Abstract
Evidence for an immunosuppressive function of indoleamine 2,3-dioxygenase (IDO) has been accumulating. However, the unusual distribution of IDO1 in gynecologic cancer cells suggests that modulating immunity may not its only function. To clarify the physiological importance of IDO1 in endometriosis, a tumor-like benign disease, we have investigated the potential mechanism by which IDO1 modulated endometrial stromal cells (ESCs) proliferation and invasion. ESCs were obtained from 16 control women (normal) and 14 patients with ovarian endometrioma, then the normal ESCs were treated with plasmid pEGFP-N1-IDO1 or SD11-IDO1 short hairpin RNA (shRNA) alone, or in combination with c-Jun N-terminal kinase (JNK) inhibitor (SP600125), and subjected to cell viability, proliferation, apoptosis assay and Matrigel invasion assay. IDO1 mRNA expression was evaluated by quantitative real-time reverse transcription-polymerase chain reaction (real-time PCR), and protein levels of IDO1, survivin, protein 53 (p53), matrix metalloproteinase (MMP)-2, MMP-9, tissue-inhibitor of metalloproteinase-1 (TIMP-1) and cyclooxygenase-2 (COX-2) in IDO1-overexpressing and IDO1-deficiency ESCs were analyzed by in-cell Western. We found that IDO1 expression was higher in endometriosis-derived eutopic and ectopic ESCs, compared with endometriosis-free normal ESCs. As a result, IDO1-overexpression in ESCs was markedly linked to reduction of apoptosis and p53 expression, and upregulation of survival, proliferation, invasion, as well as expression of MMP-9, COX-2 expression, rather than expression of survivin, MMP-2 and TIMP-1. Reversely, JNK blockage could abrogate these alterations of ESCs in IDO1-overexpressing milieu, suggesting that JNK signaling pathway was indispensable for ESCs survival, proliferation and invasion enhanced by IDO1, which may contribute to the pathophysiology of endometriosis.
Keywords: Endometriosis, indoleamine 2, 3-dioxygenase, endometrial stromal cells
Introduction
Endometriosis, the presence of endometrium outside the uterine cavity, is a common gynecologic disorder, causing abdominal pain, dyspareunia and infertility [1]. As a tumor-like benign disease, endometriosis and cancer are similar in several aspects such as unrestrained growth, decreased apoptosis and aggressive invasion [2].
Indoleamine 2,3-dioxygenase (IDO) is an intracellular heme enzyme that catalyses the initial and rate-limiting step in the metabolism of the essential amino acid tryptophan along the kynurenine pathway. IDO plays crucial roles in various infectious diseases, fetal rejection, organ transplantation, neuropathology, autoimmune disorder and cancer by reducing the availability of tryptophan [3-5]. IDO family consists of two members: IDO1 and IDO2. The corresponding genes have a similar genomic structure and are situated adjacent to each other on human chromosome 8. However, different enzymatic activities, diverse expression pattern in response to stimuli within tissues, suggest a distinct role for each protein [6]. Recent human studies indicate that, whereas the IDO2 gene seems to be functional in murine models, it was not found to be functional in humans [7]. Despite of the abundant evidence implicating a role for IDO1 in immunosuppression [3], the unusual distribution of IDO1 in gynecologic cancer cells suggests that modulating immune response was not its only function [8]. IDO1 has been found to be present in the human female genital tract, and its level in endometrium is physiologically regulated by the menstrual cycle [9,10]. Besides, our previous work demonstrated that IDO1 could also express in endometrial glandular, surface epithelial and stromal cells of endometrium [11]. Moreover, IDO1 was detected to be higher in eutopic endometrium from women with endometriosis by microarrays [12,13]. Therefore, we decided to test whether IDO1 plays a role in the pathogenesis of endometriosis and also have interactions with other known abnormal factors in endometriosis.
Mitogen-activated protein kinase (MAPK), intracellular signal transducers, have been shown to participate in a diverse array of cell programs, including cell proliferation, cell death, cell movement [14]. Among five distinguishable MAPK modules, which have been identified so far in mammalian systems, the most common ones are the extracellular signal-regulated kinase 1 and 2 (ERK1/2) cascade, which preferentially regulates cell growth and differentiation, as well as the c-Jun N-terminal kinase (JNK) and p38 MAPK cascades, which function mainly in stress responses like inflammation and apoptosis [15,16]. Association of MAPK activity with the pathogenesis of endometriosis has been well described [17]. It has been reported that enhanced proliferation and survival of eutopic or ectopic endometrial cells from patients with endometriosis correlated with abnormal MAPK phosphorylation [18-20]. Previous work have demonstrated that, in many cell lines and tissues, IDO1 could be induced by lipopolysaccharide (LPS)-mediated effects [9], which related to activation of MAPK [21]. The racemic mixture of IDO1 inhibitor-1-methyl-tryptophan (1-MT) has also been reported to modify the polarization of dendritic cells (DC) by modulating MAPK [22]. Thus, MAPK might exist as the downstream of IDO1. So in the present study, we’d like to explore whether inhibition of MAPK signaling could affect the ESCs biologic characteristics regulated by IDO1.
Given the role of IDO1 and MAPK in endometriosis, the present study is undertaken to explore which MAPK signaling transduction pathway might mediate IDO1-induced ESCs proliferation and invasion, and the possible downstream signals of IDO1 participating in the modulation of ESCs.
Materials and methods
Patients and tissue collection
Endometrial or endometriotic samples were obtained from patients (age 23-40 years) who underwent laparoscopy and additional curettage for treatment of endometriosis (n=18) or ovary dermoid cyst (n=18). None of the women had taken medications or received hormonal therapy for at least 6 months prior to surgery. 4 negative samples for endometriosis and 2 for dermoid cyst were excluded after confirmation by laparoscopically and histological diagnosis. The median age was 30.1±5.9 years for the group of women (n=14) with endometriosis and 31.7±9.5 years for the control group (n=16). No significant difference was found between the parity of the endometriosis group (1.26±0.66) and control group (1.69±0.35). All samples were detected histologically to be in the secretory phase of menstrual cycle. Each subject completed a signed, written consent form approved by the Research Ethics Committee in Obstetrics and Gynecology Hospital, Shanghai Medical School, Fudan University. The tissue was collected under sterile conditions and transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA).
Cell culture
We purified ESC as described previously elsewhere [23] with slight modification. Tissues were minced into 2- to 3-mm pieces and incubated in DMEM/F12 containing collagenase type IV (0.1%; Sigma, USA) and deoxyribonuclease type I (DNase I; 3000 U; Sigma, USA) with constant agitation for 70 min at 37°C. The resulting dispersed was filtrated through sterile 100 μm and 70 μm nylon strainers (Becton Dickinson) in turn to remove undigested tissue and epithelial cells. The filtrate was then centrifuged at 800×g for 15 min to further remove leukocytes and erythrocytes, and washed with phosphate-buffered saline (PBS). The ESCs were resuspended in DMEM/F-12 containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), and plated into culture flask in 5% CO2 at 37°C. The culture medium was replaced every 3 days. Cell viability was assessed by Trypan Blue exclusion assay (approximately 89.7%). The purity of ESCs was more than 95%, as judged by diffuse and strong immunostaining for vimentin and negative for cytokeratin-7 (CK7) in immunocytochemistry.
Real-time reverse transcriptase-polymerase chain reaction
Total RNA was extracted from normal, eutopic and ectopic ESCs with Trizol reagent (Invitrogen; USA). The real-time PCR was performed using the SYBR Green PCR Mix (Fermentas Inc, Glen Burnie, Maryland), according to the manufacturer’s instructions. The housekeeping gene glyceraldehydes 3-phosphate dehydrogenase (GAPDH) was used as the normalizer. The forward primer of IDO1 was 5’-TCACAGACCACAAGTCACAGC-3’. The reverse primer was 5’-AGTTGGCAGTAAGGAACAGCA-3’. The forward primer of GAPDH was 5’-AACAGCGACACCCACTCCTC-3’. The reverse primer was 5’-CATACCAGGAAATGAGCTTGACAA-3’. The real-time PCR reaction was carried out for 40 cycles. Polymerase chain reactions were run on the Mx4000 and Mx3005 quantitative real-time PCR Stratagene systems (Agilent Technologies). Pair-wise comparisons between target and control at each time point were performed. All validation experiments used four-subject samples in each group. The values were normalized to the GAPDH controls.
IDO1 overexpression or shRNA plasmids transfection
Normal ESCs were grown in culture medium with 10% FBS. When cells had reached confluency, Lipofectamine 2000 (Invitrogen; USA), OPTI-MEM (Gibco, USA) and plasmid pEGFP-N1-IDO1 or SD11-IDO1 shRNA (short hairpin RNA) (GeneChem, Shanghai, China) were mixed and incubated for 20 min and added to the cells at room temperature according to the manufacturer’s protocol. The vector-only plasmid pEGFP-N1 and SD11 (GeneChem, Shanghai, China) were used as the negative controls, respectively. And the normal ESCs without plasmid transfection were treated as the blank control. After 6 h of incubation, these cells were then incubated in DMEM/F-12 containing 10% FBS in 5% CO2 at 37°C.
In vitro treatment of ESCs
To evaluate the effect of JNK MAPK signaling pathway on IDO1-overexpression (pEGFP-N1-IDO1) or interference (SD11-IDO1 shRNA) normal ESCs survival, proliferation, invasion and target protein expressions, after serum starvation for 12h, the transfected cells were incubated with SP600125 (JNK inhibitor, 10 μM, Sigma, USA), or vehicle (1‰ dimethyl sulfoxide, DMSO; Sigma, USA ) as negative control for 24h.
In-cell western
According to the description by Egorina [24], we used a newly set-up assay called in-cell Western to determine the in-cell protein level of interest. Vector-only transfected ESCs, IDO1-overexpressing or interference ESCs were growing with DMEM/F-12 containing 10% FBS in 96-well plate for 36 h. After 12h serum starvation, the cells were incubated with SP600125 (10 μM) or vehicle (1‰ DMSO) for 24h, respectively. Then they were fixed with 4% formaldehyde 10 min, washed with 0.1% Triton in PBS for 5 times, and blocked by 150 μl of LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, Nebraska, USA) for 90 min at room temperature. Subsequently, to detect the MAPK signaling pathway IDO1 activated, the cells were incubated with mouse anti-human phospho-Erk1/2 (1:50, Santa Cruz Biotechnology, USA), mouse anti-human phospho-JNK (1:50, Santa Cruz Biotechnology, USA), mouse anti-human phospho-p38 (1:50, Santa Cruz Biotechnology, USA). And rabbit anti-human Erk1/2 (1:80, Santa Cruz Biotechnology, USA), rabbit anti-human JNK (1:80, Santa Cruz Biotechnology, USA), rabbit anti-human p38 (1:80, Santa Cruz Biotechnology, USA) were added as homologous control, respectively. Additionally, the cells were incubated with mouse anti-human IDO1 (1:30; Abcam, USA), mouse anti-human monoclonal survivin (clone 91630, 20ug/ml; R&D Systems, Abingdon, UK), mouse anti-human monoclonal Protein 53 (p53; Clone 184721, 20ug/ml; R&D Systems, Abingdon, UK), mouse anti-human MMP-2 (clone 101721, 20ug/ml; R&D Systems, Abingdon, UK), mouse anti-human TIMP-1 (clone 151620, 20ug/ml; R&D Systems, Abingdon, UK). The polyclonal antibody of housekeeping protein actin, rabbit anti-human actin (1:50, Santa Cruz Biotechnology, USA) was meanwhile added to each well as an internal control. However, for rabbit anti-human polyclonal COX-2 (1:30, Cell Signaling Technology, USA), rabbit anti-human polyclonal MMP-9 (20ug/ml, R&D Systems, Abingdon, UK) detection group, homologue mouse anti-human polyclonal GAPDH (1:50, Santa Cruz Biotechnology, USA) was served as an internal control. After overnight treatment at 4°C, the wells were then incubated with corresponding IRDyeTM 700DX (red fluorescence)-conjugated goat anti-mouse fluorescence secondary antibody (1:50, Rockland Inc, USA) and IRDyeTM 800DX (green fluorescence)-conjugated goat anti-rabbit fluorescence secondary antibody (1:50, Rockland Inc, USA) in the dark (as shown in Table 1). The signal was detected and the protein was analyzed semiquantitatively using the Odyssey Infrared Imaging System (LI-COR Biosciences German version of Ltd.). The expression level of the correspondent molecules was calculated as the ratio of the intensity of target proteins to actin or GAPDH.
Table 1.
Antibody used in in-cell Western
| Target protein | Primary antibody | Fluorescence secondary antibody | ||
|---|---|---|---|---|
|
| ||||
| Target | Internal control | Detect target protein | Detect internal protein | |
| P-Erk1/2 | mouse anti-human P-Erk1/2 | rabbit anti-human Erk1/2 | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| P-JNK | mouse anti-human P-JNK | rabbit anti-human JNK | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| P-p38 | mouse anti-human P-p38 | rabbit anti-human p38 | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| IDO1 | mouse anti-human IDO1 | rabbit anti-human actin | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| survivin | mouse anti-human survivin | rabbit anti-human actin | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| p53 | mouse anti-human p53 | rabbit anti-human actin | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| MMP-2 | mouse anti-human MMP-2 | rabbit anti-human actin | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| MMP-9 | rabbit anti-human MMP-9 | mouse anti-human GAPDH | IRDyeTM 800DX -goat anti-rabbit antibody (green) | IRDyeTM 700DX -goat anti-mouse antibody (red) |
| TIMP-1 | mouse anti-human TIMP-1 | rabbit anti-human actin | IRDyeTM 700DX -goat anti-mouse antibody (red) | IRDyeTM 800DX -goat anti-rabbit antibody (green) |
| COX-2 | rabbit anti-human COX-2 | mouse anti-human GAPDH | IRDyeTM 800DX -goat anti-rabbit antibody (green) | IRDyeTM 700DX -goat anti-mouse antibody (red) |
Cell viability assay
To detect cell viability, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) assay was used. The IDO1-overexpression or blockage ESCs (1×104 cell/per well in 96 well plate) were cultured without serum for 12h and then incubated with SP600125 (20 μM) or vehicle for 24h in cell-growing media. Cells were then incubated for 4 h in the presence of 2.5 mg/ml MTT and thereafter 100 μl DMSO was added. Absorbance (450 nm) was determined using the DigiScan Microplate Reader (ASYS Hitech GmbH, Eugendorf, Austria). These values were normalized to the vector-only controls whose absorbance was set to 1.
Proliferation assay
The ability of ESCs proliferation was detected by 5-bromo-2’-deoxyuridine (BrdU) cell proliferation enzyme-linked immunosorbent assay (ELISA) system (Millipore, USA) according to the manufacturer’s instruction. The transfected ESCs (1×104 cell/ per well in 96 well plate) were cultured without serum for 12h and then incubated with SP600125 (20 μM) or vehicle for 24h in cell-growing media. The proliferation assay was performed 12 h following the addition of BrdU reagan (10 ng/ml). The absorbance values measured at 450 nm wavelength represent the rate of DNA synthesis and correspond to the number of proliferating cells. These values were normalized to the experimental controls that set to 1.
Flowcytometry
The different stages of apoptosis were analyzed by flowcytometry with allophycocyanin (APC) conjugate-annexin V (eBioscience, USA) and propidium iodide (PI) staining. The ESCs transfected with pEGFP-N1-IDO1 or SD11-IDO1 shRNA were cultured without serum for 12h and then incubated with SP600125 (20 μM) or not for 24h in cell-growing media. A minimum of 30,000 ESCs were harvested at the same concentration and washed in cold PBS. Then Annexin V-Alexa Fluor 750 and PI working solution were added into cell suspension for 15 min in the dark at room temperature. After staining, cells were washed twice with cold PBS and then applied to flowcytometry (Becton Dickson, San Jose, CA). Data were acquired in the list mode, and the relative proportions of cells within different areas of the fluorescence profile were quantified using the LYSYS II software program (Becton Dickson, Franklin Lakes, NJ). Data were revealed as a percentage of the controls.
Matrigel invasion assay
Cells were analyzed for invasion using the Matrigel invasion assay with polycarbonate membranes as previously described [25]. An equal number of transfected ESCs (5×104 resuspended in 200 μl DMEM/F12 with 10% FBS) were seeded in the upper Matrigel-coated chambers (8 μm pore size, 6.5 mm diameter, Corning, USA) and allowed to invasion for 24 h in 5% CO2 at 37°C, while SP600125 (20 μM) or vehicle was added in the lower chambers. The cells attached to the upper surface of filter were removed by scrubbing with cotton swab, and cells on the underside of the membrane were fixed, stained with hemotoxylin, and counted (five random fields) by two independent investigators. The results were expressed as a percentage of the controls.
Statistical analysis
Data were analyzed by Student’s t-test and One-way analysis of variance (ANOVA) with post hoc test. Differences were considered as statistically significant at P<.05.
Results
IDO1 expression in endometriosis-derived eutopic and ectopic ESCs was higher than the normal ones
The expression of IDO1 in ESCs was determined by real-time PCR and in-cell Western. The level of IDO1 in eutopic and ectopic ESCs was higher than normal ones (Figure 1A, P<.05). Moreover, the protein level of IDO1 in endometriosis-derived ESCs elevated significantly compared with that of endometriosis-free ESCs, indicating that IDO1 upregulation in ESCs may be involved in the pathogenesis of endometriosis. However, no statistically significant differences of IDO1 expression between eutopic and ectopic ESCs were observed here (P>.05; Figure 1A and 1B).
Figure 1.

Higher IDO1 expression in endometriosis-derived ESCs. The expression of IDO1 was statistically significantly higher in eutopic and ectopic ESCs than in normal ones as analyzed by real-time PCR (A) and in-cell Western (B). Normal: ESCs from endometriosis-free patients; Eutopic: ESCs from endometriosis-derived endometrium; Ectopic: ESCs from endometriosis-derived endometriotic tissue. Representative data were shown as well as the mean ± SD of 14 different experiments. #P<.05, vs. control (normal ESCs).
JNK pathway was involved in IDO1 expression of ESCs
We then explored the signalling pathways involved in the upregulation of IDO1 in endometriosis-derived ESCs. To clarify IDO1’s role in ESCs, we transfected normal ESCs with plasmid pEGFP-N1-IDO1 or SD11-IDO1 shRNA (ensemble ID ENSG00000131203) respectively. First we analyzed the effect of plasmid transfection on IDO1 protein expression in these ESCs. In-cell Western analysis showed that IDO1 protein level in ESCs was obviously increased to 1.81 fold after pEGFP-N1-IDO1 transfection, and on the contrary, it was markedly attenuated to 29.80% by the introduction of SD11-IDO1 shRNA, compared with vector pEGFP-N1 or SD11 transfection respectively (Figure 2A, P<.01). Moreover, IDO1 protein level of IDO1-overexpression ESCs was similar to that of ectopic ones (P>.05, Figure 1B and 2A), suggesting that the normal ESCs transfected by pEGFP-N1-IDO1 could well mimic the ectopic ESCs as respect of IDO1 expression. Compared with the normal ESCs without transfection (blank controls), pEGFP-N1 and SD11 vector transfected ESCs (negative controls) had effect on neither ESCs’ expression of our detected proteins (such as IDO1, P-Erk1/2, Erk1/2, P-JNK, JNK, p38, P-p38, MMP2, MMP-9, TIMP-1, COX-2, survivin and p53) (P>.05, data not shown), nor ESCs viability, proliferation, apoptosis and invasion (P>.05, data not shown).
Figure 2.
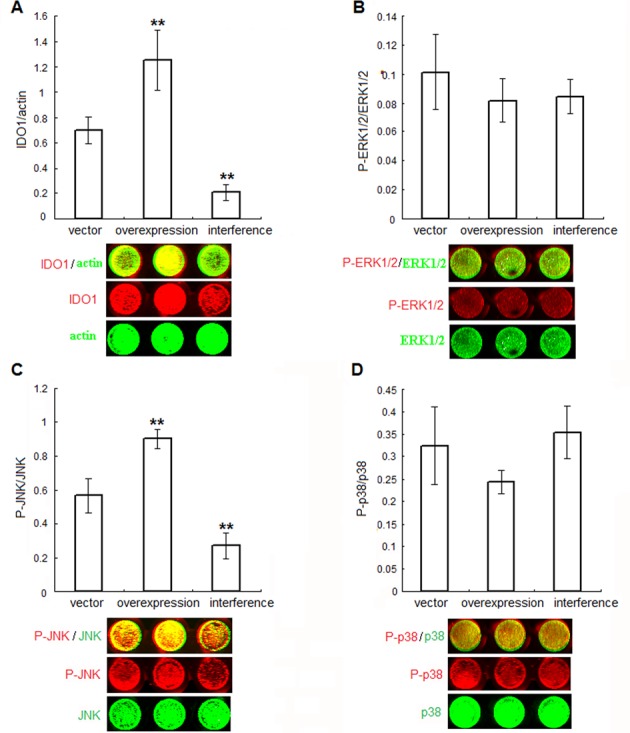
IDO1 activates JNK signaling pathway in ESCs. Normal ESCs from endometriosis-free women were treated with plasmid pEGFP-N1-IDO1 or SD11-IDO1 shRNA, and vector-only plasmid as control. The efficiency of plasmid transfection (A), as well as the phosphorylation level of Erk1/2 (B), JNK (C) and p38 (D) was demonstrated by in-cell Western analysis. Herein, vector control: normal ESCs transfected with vector-only plasmid; IDO1 overexpression: normal ESCs transfected with plasmid pEGFP-N1-IDO1; IDO1 interference: normal ESCs transfected with SD11-IDO1 shRNA. *P<.01, vs. normal group, **P<.01, vs. vector control group. Representative data as well as the mean ± SD of 12 different experiments were shown.
Since the higher MAPK phosphorylation in eutopic or ectopic endometrial cells from patients with endometriosis has been confirmed by others [18-20], then we studied whether IDO1 expression has any effect on change of MAPK phosphorylation in ESCs. As showed in Figure 2, P-JNK levels elevated to 1.60 fold in IDO1-overexpression ESCs, while dramatically decreased to 47.5% in IDO1-deficient ESCs, compared with vector-only control (P<.01; Figure 2C). No statistically difference of P-p38 or P-ERK1/2 levels upon IDO1 overexpression or knockdown was observed in ESCs (versus vector-only control; P>.05; Figure 2B and 2D), indicating that JNK pathway, but not ERK1/2 or p38 pathway, was activated by IDO1 overexpression in ESCs.
IDO1 regulated ESCs viability, proliferation, apoptosis and invasion via JNK signaling pathway
Based on the results described above, and to further demonstrate the effect of JNK signaling pathway in IDO1-influenced ESCs biological behavior, we analyzed the effects of the JNK inhibitor, SP600125 (20 μM) on transfected ESCs viability, proliferation, apoptosis and invasion 24h after its administration. Normal ESCs transfected with or without pEGFP-N1/SD11 vector had the similar effects on ESCs biological characteristics (P>.05, data not shown). Compared with vector-only transfected ESCs, IDO1-overexpressing ESCs was linked to upregulation of cell survival and growth levels to 128% and 159%, respectively. Additionally, overexpression of IDO1 in ESCs could reduce cell apoptosis to 43% (vs. control; P<.05; Figure 3A-C). SP600125, an inhibitor of JNK, could reduce viability and proliferation of vector-only and pEGFP-N1-IDO1 transfected ESCs, while triggered their apoptosis (P<.05; Figure 3A-C). However, SP600125 had no significant effect on IDO1-knockdown ESCs growth. Moreover, compared to the control, IDO1 overexpression significantly increased ESCs invasion ability (P<.01; Figure 3D), and the migration could be attenuated by JNK signaling inhibitor SP600125 (P<.05; Figure 3D). Collectively, these data strongly suggest that IDO1 affects cell viability, proliferation, apoptosis and invasion by a mechanism depended on JNK signaling.
Figure 3.
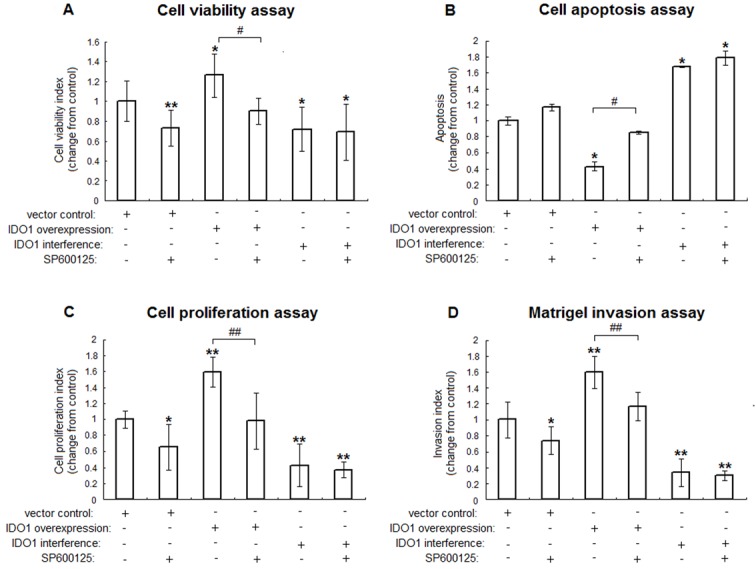
JNK signaling pathway responsible for enhancement of the ESCs viability, proliferation and invasion by IDO1. IDO1-overexpressing and IDO1-deficiency ESCs were treated with JNK inhibitor (SP600125, 20 μM) or vehicle control (1‰ DMSO) for 24 h. Thereafter, MTT assay (A), apoptosis assay (B), BrdU assay (C) and Matrigel invasion assay (D) were applied to analyze the viability, apoptosis, proliferation and invasion of transfected ESCs, respectively. The columns represent the mean ± SD calculus of 8 different experiments. *P<.05 vs. vector control transfected ESCs without SP600125; **P<.01 vs. vector control transfected ESCs without SP600125; #P<.05, IDO1-overexpressing ESCs with SP600125 vs. IDO1-overexpressing ESCs without SP600125; ##P<.01, IDO1-overexpressing ESCs with SP600125 vs. IDO1-overexpressing ESCs without SP600125.
P53 was indispensable for IDO1-regulated JNK dependent cell growth in ESCs
To get an insight into the mechanism of JNK-dependent proliferation in IDO1-overexpressing or deficiency ESCs, we detected the proliferation-associated proteins survivin and apoptosis-related protein p53 in transfected ESCs by in-cell Western. Our data showed that IDO1-activated JNK signaling pathway suppressed expression of p53 to 77.1% (vector control without SP600125 vs. IDO1-overexpressing ESCs without SP600125, P<.05; Figure 4B), and its expression was elevated to 117% by SP600125 (IDO1-overexpressing ESCs with SP600125 vs. IDO1-overexpressing ESCs without SP600125, P<.01; Figure 4B). Besides, p53 expressions in IDO1-deficency ESCs with or without SP600125 were stimulated to 185% and 190% (P<.01 vs. vector control without SP600125; Figure 4A). Conversely, no statistical changes in survivin levels upon IDO1 transfection or JNK inhibitor were observed (P>.05, Figure 4A). Thus, IDO1 regulated p53 expression in normal ESCs via JNK signaling pathway.
Figure 4.
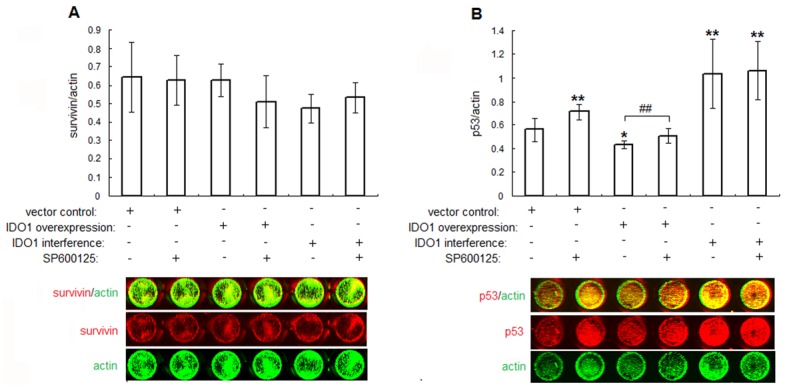
IDO1 inhibits p53 expression through JNK signaling pathway. The transfected ESCs were treated with JNK inhibitor (SP600125, 20 μM) or vehicle control (1‰ DMSO) for 24 h. Thereafter, the expressions of proliferation associated proteins survivin (A), p53 (B) were determined by in-cell Western. The columns represent the mean ± SD calculus of 8 different experiments. *P<.05 vs. vector control transfected ESCs without SP600125; **P<.01 vs. vector control transfected ESCs without SP600125; #P<.05, IDO1-overexpressing ESCs with SP600125 vs. IDO1-overexpressing ESCs without SP600125.
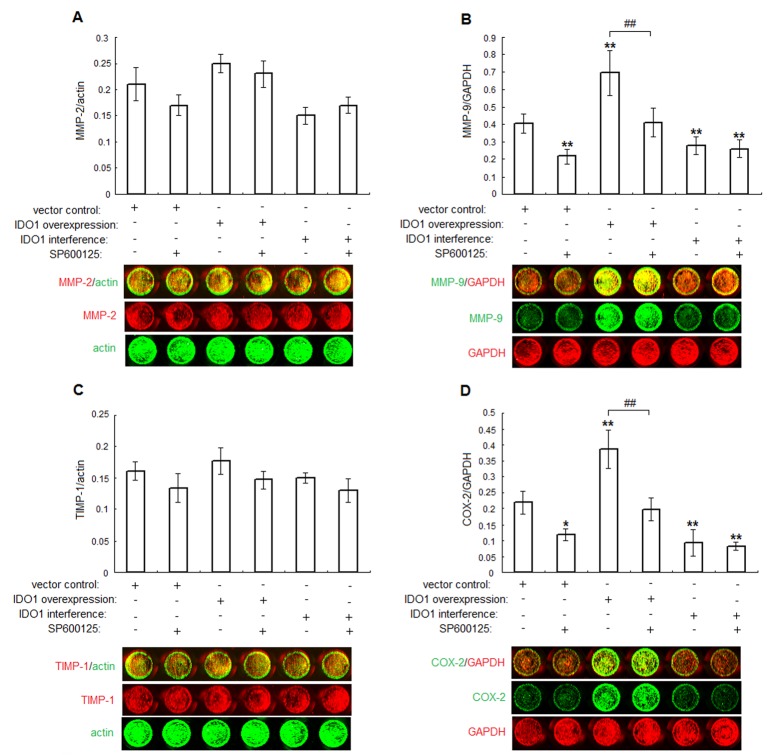
JNK inhibitor on IDO1-induced MMP-2, MMP-9, TIMP-1 and COX-2 expression
To rule out how IDO1 participated in the regulation of ESCs invasion, we analyzed the influence of IDO1 overexpression or knockdown on ESCs MMPs, TIMP-1 and COX-2 expression. Data were presented in Figure 5 that, JNK inhibitor could abrogate IDO1-stimulated MMP-9 and COX-2 expression in the IDO1-overexpressing ESCs (IDO1-overexpressing with SP600125 administration vs. IDO1-overexpressing without SP600125 administration; P<.05; Figure 5B and 5D). Conversely, IDO1-deficiency ESCs had lower MMP-9, COX-2 expression (P<.01, Figure 5B and 5D) compared with ESCs transfected with vector only, and that couldn’t be influenced by SP600125 (P>.05; Figure 5B and 5D). Surprisingly, neither IDO1 nor JNK inhibitor could affect MMP-2, TIMP-1 expression (P>.05; Figure 5A and 5C). These findings suggested that IDO1 might be an upstream signal participating in the regulation of MMP-9 and COX-2, thereby possibly controlling the invasion of ESCs. However, further work should be done to confirm this causation.
Figure 5.
IDO1 stimulates COX-2, MMP-9 expression in ESCs through activation of JNK signaling pathway. IDO1 in normal ESCs was overexpressed or blockaded by plasmid transfection, and treated with or without JNK inhibitor (SP600125, 20μM). The expression of invasion-related proteins MMP-2 (A), MMP-9 (B), TIMP-1 (C) and COX-2 (D) were determined by in-cell Western. Data were shown as the mean ± SD calculus of 8 different experiments; *P<.05 vs. vector control transfected ESCs without SP600125; **P<.01 vs. vector control transfected ESCs without SP600125; #P<.05, IDO1-overexpressing ESCs with SP600125 vs. IDO1-overexpressing ESCs without SP600125; ##P<.01, IDO1-overexpressing ESCs with SP600125 vs. IDO1-overexpressing ESCs without SP600125.
Discussion
The results presented establish unambiguously that IDO1 highly expresses in eutopic and ectopic ESCs from patients with endometriosis than normal ones, and overexpression of IDO1 in normal ESCs elicits an increase in the phosphorylation of the JNK signaling pathway. Via JNK pathway, IDO1 regulates ESCs expression of p53, MMP-9 and COX-2, which were accompanied by the enhancement of cell survival, proliferation, invasion, and coupled to inhibitory effects on cell apoptosis.
Traditionally, IDO is thought to be an immune modulator through tryptophan depletion and via the generation of proapoptotic metabolites [4]. It has also been pointed out to be participating in tumor progression [8]. Since endometriosis is a gynecological tumor-like disease, we supposed that IDO1 is a potential candidate which facilitates endometriosis development. Burney [12] and Aghajanova [13] have mentioned that IDO1 gene expression increased in endometriosis-derived eutopic endometrium, and was relevant to the patients’ clinical stage. And our previous result also revealed that IDO1 present in the stromal cells of endometrium or endometriotic tissue, and especially highly expressed in endometriosis-derived ESCs [11]. To further test the mechanism of IDO1 in origin of endometriosis, we regulated IDO1 expression by transfection of plasmid pEGFP-N1-IDO1 or SD11-IDO1 shRNA, which could well reflect the role of IDO1 in endometriosis-derived ESCs, and re-evaluated the effect of IDO1 on ESCs biologic functions. We found that overexpressing of IDO1 significantly increase the P-JNK in ESCs, which is in agreement with others’ work in CD11+ dendritic cells [26].
JNK belong to the MAPK family, which is critical for cellular functions in eukaryotic cells. Each pathway is preferentially recruited by distinct sets of stimuli, thereby allowing cells to response to multiple divergent inputs in a coordinate manner. Recently, clusters of researches have indicated the importance of MAPK in functions of human eutopic and ectopic endometrial cells [17,27,28]. And the enhanced proliferation and survival of eutopic or ectopic endometrial cells from endometriosis patients have been confirmed to correlate with higher level of MAPK phosphorylation [18-20]. The JNK protein kinases are collectively referred to as stress-activated MAP kinase (SAPKs), and encoded by three distinct genes. JNK1 and JNK2 are ubiquitously expressed, while JNK3 is selectively expressed in the brain [29]. JNK phosphorylation and activation occur in response to a variety of environmental, developmental, and inflammatory stimuli [30]. In the canonical JNK pathway, activated JNK acts to phosphorylate the transcriptional activation domain of c-Jun, which then constitutes the activator protein-1 (AP-1) transcription factor with c-Fos [31]. Subsequently, G protein-coupled receptors regulate MAPK signaling pathways that result in the expression of specific response genes involved in cell proliferation, invasion and apoptosis [32]. Since the regulatory elements such activator protein-1 (AP-1) was located on the human IDO gene promoter region, it could better explain the role of JNK in IDO1-regulated ESCs [21]. Because JNK has been shown to be required for IDO1 expression, we used SP600125 as it is a potent, cell-permeable and selective inhibitor of JNK. It competitively targets the AP binding site of JNK1, JNK2 and JNK3, exhibiting over 300-fold greater selectivity for JNK [33].
Endometriotic cells are identified with altered growth potency and lower susceptibility to apoptosis. However, SP600125, the blocker of JNK, leaded to the inhibitory activity in survival and proliferation, while offered greater level of apoptosis, as well as the expression of p53 in IDO1-overexpressing ESCs. The role of apoptosis in the physiopathology of endometriosis is increasingly apparent [34,35]. It can be initiated by extracellular and intracellular “death signals” that increase p53 protein expression [36]. Evidences for p53 as a marker of anomalous apoptosis in endometriosis has been accumulating, especially in ovarian endometriosis [37,38]. And experiments also suggested that JNK pathway is associated with inhibition of p53 in human [39,40]. Similarly, our findings suggest that IDO1 could downregulate the expression of p53, as well as ESCs apoptosis through JNK pathway. Survivin has also been revealed to participate in the endometriosis, and correlated with apoptosis and invasive phenotype of endometriotic tissues [2]. It has been defined to be regulated mainly through the Raf-1/MEK/ERK pathway in human cells but not JNK pathway [41,42], indicating that increase of survivin in endometriotic tissue may due to the other factors rather than IDO1.
Invasion, controlled by cross-talk mechanisms between cells and extracellular microenvironment, has been investigated in the pathogenesis of endometriosis [43]. We demonstrated that IDO1-overexpression ESCs had an elevated invasiveness compared to that of normal ESCs. Moreover, JNK inhibitor could abolish the increase invasion capability and MMP-9, COX-2 expressions of ESCs induced by IDO1 in a significant manner. Our findings were in line with previous findings that MMPs and COX-2 are involved in the regulation of endometriotic cells [44,45]. It has been reported that product of COX-2, prostaglandins (PGs), can explain most of the symptoms of endometriosis [46]. Conversely, selective inhibition of PGE2 receptors could decreases migration and invasion of human immortalized endometriotic epithelial and stromal cells into Matrigel [47]. Another essential proteinase-MMP, the enzymes for extracellular matrix (ECM) degradation was also play a vital role in the invasion of endometriotic lesions. The retrograde endometrial tissue could be more prone to peritoneal implantation and invasion due to the altered production of MMPs in eutopic endometrium from endometriosis affected women [2]. Upregulation of COX-2 and MMPs secretion response to various stimuli through JNK pathway has been reported yet [48,49]. We conjecture that, MMP-9 and COX-2 secreted from IDO1-stimulated ESCs may contribute to the invasion of ESCs and may be activated in the disease of ESCs via JNK pathway, though a further study needed to reinforce the thesis.
In conclusion, abnormal expression of IDO1 in ESCs is associated with aberrant activation of JNK pathway, which contributed to the down-regulation of p53 and coupled to inhibitory of cell apoptosis. Besides, through JNK pathway, IDO1 induced the expression of MMP-9 and COX-2, and leaded to the increased invasion of ESCs. Based on our previous work, the present study further probed into the potential signaling pathway through which IDO1 involved in the origin of endometriosis, as well as its downstream effect molecules. However, the evidences are still insufficient to confirm that, whether increased IDO1 in eutopic endometrium of women with endometriosis precedes the development of disease or results afterwards from development of ectopic lesions. So animal model should next be established to help us to understand and elude how IDO1 participates in the pathophysiology of endometriosis after all. Therefore, this information would be helpful in further investigation on the pathogenesis and therapeutics of endometriosis.
Acknowledgement
This work was supported by Foundation for Talents of Shanghai funded by Shanghai Municipal Human Resources and Social Security Bureau (No. 2009008 to X.-Y.Z.) and Natural Science Foundation of China (No. 81270677 to X.-Y.Z.).
References
- 1.Strathy JH, Molgaard CA, Coulam CB, Melton LJ 3rd. Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril. 1982;38:667–72. doi: 10.1016/s0015-0282(16)46691-4. [DOI] [PubMed] [Google Scholar]
- 2.Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Hung YC, Ueki M. Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 2002;87:3452–9. doi: 10.1210/jcem.87.7.8682. [DOI] [PubMed] [Google Scholar]
- 3.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16:354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palafox D, Llorente L, Alberú J, Torres-Machorro A, Camorlinga N, Rodríguez C, Nyström J. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance in organ transplantation. Transplant Rev (Orlando) 2010;24:160–5. doi: 10.1016/j.trre.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Entrican G, Wattegedera S, Rocchi M, Wheelhouse N. Pregnancy, indoleamine 2,3-dioxygenase (IDO) and chlamydial abortion: an unresolved paradox. Vet Microbiol. 2009;135:98–102. doi: 10.1016/j.vetmic.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–4. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 8.Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S, Nagasaka T, Takikawa O, Kikkawa F. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555–61. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King NJ, Thomas SR. Molecules in focus: Indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–72. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Sedlmayr P, Blaschitz A, Wintersteiger R, Semlitsch M, Hammer A, Mackenzie CR, Walcher W, Reich O, Takikawa O, Dohr G. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod. 2002;8:385–91. doi: 10.1093/molehr/8.4.385. [DOI] [PubMed] [Google Scholar]
- 11.Mei J, Jin LP, Ding D, Li MQ, Li DJ, Zhu XY. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion, invasion of endometrial stromal cells. Mol Hum Reprod. 2012;18:467–76. doi: 10.1093/molehr/gas021. [DOI] [PubMed] [Google Scholar]
- 12.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 13.Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci. 2011;18:229–51. doi: 10.1177/1933719110386241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–44. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10:205–19. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 16.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 17.Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol. 2004;52:306–11. doi: 10.1111/j.1600-0897.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng W, Chen L, Yang S, Han J, Zhai D, Ni J, Yu C, Cai Z. Puerarin Suppresses Proliferation of endometriotic stromal cells partly via the MAPK signaling pathway induced by 17ß-estradiol-BSA. PLoS One. 2012;7:e45529. doi: 10.1371/journal.pone.0045529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makker A, Goel MM, Das V, Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol Endocrinol. 2012;28:175–81. doi: 10.3109/09513590.2011.583955. [DOI] [PubMed] [Google Scholar]
- 20.Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011;26:885–97. doi: 10.1093/humrep/der010. [DOI] [PubMed] [Google Scholar]
- 21.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1α and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-κB Pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–62. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 22.Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V. 1-Methyltryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol. 2006;177:2061–71. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirota Y, Osuga Y, Hirata T, Harada M, Morimoto C, Yoshino O, Koga K, Yano T, Tsutsumi O, Taketani Y. Activation of protease-activated receptor 2 stimulates proliferation and interleukin (IL)-6 and IL-8 secretion of endometriotic stromal cells. Hum Reprod. 2005;20:3547–53. doi: 10.1093/humrep/dei255. [DOI] [PubMed] [Google Scholar]
- 24.Egorina EM, Sovershaev MA, Osterud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–20. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Fujiwara H, Higuchi T, Yoshioka S, Tatsumi K, Maeda M, Fujii S. Involvement of dipeptidyl peptidase IV in extravillous trophoblast invasion and differentiation. J Clin Endocrinol Metab. 2002;87:4287–96. doi: 10.1210/jc.2002-020038. [DOI] [PubMed] [Google Scholar]
- 26.Jung ID, Lee CM, Jeong YI, Lee JS, Park WS, Han J, Park YM. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007;581:1449–56. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 27.Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Yano T, Ayabe T, Tsutsumi O, Taketani Y. Endometrial stromal cells undergoing decidualization down-regulate their properties to produce proinflammatory cytokines in response to interleukin-1 beta via reduced p38 mitogen-activated protein kinase phosphorylation. J Clin Endocrinol Metab. 2003;88:2236–41. doi: 10.1210/jc.2002-021788. [DOI] [PubMed] [Google Scholar]
- 28.Krikun G, Critchley H, Schatz F, Wan L, Caze R, Baergen RN, Lockwood CJ. Abnormal uterine bleeding during progestin-only contraception may result from free radical-induced alterations in angiopoietin expression. Am J Pathol. 2002;161:979–86. doi: 10.1016/S0002-9440(10)64258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta. 2010;1804:463–75. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Lue H, Dewor M, Leng L, Bucala R, Bernhagen J. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell Signal. 2011;23:135–44. doi: 10.1016/j.cellsig.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–70. [PMC free article] [PubMed] [Google Scholar]
- 32.Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE. 2000;2000:re1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Lawson MA, Dantzer R, Kelley KW. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain Behav Immun. 2010;24:201–9. doi: 10.1016/j.bbi.2009.06.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González-Ramos R, Defrère S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–8. doi: 10.1016/j.fertnstert.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Namkung J, Song JY, Jo HH, Kim MR, Lew YO, Donahoe PK, Maclaughlin DT, Kim JH. Mullerian inhibiting substance induces apoptosis of human endometrial stromal cells in endometriosis. J Clin Endocrinol Metab. 2012;97:3224–30. doi: 10.1210/jc.2012-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldwin AS. Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol Rev. 2012;246:327–45. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 37.Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C. The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril. 2008;90:1559–70. doi: 10.1016/j.fertnstert.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Dufournet C, Uzan C, Fauvet R, Cortez A, Siffroi JP, Daraï E. Expression of apoptosis-related proteins in peritoneal, ovarian and colorectal endometriosis. J Reprod Immunol. 2006;70:151–62. doi: 10.1016/j.jri.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhao L, Sun H, Yu J, Li N, Liang J, Wang Y, He M, Bai X, Yu Z, Zheng Z, Mi X, Wang E, Wei M. Gene Silencing of FANCF Potentiates the Sensitivity to Mitoxantrone through Activation of JNK and p38 Signal Pathways in Breast Cancer Cells. PLoS One. 2012;7:e44254. doi: 10.1371/journal.pone.0044254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208–25. doi: 10.1038/sj.neo.7900073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Gambosova K, Cooper ZA, Holloway MP, Kassai A, Izquierdo D, Cleveland K, Boney CM, Altura RA. EGF regulates survivin stability through the Raf-1/ERK pathway in insulin-secreting pancreatic β-cells. BMC Mol Biol. 2010;11:66. doi: 10.1186/1471-2199-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, Zhang B. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–68. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 43.Alessandro R, Kohn EC. Signal transduction targets in invasion. Clin Exp Metastasis. 2002;19:265–73. doi: 10.1023/a:1015547804511. [DOI] [PubMed] [Google Scholar]
- 44.Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arosh JA. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 2008;149:1180–9. doi: 10.1210/en.2007-1168. [DOI] [PubMed] [Google Scholar]
- 45.Collette T, Maheux R, Mailloux J, Akoum A. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum Reprod. 2006;21:3059–67. doi: 10.1093/humrep/del297. [DOI] [PubMed] [Google Scholar]
- 46.Chishima F, Hayakawa S, Sugita K, Kinukawa N, Aleemuzzaman S, Nemoto N, Yamamoto T, Honda M. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am J Reprod Immunol. 2002;48:50–6. doi: 10.1034/j.1600-0897.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee J, Banu SK, Subbarao T, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol. 2011;332:306–13. doi: 10.1016/j.mce.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Chen CC, Cheng YY, Chen SC, Tuan YF, Chen YJ, Chen CY, Chen LC. Cyclooxygenase-2 expression is up-regulated by 2-aminobiphenyl in a ROS and MAPK-dependent signaling pathway in a bladder cancer cell line. Chem Res Toxicol. 2012;25:695–705. doi: 10.1021/tx2004689. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z, Chen M, Liu Y, Zhu Y, Wu J. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. 2011;187:4844–60. doi: 10.4049/jimmunol.1100998. [DOI] [PubMed] [Google Scholar]