Abstract
Small cell neuroendocrine carcinoma of the esophagus (SCNECE) is a very rare, but a highly aggressive tumor. Six cases of SCNECE (0.25%) were found in the 2,438 archival pathologic specimens of esophagus in the last 20 years in our pathology laboratory. The ages ranged from 62 years to 81 years with a mean of 73 years. All cases were male. The presenting symptoms were dysphagia in 5 cases and vomiting in 1 case. The locations were lower esophagus in 4 cases and middle esophagus in 2 cases. Endoscopically, the tumor was ulcerated in 3 cases and polypoid in 3 cases. All the 6 patients were treated by chemoradiation therapy, and the survival ranged from 6 months to 25 months with a mean of 13 months. Histologically, 5 cases were pure SCNECE, 1 case showed triplicate differentiation into small cell carcinoma, adenocarcinoma and squamous cell carcinoma. Immunohistochemically, each SCNECE showed at least one of the neurocrine antigens. Cytokeratins were positive in 6/6, vimentin 0/6, synaptophysin in 4/6, CD56 4/6, neuron-specific enolase 3/6, chromogranin 0/6, p53 protein in 6/6, KIT in 6/6, and platelet-derived growth factor receptor-α (PDGFRA) in 6/6. Ki-67 labeling ranged from 56% to 100% with a mean of 79%. A retrospective genetic analysis using PCR-direct sequencing method in paraffin sections identified no mutations of KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes in all the 6 cases.
Keywords: Esophagus, small cell neuroendocrine carcinoma, KIT, PDGFRA
Introduction
Small cell neuroendocrine carcinoma of the esophagus (SCNECE) is a very rare entity. Several comprehensive studies of SENECE have been reported in the English literature [1-9]. However, there have been no reports of SCNECE investigating protein expression and gene mutations of KIT and platelet-derived growth factor receptor-α (PDGFRA). The author reports herein 6 cases primary SCNECE of with an examination of protein expressions of KIT and PDGFRA and gene status of KIT and PDFRRA genes. KIT and PDGFRA genes, both mapped to 4q12, encode receptor tyrosine kinase oncoproteins called KIT (CD117) and PDGFRA, respectively [10-15]. Both molecules are transmembranous oncoproteins, and play important roles in the carcinogenesis of several tumors such as gastrointestinal stromal tumor (GIST) [10-22].
Materials and methods
The author retrospectively reviewed 2,438 esophageal pathologic specimens in the last 20 years in our pathology laboratory. As the results, six cases of SCNEC (0.25%) were found. Of the six cases, two cases had been reported [8,9] as case reports.
Many 3-μm sections were cut from each paraffin block of these 6 cases, and one of them was stained with HE. The others were subjected to immunohistochemical staining and molecular genetic analysis. The immunohistochemical analysis was performed by Dako Envision methods (Dako Corp, Glostrup, Denmark), as previously reported [23-29]. The antibodies employed were cytokeratins (AE1/3 and polyclonal, Dako, Glostrup, Denmark), synaptophysin (polyclonal, Dako), neuron-specific enolase (polyclonal, Dako), chromogranin (DAK-A3, Dako), CD56 (MOC-1, Dako), p53 protein (DO-7, Dako), Ki-67 antigen (MIB1, Dako), KIT (polyclonal, Dako), and PDGFRA (polyclonal, Santa Cruz, CA, USA).
A molecular genetic analysis of KIT gene (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) gene were performed by the PCR direct sequencing method, as previously reported [16-22]. The exons of both genes were selected because they are frequent mutation sites [10-15]. The primers are shown in Table 1. In brief, genomic DNA was extracted from paraffin blocks with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C for one minute, 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The annealing temperature was 53°C. PCR products were extracted, and subjected to a computed automatic DNA sequencer (ABI PRIZM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA).
Table 1.
Primer sequence
| Forward | Reverse |
|---|---|
| KIT exon 9 | |
| 5’-TCC TAG AGT AAG CCA GGG CTT-3’ | 5’-TGG TAG ACA GAG CCT AAA CAT CC-3’ |
| KIT exon11 | |
| 5’-GAT CTA TTT TTC CCT TTC TC-3’ | 5’AGC CCC TGT TTC ATA CTG AC-3’ |
| KIT exon 13 | |
| 5’-GCT TGA CAT CAG TTT GCC AG -3’ | 5’-AAA GGC AGC TTG GAC ACG GCT TTA-3’ |
| KIT exon 17 | |
| 5’-CTC CTC CAA CCT AAT AGT GT-3’ | 5’-GTC AAG CAG AGA ATG GGT AC-3’ |
| PDGFRA exon12 | |
| 5’-TTG GAT ATT CAC CAG TTA CCT GTC-3’ | 5’-CAA GGG AAA AGC TCT TGG-3’ |
| PDGFRA exon 18 | |
| 5’-ACC ATG GAT CAG CCA GTC TT-3’ | 5’-TGA AGG AGG ATG AGC CTG ACC-3’ |
Results
Clinically, the ages of patients with SENECE ranged from 62 years to 81 years with a mean of 73 years. All patients were male. The presenting symptoms were dysphagia in 5 cases and vomiting in 1 case. The locations of SCNECE were lower esophagus in 4 cases and middle esophagus in 2 cases. Endoscopically, the SCNECE was ulcerated in 3 cases and polypoid in 3 cases. All the 6 patients were treated by chemoradiation therapy, and the prognosis ranged from 6 months to 25 months with a mean of 13 months.
Histologically, 5 cases were pure SCNECE, and 1 case showed triplicate differentiation into small cell carcinoma, adenocarcinoma and squamous cell carcinoma. The pure SENECE was composed of medullary small malignant cells with hyperchromatic nuclei, molded nuclei, fine chromatin, scant cytoplasm, and absent or inconspicuous nucleoli (Figure 1). One SCNECE showed triplicate differentiations into small cell carcinoma (Figure 2A), adenocarcinoma (Figure 2B), and squamous cell carcinoma (Figure 2C). Immunohistochemically, each SCNECE showed at least one of the neuroendocrine antigens. Cytokeratins (Figure 3A) were positive in 6/6, vimentin 0/6, synaptophysin (Figure 3B) in 4/6, CD56 (Figure 3C) 4/6, neuron-specific enolase (Figure 3D) 3/6, chromogranin 0/6, p53 protein (Figure 3E) in 6/6, KIT (Figure 3F) in 6/6, and PDGFRA (Figure 3G) in 6/6. Ki-67 labeling (Figure 3H) ranged from 56% to 100% with a mean of 79%.
Figure 1.
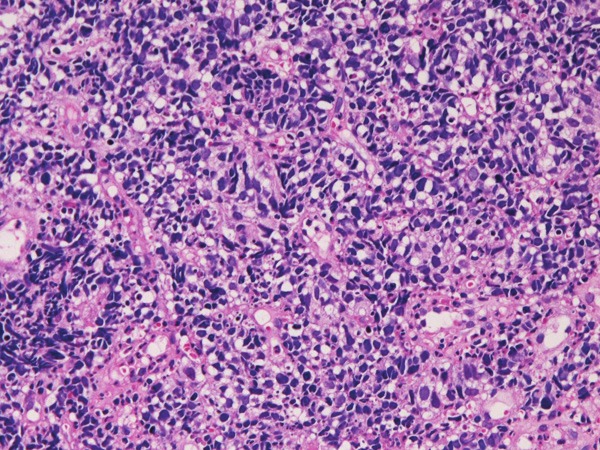
HE histology of small cell neuroendocrine carcinoma of the esophagus. HE, x200.
Figure 2.
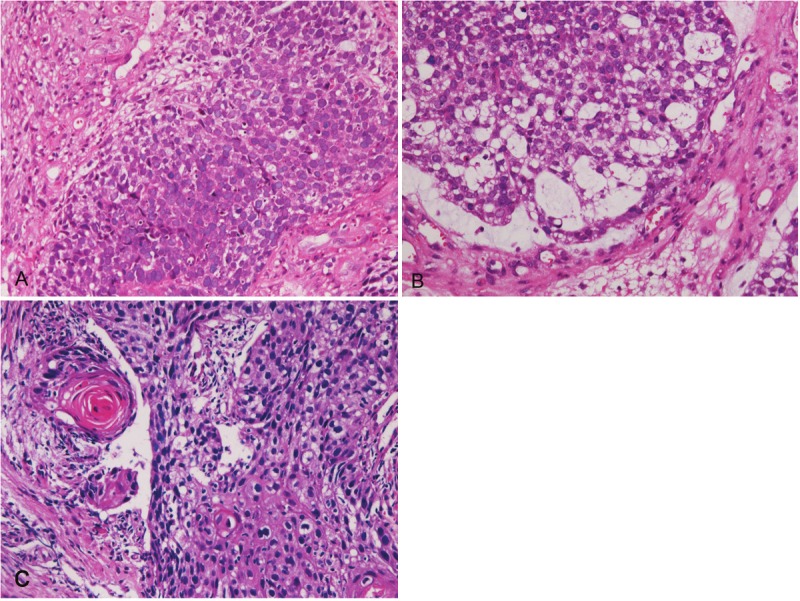
A: Small cell carcinoma area of a caseof esophageal carcinoma with differentiations intosmall cell carcinoma, adenocarcinoma and squamouscell carcinoma. HE, x200. B: Adenocarcinomaarea of a case of esophageal carcinoma with differentiationsinto small cell carcinoma, adenocarcinomaand squamous cell carcinoma. There aretransition between adenocarcinoma and small cellcarcinoma. HE, x200. C: Squamous cell carcinomaarea of a case of esophageal carcinoma with differentiationsinto small cell carcinoma, adenocarcinomaand squamous cell carcinoma. There are transitionbetween squamous cell carcinoma and smallcell carcinoma. HE, x200.
Figure 3.
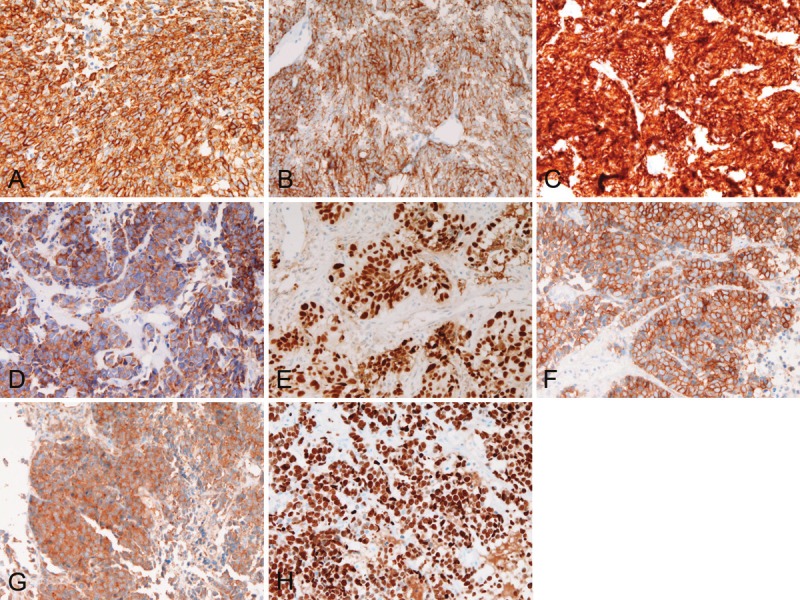
A: Cytokeratins are positive in tumor cells. Immunostaining (AE1/3), x200. B: Synaptophysin is positivein tumor cells. Immunostaining x200. C: CD56 is positive in tumor cells. Immunostaining x200. D: Neuron-specificenolase is positive in tumor cells. Immunostaining, x200. E: p53 protein is positive in tumor cells’ nuclei. Immunostainingx200. F: KIT is strongly positive. Immunostaining, x200. G: PDGFRA is positive. Immunostaining, x200. H:The Ki-67 labeling is 100%. Immunostaining, x200.
A retrospective genetic analysis using PCR-direct sequencing method in paraffin sections identified no mutations of KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes in all the 6 SCNECE cases.
Discussion
In the present study, only 6 cases of SCNECE were found in the 2,438 esophageal specimens; the frequency was 0.25%, suggesting that SCNECE is a very rare tumor. Clinically, the ages of patients with SCNECE ranged from 62 years to 81 years with a mean of 73 years, and all patients were male. This suggests that SCNECE affect mainly old male. In the present series, the presenting symptoms were dysphagia in 5 cases and vomiting in 1 case, similar to esophageal squamous cell carcinoma. In the present series, the locations of SENECS were lower esophagus in 4 cases and middle esophagus in 2 cases, suggesting that SCNECE mainly affects distal esophagus. In the present series, the survival of the patients ranged from 6 months to 25 months with a mean of 13 months, indicating that SCNEC is a very aggressive tumor.
Small cell carcinoma is defined by only HE histology [30]. According to WHO criteria [30], it is defined as a malignant epithelial tumor consisting of small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, and absent or inconspicuous nucleoli. The tumor cells are round, oval and spindle-shaped. Nuclear molding is prominent. Necrosis is typically extensive and mitotic count is high [30]. More than 90% of small cell carcinoma has neuroendocrine features [30]. The present cases fulfill the criteria of small cell carcinoma. The present SCNECE cases had neuroendocrine features. Thus, the present cases are small cell carcinomas in strict criteria.
In the present study, 5 cases were pure SCNECE, 1 case showed triplicate differentiation into small cell carcinoma, adenocarcinoma and squamous cell carcinoma. As to the latter one case, some reports revealed that small cell carcinoma of the esophagus coexisted with squamous cell carcinoma and adenocarcinoma [6,31]. It has been considered that small cell carcinoma may arise from totipotential stem cell with triple differentiations [31]. In the present cases, SCNECE may arise from such totipotential stem cells.
In the present cases, immunohistochemically, cytokeratins were positive in 6/6, vimentin 0/6, synaptophysin in 4/6, CD56 4/6, neuron-specific enolase 3/6, chromogranin 0/6, p53 protein in 6/6, KIT in 6/6, and PDGFRA in 6/6. Ki-67 labeling ranged from 56% to 100% with a mean of 79%. The positivity of cytokeratin and negativity of vimentin indicate the epithelial nature of the present SCNECE. Among the neuroendocrine markers, synaptophysin and CD56 was most sensitive followed by neuron-specific enolase. In the present series, no chromogranin-positive SCNECE was present. The expression of p53 indicates p53 gene mutation. The high Ki-67 labeling shows highly proliferative activity of the present SCNECE cases.
The novel findings in the present study are that immunoreactive KIT and PDGFRA were present in all cases. The present study is the first report of SCNECE that examined KIT and PDGFRA proteins and KIT and PDGFRA genes. KIT and PDGFRA proteins and KIT and PDGFRA genes have rarely been investigated in extrapulmonary small cell carcinoma [32-45], while several comprehensive reports are present in small cell lung carcinoma. KIT has been reported to be expressed in 30-80% of the small cell lung carcinoma [43,46,47]. The present case shows that esophageal SCNECE also expresses KIT protein. Only one study of PDGFRA protein has been reported in small cell lung carcinoma [43], and only two studies of KIT and PDGFRA in SCNECE have been reported [8,9]. The present study showed PDGFRA expression, suggesting that SCNECE expresses this oncoprotein.
The present cases did not identify mutations of KIT and PDFGRA genes. Most reports of small cell lung carcinoma have shown no mutations in KIT genes [43,46], except for Boldrini et al. [47] who found five mutations in 60 small cell lung carcinomas. On the other hand, Terada [43] and Sihto et al. [46] identified no KIT mutations in many cases of small cell lung carcinomas. More studies of KIT mutations remain to be performed in the SCNECE. With regard to PDFGRA mutations, Terada [43] and Sihto et al. [46] found no mutations in many cases of small cell lung carcinomas. Sihto et al. [46] insisted that KIT expression in small cell lung carcinoma is due not to KIT gene mutations but to KIT gene amplification.
Among many KIT-positive tumors, GIST is representative [10-15]. It is thought that GIST arises from interstitial cell of Cajal, a pacemaker neuronal cell that normally expresses KIT protein [10-15]. In contrast, SCNECE is an undifferentiated carcinoma with neuroendocrine phenotypes. The original cell of SCNECE is unknown. Recently, Blumming et al. [48] found that GIST expresses synaptic vesicle proteins, and suggested that GIST has endocrine features. Therefore, it is suggested that there may be an association between GIST and SCNECE in that the both entities have neuroendocrine features.
Several studies of GIST have revealed that there are minute subclinical microGISTs or “GIST tumorlets” in the gastrointestinal tract [49-51]. The incidence of these is about 20%, and these are considered as GIST precursors. Frequent KIT mutations (about 46%) and occasional PDGFRA mutations (about 4%) are present in these “GIST tumorlets” [49-51]. However, these “GIST tumorlets” do not always develop into clinical GIST. Other genetic events are necessary for the development of clinical GIST. In contrast, little is known about the precursor lesions in SENECE.
Recently, the phosphorylation (activation) status of KIT and PDGFRA has been studies [52,53]. This is particularly important in KIT mutation-negative tumors as in the present case. KIT kinase activation and downstream signaling proteins leading to tumorigenesis have been studied, but little is known as yet. Protein kinase C-theta and PI3-kinase/AKT are activated in imatinib-resistant GIST [52,53], and analyses of these KIT signaling molecules may be important in the treatment of GIST. Such studies are not performed in SCNECE. In the present study, the author could not investigate these molecules, because no relevant antibodies were available. KIT tyrosine kinase activity and KIT signaling abnormalities in SCNECE remain to be elucidated.
In summary, the author reported 6 very rare cases of SCNECE with KIT and PDGFRA expressions but without KIT and PDGFRA mutations.
Acknowledgements
This work was approved by Ethics Committee of our hospital.
Conflict of interest statement
The author declares no conflict of interest.
References
- 1.Yun JP, Zhang MF, Hou JH, Tian QH, Fu J, Liang XM, Wu QL, Rong TH. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer. 2007;7:38. doi: 10.1186/1471-2407-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medgyesy CD, Wolff RA, Putnam JB Jr, Ajani JA. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer. 2000;88:262–267. [PubMed] [Google Scholar]
- 3.Bennouna J, Bardet E, Deguiral P, Douillard JY. Small cell carcinoma of the esophagus: analysis of 10 cases and review of the published data. Am J Clin Oncol. 2000;23:455–459. doi: 10.1097/00000421-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Law SY, Fok M, Lam KY, Loke SL, Ma LT, Wong J. Small cell carcinoma of the esophagus. Cancer. 1994;73:2894–2899. doi: 10.1002/1097-0142(19940615)73:12<2894::aid-cncr2820731204>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Ma JY, Yang JJ, Zhao YF, Zhang SF. Primary small cell carcinoma of esophagus: report of 9 cases and review of the literature. World J Gastroenterol. 2004;10:3680–3682. doi: 10.3748/wjg.v10.i24.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto J, Ohshima K, Ikeda S, Iwashita A, Kikuchi M. Primary esophageal small cell carcinoma with concomitant invasive squamous cell carcinoma or carcinoma in situ. Hum Pathol. 2003;34:1108–1115. doi: 10.1053/j.humpath.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Takubo K, Nakamura K, Sawabe M, Arai T, Esaki Y, Miyashita M, Mafune K, Tanaka Y, Sasajima K. Primary undifferentiated small cell carcinoma of the esophagus. Hum Pathol. 1999;30:216–221. doi: 10.1016/s0046-8177(99)90279-4. [DOI] [PubMed] [Google Scholar]
- 8.Terada T. Primary esophageal small cell carcinoma with brain metastasis and with CD56, KIT, and PDGFRA expressons. Pathol Oncol Res. 2012;18:1091–1093. doi: 10.1007/s12253-011-9374-y. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. KIT and PDGFRA in esophageal pure small cell carcinoma. Int J Clin Exp Pathol. 2011;4:718–721. [PMC free article] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplasmic tissue, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 11.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumor. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 12.Losota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diagn Pathol. 2006;23:91–102. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Lasota J. Gastrointestinal stromal tumor: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 14.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shimomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumor. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 15.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumor. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 16.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol. 2011;19:450–453. doi: 10.1097/PAI.0b013e31820d2872. [DOI] [PubMed] [Google Scholar]
- 17.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15:453–456. doi: 10.1007/s10147-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 18.Terada T. Gastrointestinal stromal tumor of the digestive organs: a histopathologic study of 31 cases in a single Japanese institute. Int J Clin Exp Pathol. 2010;3:162–168. [PMC free article] [PubMed] [Google Scholar]
- 19.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 20.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 21.Terada T. Gastrointestinal stromal tumor of the uterus: A case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 22.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 24.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;271:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 25.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 26.Terada T, Takeuchi T, Taniguchi M. Hepatobiliary cystadenocarcinoma with cystadenoma elements of the gall bladder in an old man. Pathol Int. 2003;53:790–795. doi: 10.1046/j.1440-1827.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- 27.Terada T. Ductal adenoma of the breast: Immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 28.Terada T. Gall bladder adenocarcinoma arising in Rokitansky-Schoff sinuses. Pathol Int. 2008;58:806–809. doi: 10.1111/j.1440-1827.2008.02316.x. [DOI] [PubMed] [Google Scholar]
- 29.Terada T. Intraductal tubular carcinoma, intestinal type, of the pancreas. Pathol Int. 2009;59:53–58. doi: 10.1111/j.1440-1827.2008.02325.x. [DOI] [PubMed] [Google Scholar]
- 30.Travis W, Petersen I, Nicholson S, Meyerson M, Hisrch FR, Hanash SM, Pugatch B, Jen J, Geisinger K, Takahashi K, Brambillia E, Fernandez EA, Gazdar A, Capron F. Small cell carcinoma. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. WHO Classification of tumous. Pathology and genetics, Tumours of the lung, pleura, thymus and hear. Ryon: IARC Press; pp. 31–34. [Google Scholar]
- 31.Ho KJ, Herrera GA, Jones JM, Alexander CB. Small cell carcinoma of the esophagus: evidence for a unified histogenesis. Hum Pathol. 1984;15:460–468. doi: 10.1016/s0046-8177(84)80081-7. [DOI] [PubMed] [Google Scholar]
- 32.Terada T. Primary small cell carcinoma of the mediastinum: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2009;26:247–250. doi: 10.1007/s12032-008-9116-5. [DOI] [PubMed] [Google Scholar]
- 33.Terada T. Primary small cell carcinoma of the ureter: A case report involving immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Pathology. 2010;42:101–102. doi: 10.3109/00313020903443018. [DOI] [PubMed] [Google Scholar]
- 34.Terada T. An autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int. 2009;59:247–250. doi: 10.1111/j.1440-1827.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 35.Terada T. Primary small cell carcinoma of the pleura: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol. 2010;27:1119–1122. doi: 10.1007/s12032-009-9345-2. [DOI] [PubMed] [Google Scholar]
- 36.Terada T. KIT-positive primary small cell carcinoma of the endometrium: A case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Arch Gynecol Obstet. 2010;282:413–416. doi: 10.1007/s00404-009-1324-5. [DOI] [PubMed] [Google Scholar]
- 37.Terada T. Large cell neuroendocrine carcinoma with sarcomatous changes of the endometrium: a case report with immunohistochemical studies and molecular genetic study of KIT and PDGFRA. Pathol Res Pract. 2010;206:420–425. doi: 10.1016/j.prp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Terada T. Pulmonary large cell neuroendocrine carcinoma diagnosed in a brain metastasis. Int J Clin Exp Pathol. 2012;5:159–162. [PMC free article] [PubMed] [Google Scholar]
- 39.Terada T. Neuroendocrine carcinoma of the esophagus: a case report with immunohistochemical and molecular genetic analysis of KIT and PDGFRA. Medical Oncology. 2011;28:509–512. doi: 10.1007/s12032-010-9499-y. [DOI] [PubMed] [Google Scholar]
- 40.Terada T. Composite carcinoid and small cell carcinoma of the duodenum. Scand J Gastroenterol. 2010;45:1387–1392. doi: 10.3109/00365521.2010.505661. [DOI] [PubMed] [Google Scholar]
- 41.Terada T. Small cell carcinoma of the ileum that developed 10 years after total gastrectomy for gastric signet ring cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:618–619. doi: 10.1097/PAI.0b013e31823eb34f. [DOI] [PubMed] [Google Scholar]
- 42.Terada T. Small cell neuroendocrine carcinoma of the prostate: Incidence and a report of four cases with an examination of KIT and PDGFRA. Prostate. 2012;72:1150–1156. doi: 10.1002/pros.22464. [DOI] [PubMed] [Google Scholar]
- 43.Terada T. An immunohistochemical and molecular genetic analysis of KIT and PDGFRA in small cell lung carcinoma in Japanese. Int J Clin Exp Pathol. 2012;5:331–338. [PMC free article] [PubMed] [Google Scholar]
- 44.Terada T. Primary small cell carcinoma of the maxillary sinus: a case report with immunohistochemical and molecular genetic study involving KIT and PDGFRA. Int J Clin Exp Pathol. 2012;5:264–269. [PMC free article] [PubMed] [Google Scholar]
- 45.Terada T. Small cell carcinoma of the urinary bladder. Int J Clin Exp Pathol. 2012;5:596–600. [PMC free article] [PubMed] [Google Scholar]
- 46.Sihto H, Sarlomo-Rikara M, Tynnienen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 47.Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camcci T, Lucchi M, Mussi A, Basolo F, Pingitore R, Fontanini G. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;15:4101–4108. doi: 10.1158/1078-0432.CCR-03-0664. [DOI] [PubMed] [Google Scholar]
- 48.Blumming P, Nilsson O, Ahlman H, Welbencer A, Andersson MK, Sjolund K, Nilsson B. Gastrointestinal strumal tumor regularly express synaptic vesicle proteins: evidence of a neuroendocrine phenotype. Endocr Relat Cancer. 2007;14:853–863. doi: 10.1677/ERC-06-0014. [DOI] [PubMed] [Google Scholar]
- 49.Agaimy A, Wunsch PH, Hofstaedter F, Blaszyk H, Rummele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–120. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 50.Kawanoya K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Kanajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Agaimy A, Wunsch PH, Dirnhofer S, Bihl MP, Terracciano LM, Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and interstinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 cases. Am J Surg Pathol. 2008;32:867–873. doi: 10.1097/PAS.0b013e31815c0417. [DOI] [PubMed] [Google Scholar]
- 52.Ou WB, Zhu MJ, Demetri GD, Fletcher CD, Fletcher JA. Protein kinase C-theta regulates KIT expression and proliferation in gastrointestinal stromal tumor. Oncogene. 2008 Sep 18;27:5624–34. doi: 10.1038/onc.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in inatinib-resistant gastrointestinal stromal tumor: PI1-kinase/AKT is a crucial survival pathway. Oncogene. 2007;29:7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]


