-0173(03)00189-9. [DOI] [PubMed] [Google Scholar]
- 18.Nuttall AL. Sound-Induced Cochlear Ischemia/Hypoxia as a Mechanism of Hearing Loss. Noise Health. 1999;2:17–32. [PubMed] [Google Scholar]
- 19.Wangemann P. Cochlear blood flow regulation. Adv Otorhinolaryngol. 2002;59:51–57. doi: 10.1159/000059241. [DOI] [PubMed] [Google Scholar]
- 20.Chen GD, Liu Y. Mechanisms of noise-induced hearing loss potentiation by hypoxia. Hear Res. 2005;200:1–9. doi: 10.1016/j.heares.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek B, Rheinlander C, Fuchs FU, Amarjargal N, Kuban RJ, Ungethum U, Haupt H, Kietzmann T, Gross J. [Influence of ischemia/hypoxia on the HIF-1 activity and expression of hypoxia-dependent genes in the cochlea of the newborn rat] . HNO. 2006;54:689–697. doi: 10.1007/s00106-005-1371-6. [DOI] [PubMed] [Google Scholar]
- 22.Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Axelsson A, Dengerink H. The effects of noise on histological measures of the cochlear vasculature and red blood cells: a review. Hear Res. 1987;31:183–191. doi: 10.1016/0378-5955(87)90125-0. [DOI] [PubMed] [Google Scholar]
- 24.Prazma J, Vance SG, Bolster DE, Pillsbury HC, Postma DS. Cochlear blood flow. The effect of noise at 60 minutes’ exposure. Arch Otolaryngol Head Neck Surg. 1987;113:36–39. doi: 10.1001/archotol.1987.01860010040011. [DOI] [PubMed] [Google Scholar]
- 25.Scheibe F, Haupt H, Ludwig C. Intensity-related changes in cochlear blood flow in the guinea pig during and following acoustic exposure. Eur Arch Otorhinolaryngol. 1993;250:281–285. doi: 10.1007/BF00186226. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima T. Autoregulation of cochlear blood flow. Nagoya J Med Sci. 1999;62:1–9. [PubMed] [Google Scholar]
- 27.Brechtelsbauer PB, Ren TY, Miller JM, Nuttall AL. Autoregulation of cochlear blood flow in the hydropic guinea pig. Hear Res. 1995;89:130–136. doi: 10.1016/0378-5955(95)00130-4. [DOI] [PubMed] [Google Scholar]
- 28.Ren TY, Nuttall AL, Miller JM. Provoked flux motion of cochlear blood flow measured with laser Doppler flowmetry in guinea pig. Acta Otolaryngol. 1993;113:609–614. doi: 10.3109/00016489309135872. [DOI] [PubMed] [Google Scholar]
- 29.Cave KM, Cornish EM, Chandler DW. Blast injury of the ear: clinical update from the global war on terror. Mil Med. 2007;172:726–730. doi: 10.7205/milmed.172.7.726. [DOI] [PubMed] [Google Scholar]
- 30.Darley DS, Kellman RM. Otologic considerations of blast injury. Disaster Med Public Health Prep. 2010;4:145–152. doi: 10.1001/dmphp.d-08-00057r2. [DOI] [PubMed] [Google Scholar]
- 31.Nageris BI, Attias J, Shemesh R. Otologic and audiologic lesions due to blast injury. J Basic Clin Physiol Pharmacol. 2008;19:185–191. doi: 10.1515/jbcpp.2008.19.3-4.185. [DOI] [PubMed] [Google Scholar]
- 32.van de Weyer PS, Praetorius M, Tisch M. [Update: blast and explosion trauma] . HNO. 2011;59:811–818. doi: 10.1007/s00106-011-2352-6. [DOI] [PubMed] [Google Scholar]
- 33.Lasso-Luis O, Chacon-Martinez J. [Tympanic blast injury] . Acta Otorrinolaringol Esp. 2009;60:380–381. doi: 10.1016/j.otorri.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 1996;23:1082–1090. doi: 10.1111/j.1440-1681.1996.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 35.Brechtelsbauer PB, Nuttall AL, Miller JM. Basal nitric oxide production in regulation of cochlear blood flow. Hear Res. 1994;77:38–42. doi: 10.1016/0378-5955(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 36.Michel O, Hess A, Bloch W, Stennert E, Su J, Addicks K. Localization of the NO/cGMP-pathway in the cochlea of guinea pigs. Hear Res. 1999;133:1–9. doi: 10.1016/s0378-5955(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 37.Tian F, Fessenden JD, Schacht J. Cyclic GMP-dependent protein kinase-I in the guinea pig cochlea. Hear Res. 1999;131:63–70. doi: 10.1016/s0378-5955(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 38.Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvasc Res. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- 39.Koss MC. Role of nitric oxide in maintenance of basal anterior choroidal blood flow in rats. Invest Ophthalmol Vis Sci. 1998;39:559–564. [PubMed] [Google Scholar]
- 40.Hoshijima H, Makimoto K, Noi O, Ohinata Y, Takenaka H. Effects of nitric oxide synthase inhibitor on cochlear blood flow. Hear Res. 2002;171:32–42. doi: 10.1016/s0378-5955(02)00328-3. [DOI] [PubMed] [Google Scholar]