Abstract
Polyomavirus BK (BKV)-associated nephropathy causes premature kidney transplant (KT) failure. BKV viruria and viremia are biomarkers of disease progression, but associated risk factors are controversial. A total of 682 KT patients receiving basiliximab, mycophenolic acid (MPA), corticosteroids were randomized 1:1 to cyclosporine (CsA) or tacrolimus (Tac). Risk factors were analyzed in 629 (92.2%) patients having at least 2 BKV measurements until month 12 posttransplant. Univariate analysis associated CsA-MPA with lower rates of viremia than Tac-MPA at month 6 (10.6% vs. 16.3%, p = 0.048) and 12 (4.8% vs. 12.1%, p = 0.004) and lower plasma BKV loads at month 12 (3.9 vs. 5.1 log10 copies/mL; p = 0.028). In multivariate models, CsA-MPA remained associated with less viremia than Tac-MPA at month 6 (OR 0.60; 95% CI 0.36–0.99) and month 12 (OR 0.33; 95% CI 0.16–0.68). Viremia at month 6 was also independently associated with higher steroid exposure until month 3 (OR 1.19 per 1 g), and with male gender (OR 2.49) and recipient age (OR 1.14 per 10 years) at month 12. The data suggest a dynamic risk factor evolution of BKV viremia consisting of higher corticosteroids until month 3, Tac-MPA compared to CsA-MPA at month 6 and Tac-MPA, older age, male gender at month 12 posttransplant.
Keywords: BK virus, cyclosporine, immunosuppression, polyomavirus, risk factor, steroids, tacrolimus, transplantation
Introduction
In the last decade, polyomavirus BK-associated nephropathy (PyVAN) has emerged as significant cause of premature kidney transplant (KT) failure in many transplant centers around the world 1–3. PyVAN rates range from 1% to 10%, and progressive graft failure is seen in more than half of the cases 4–6. In recent analyses of large US databases covering approximately 40 000 KT patients during the period 2003–2006, treatment for BKV was reported in 6.6% during the first 5 years posttransplant and the adjusted risk of graft loss was at least twofold higher compared to unaffected patients 7,8. In the absence of specific antiviral therapy, current treatment relies on reducing immunosuppression using rising plasma BKV loads as a surrogate marker of disease 9–12. This approach can result in good clinical outcomes when performed early posttransplant 13,14. Accordingly, plasma BKV loads are currently recommended for screening and monitoring KT patients with presumptive and proven PyVAN 5,15,16. Despite growing consensus about screening, the risk factors for BKV viremia and nephropathy are not well defined 16. Most likely, nonmodifiable donor and recipient determinants synergize with potentially modulating factors such as immunosuppression 17. In the face of the unchanged seroepidemiology of BKV infection 9,18, increasing use of tacrolimus (Tac) compared to cyclosporine (CsA) has been discussed as a potential factor 19. However, while some studies reported a higher risk of BKV viruria, viremia and/or nephropathy in Tac-treated patients compared to CsA-treated patients 2,20, other studies were unable to identify such relation 11,21. To investigate the impact of the calcineurin inhibitor (CNI) directly, we examined BKV viruria and viremia in more than 600 de novo kidney transplant patients randomized 1:1 to Tac or CsA as part of the Diabetes Incidence after REnal Transplantation: Cyclosporine C2 monitoring versus Tacrolimus (DIRECT) study 22.
Methods
Patients
The DIRECT study is a prospective 6-month, open-label multicenter study with a follow-up visit at month 12 randomizing de novo KT patients to CsA or Tac. The study methodology has been described elsewhere 22. In brief, de novo renal transplant recipients aged 18–70 years (deceased, living-related or living-unrelated donor) were randomized 1:1 to CsA or Tac. Randomization was automated and investigators were notified via an interactive voice response system. The coprimary endpoints of this study were new onset diabetes or impaired fasting glucose, and biopsy-proven acute rejection, graft loss or death 22, and BKV replication was a secondary endpoint (see NCT00171496 at http://ClinicalTrials.gov). The study was performed in 59 transplant centers in 15 countries during October 2003 to March 2005 (see the Appendix). CsA (Neoral®, Novartis Pharma AG, Basel, Switzerland) dose was adjusted targeting C2 ranges: 1400–1800 ng/mL during month 1, 1200–1600 ng/mL during months 2–3 and 800–1200 ng/mL during months 4–6. Tac (Prograf®, Astellas Pharma, Tokyo, Japan) dosing was based on C0 targets: 10–15 ng/mL during months 1–3 and 5–10 ng/mL during months 4–6. All patients received mycophenolic acid (MPA) in the form of mycophenolate mofetil (MMF, Cellcept®, Roche Pharmaceuticals, Basel, Switzerland) or enteric-coated mycophenolate sodium (EC-MPS, myfortic®, Novartis Pharma AG) administered according to local practice, with corticosteroids (intravenous methylprednisolone 500 mg followed by oral prednisone tapered from 100–200 mg/day on day 1 to 5–10 mg/day from month 3 onward). Induction therapy consisted of two 20 mg doses of basiliximab (Simulect®, Novartis Pharma AG) given on days 0 and 4.
Virological analysis
Collection of urine and EDTA blood samples was scheduled at baseline (i.e. pretransplantation or on the day of transplantation) and at months 1, 2, 3, 6 and 12. All samples were frozen at −20°C until analyzed by a quantitative real-time polymerase chain reaction 23 in the Division Infection Diagnostics, University of Basel (STS217 ISO/IEC-17025). BKV viruria was defined as detecting BKV DNA above a diagnostic threshold of 2500 copies/mL, high-level BKV viruria as urine DNA loads of >7 log10 copies/mL 5. BKV viremia was defined as plasma BKV loads above the lower diagnostic limit of detection of 1000 copies/mL, high-level BKV viremia as plasma BKV loads of >4 log10 copies/mL 5.
Statistical analysis
Kaplan–Meier analyses were applied to determine cumulative incidences omitting patients with detectable BKV at baseline. Standard summary statistics were determined for numerical results. Missing samples were not imputed and not included in the analyses. In univariate analyses, we investigated potential determinants of BKV replication including age, gender, race, preexisting diabetes, HLA-mismatches, cold ischemia time, delayed graft function, donor status (living versus deceased), type of dialysis prior to transplantation, CMV status of donor and recipient, recipients’ hepatitis C virus (HCV) status and CNI. Multivariate logistic regression modeling was performed to investigate risk factors of BKV viruria and viremia at months 6 and 12. Odds ratios (OR) were calculated for 10 years of age and for 1 g of cumulative steroid dose, respectively. Cumulative steroid doses >10 g were censored at 10 g to avoid undue influence of exceptional outliers. Binary variables were used for CNI type (CsA vs. Tac), gender (male vs. female), race (white vs. nonwhite), history of diabetes mellitus (yes vs. no), sum of HLA mismatches at loci A, B and DR (>4 vs. ≤ 4) and delayed graft function (yes vs. no). In sensitivity analyses, BKV samples were omitted from analysis if they were acquired after discontinuation of the study medication. All p-values were two-tailed and considered significant at <0.05. Analyses were performed using SAS statistical software version 8.2 (SAS Institute, Cary, NC, USA).
Results
A total of 3213 urine and 3531 plasma samples were obtained from 682 patients at the scheduled time points for BKV DNA testing. No posttransplant sample had been collected in 39 patients, and only one sample in 14 patients, together 53 patients excluded from further analysis. Thus, the BKV study population consisted of 629 kidney transplant recipients (92.2%) for whom a total of 3156 urine (98.2%) and 3465 plasma samples (98.1%) were obtained from at least two visits between months 1 and 6 posttransplant.
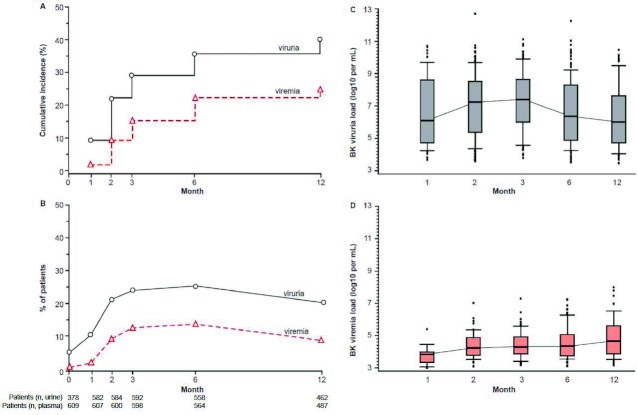
At baseline, BKV viruria was detected in 19 (5.0%) of 378 patients with residual urine production. None of these developed viremia, and only 8 remained viruric posttransplant at a low level of less than 5 log10 copies/mL. Baseline BKV viremia was found in 3 (0.5%) of 609 patients, but none had detectable viruria or viremia posttransplant. Kaplan–Meier estimates showed that the incidence of new onset BKV viruria and viremia at month 12 increased to 39.5% (95% CI 35.4%, 43.5%) and 23.9% (95% CI 20.4%, 27.3%), respectively (Figure 1A). Comparing different time points posttransplant, the highest rates of viruria and viremia were observed at month 6 (25.4% and 13.7%, respectively) which then decreased at month 12 (20.3% and 8.6%, respectively) (Figure 1B). Median urine BKV loads increased from 6.1 log10 copies/mL at month 1 to 7.4 log10 copies/mL at month 3 before declining to 6.0 log10 copies/mL at month 12 (Figure 1C). At that time point, one fourth of the samples (75th percentile) had very high urine viral loads above 8 log10 copies/mL. Plasma BKV loads increased from a median 3.8 log10 copies/mL at month 1 to 4.7 log10 copies/mL at month 12 (Figure 1D). Biopsy-proven acute rejection episodes were more frequent in patients with BKV viremia at month 6 (13.0% vs. 6.1%, p = 0.030) while no statistically significant association was found for viruria. The estimated glomerular filtration rate was not different for patients with or without viruria, but at month 12, viremic patients had a significantly impaired function compared to those without viremia (median GFR 60.4 mL/min [25th percentile 45.6, 75th percentile 78.2] vs. 65.7 mL/min [25th percentile 53.1, 75th percentile 83.5]; p = 0.032).
Figure 1. BKV viruria and viremia after kidney transplantation.
(A) Cumulative new-onset BKV replication posttransplant; (B) point prevalence at the times of testing (patient sample number below); (C) viral load in new-onset BKV viruria posttransplant; (D) viral load in new-onset BKV viremia postransplant
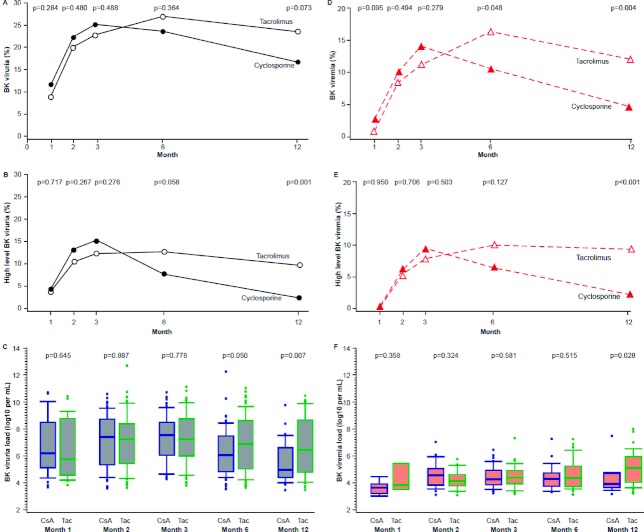
Patients randomized to either CNI arm were found to have similar baseline characteristics, including recipient male gender, white race, mean age, history of diabetes, delayed graft function, living donor and mean HLA mismatch (Table 1A). BKV viruria rates increased up to month 3 without significant differences between CsA- and Tac-randomized patients, but there was a trend toward less viruria among CsA-treated patients at month 12 (Figure 2A). At month 6, fewer patients in the CsA-treatment arm had high-level viruria of >7 log10 copies/mL compared to Tac-treated patients (p = 0.058) reaching statistically significance at month 12 (p = 0.001) (Figure 2B). Of note, median urine BKV loads were sevenfold lower (0.8 log10 copies/mL) in CsA- than in Tac-randomized patients at month 6 (p = 0.050) and approximately 30-fold lower (1.5 log10 copies/mL) at month 12 (p = 0.007; Figure 2C).
Table 1A.
Demographic and baseline determinants in patients with or without BK viruria at months 6 and 12
| Month 6 | Month 12 | |||||||
|---|---|---|---|---|---|---|---|---|
| Viruria | N | n+ (%) | n− (%) | p | N | n+ (%) | n− (%) | p |
| Total | 558 | 142 (25.4%) | 416 (74.6%) | 462 | 94 (20.3%) | 368 (79.7%) | ||
| Recipient age | 0.552 | 0.253 | ||||||
| <40 years | 183 | 47 (25.7%) | 136 (74.3%) | 156 | 25 (16.0%) | 131 (84.0%) | ||
| 40–54 years | 211 | 58 (27.5%) | 153 (72.5%) | 179 | 41 (22.9%) | 138 (77.1%) | ||
| ≥ 55 years | 164 | 37 (22.6%) | 127 (77.4%) | 127 | 28 (22.0%) | 99 (78.0%) | ||
| Gender | 0.965 | 0.747 | ||||||
| Male | 382 | 97 (25.4%) | 285 (74.6%) | 323 | 67 (20.7%) | 256 (79.3%) | ||
| Female | 176 | 45 (25.6%) | 131 (74.4%) | 139 | 27 (19.4%) | 112 (80.6%) | ||
| Race | 0.811 | 0.836 | ||||||
| White | 468 | 120 (25.6%) | 348 (74.4%) | 395 | 81 (20.5%) | 314 (79.5%) | ||
| Nonwhite | 90 | 22 (24.4%) | 68 (75.6%) | 67 | 13 (19.4%) | 54 (80.6%) | ||
| History of DM | 0.760 | 0.704 | ||||||
| Yes | 87 | 21 (24.1%) | 66 (75.9%) | 68 | 15 (22.1%) | 53 (77.9%) | ||
| No | 471 | 121 (25.7%) | 350 (74.3%) | 394 | 79 (20.1%) | 315 (79.9%) | ||
| HLA mismatches | 0.964 | 0.631 | ||||||
| 0 | 23 | 6 (26.1%) | 17 (73.9%) | 18 | 4 (22.2%) | 14 (77.8%) | ||
| 1–3 | 280 | 70 (25.0%) | 210 (75.0%) | 232 | 51 (22.0%) | 181 (78.0%) | ||
| 4–6 | 254 | 66 (26.0%) | 188 (74.0%) | 212 | 39 (18.4%) | 173 (81.6%) | ||
| DGF | 0.883 | 0.429 | ||||||
| Yes | 96 | 25 (26.0%) | 71 (74.0%) | 76 | 18 (23.7%) | 58 (76.3%) | ||
| No | 462 | 117 (25.3%) | 345 (74.7%) | 386 | 76 (19.7%) | 310 (80.3%) | ||
| Donor status | 0.095 | 0.051 | ||||||
| Living | 185 | 39 (21.1%) | 146 (78.9%) | 152 | 23 (15.1%) | 129 (84.9%) | ||
| Deceased | 373 | 103 (27.6%) | 270 (72.4%) | 310 | 71 (22.9%) | 239 (77.1%) | ||
| Dialysis | 0.738 | 0.820 | ||||||
| None | 57 | 15 (26.3%) | 42 (73.7%) | 47 | 11 (23.4%) | 36 (76.6%) | ||
| Hemodialysis | 406 | 100 (24.6%) | 306 (75.4%) | 335 | 66 (19.7%) | 269 (80.3%) | ||
| Peritoneal | 95 | 27 (28.4%) | 68 (71.6%) | 80 | 17 (21.3%) | 63 (78.8%) | ||
| CMV D/R | 0.857 | 0.661 | ||||||
| Neg./neg. | 100 | 29 (29.0%) | 71 (71.0%) | 78 | 16 (20.5%) | 62 (79.5%) | ||
| Neg./pos. | 99 | 26 (26.3%) | 73 (73.7%) | 90 | 22 (24.4%) | 68 (75.6%) | ||
| Pos./neg. | 70 | 19 (27.1%) | 51 (72.9%) | 57 | 9 (15.8%) | 48 (84.2%) | ||
| Pos./pos. | 248 | 61 (24.6%) | 187 (75.4%) | 201 | 42 (20.9%) | 159 (79.1%) | ||
| HCV D/R | 0.704 | 0.881 | ||||||
| Neg./neg. | 543 | 138 (25.4%) | 405 (74.6%) | 450 | 91 (20.2%) | 359 (79.8%) | ||
| Neg./pos. | 11 | 3 (27.3%) | 8 (72.7%) | 10 | 2 (20.0%) | 8 (80.0%) | ||
| Pos./neg. | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Pos./pos. | 2 | 0 | 2 (100.0%) | 1 | 0 | 1 (100.0%) | ||
| Cold ischemia time | 0.168 | 0.062 | ||||||
| N | 141 | 411 | 93 | 364 | ||||
| Median (h) | 14.3 | 12.0 | 15.0 | 12.0 | ||||
| IQR | (0, 35.5) | (0, 30.0) | (0.3, 35.5) | (0, 29.2) | ||||
Figure 2. BKV viruria and viremia rates according to the treatment arm.
(A) BKV viruria; (B) BKV viruria above 7 log10 geq/mL (high-level viruria); (C) urine BKV loads in viruric patients; (D) BKV viremia; (E) BKV viremia above 4 log10 geq/mL (high-level viremia); (F) plasma BKV loads in viremic patients.
Table 1B.
Demographic and baseline determinants in patients with or without BK viruria at months 6 and 12
| Month 6 | Month 12 | |||||||
|---|---|---|---|---|---|---|---|---|
| Viruria | N | n+ (%) | n− (%) | p | N | n+ (%) | n− (%) | p |
| Total | 564 | 77 (13.7%) | 487 (86.3%) | 487 | 42 (8.6%) | 445 (91.4%) | ||
| Recipient age | 0.720 | 0.104 | ||||||
| <40 years | 186 | 23 (12.4%) | 163 (87.6%) | 161 | 10 (6.2%) | 151 (93.8%) | ||
| 40–54 years | 212 | 32 (15.1%) | 180 (84.9%) | 185 | 14 (7.6%) | 171 (92.4%) | ||
| ≥ 55 years | 166 | 22 (13.3%) | 144 (86.7%) | 141 | 18 (12.8%) | 123 (87.2%) | ||
| Gender | 0.937 | 0.052 | ||||||
| Male | 386 | 53 (13.7%) | 333 (86.3%) | 342 | 35 (10.2%) | 307 (89.8%) | ||
| Female | 178 | 24 (13.5%) | 154 (86.5%) | 145 | 7 (4.8%) | 138 (95.2%) | ||
| Race | 0.633 | 0.749 | ||||||
| White | 472 | 63 (13.3%) | 409 (86.7%) | 409 | 36 (8.8%) | 373 (91.2%) | ||
| Nonwhite | 92 | 14 (15.2%) | 78 (84.8%) | 78 | 6 (7.7%) | 72 (92.3%) | ||
| History of DM | 0.732 | 0.230 | ||||||
| Yes | 88 | 11 (12.5%) | 77 (87.5%) | 78 | 4 (5.1%) | 74 (94.9%) | ||
| No | 476 | 66 (13.9%) | 410 (86.1%) | 409 | 38 (9.3%) | 371 (90.7%) | ||
| HLA mismatches | 0.983 | 0.950 | ||||||
| 0 | 24 | 3 (12.5%) | 21 (87.5%) | 19 | 2 (10.5%) | 17 (89.5%) | ||
| 1–3 | 279 | 38 (13.6%) | 241 (86.4%) | 242 | 21 (8.7%) | 221 (91.3%) | ||
| 4–6 | 260 | 36 (13.8%) | 224 (86.2%) | 226 | 19 (8.4%) | 207 (91.6%) | ||
| DGF | 0.292 | 0.718 | ||||||
| Yes | 97 | 10 (10.3%) | 87 (89.7%) | 83 | 8 (9.6%) | 75 (90.4%) | ||
| No | 467 | 67 (14.3%) | 400 (85.7%) | 404 | 34 (8.4%) | 370 (91.6%) | ||
| Donor status | 0.918 | 0.192 | ||||||
| Living | 186 | 25 (13.4%) | 161 (86.6%) | 160 | 10 (6.3%) | 150 (93.8%) | ||
| Deceased | 378 | 52 (13.8%) | 326 (86.2%) | 327 | 32 (9.8%) | 295 (90.2%) | ||
| Dialysis | 0.286 | 0.993 | ||||||
| None | 59 | 12 (20.3%) | 47 (79.7%) | 49 | 4 (8.2%) | 45 (91.8%) | ||
| Hemodialysis | 410 | 53 (12.9%) | 357 (87.1%) | 357 | 31 (8.7%) | 326 (91.3%) | ||
| Peritoneal | 95 | 12 (12.6%) | 83 (87.4%) | 81 | 7 (8.6%) | 74 (91.4%) | ||
| CMV D/R | 0.535 | 0.232 | ||||||
| Neg./neg. | 102 | 18 (17.6%) | 84 (82.4%) | 85 | 7 (8.2%) | 78 (91.8%) | ||
| Neg./pos. | 102 | 15 (14.7%) | 87 (85.3%) | 93 | 13 (14.0%) | 80 (86.0%) | ||
| Pos./neg. | 71 | 7 (9.9%) | 64 (90.1%) | 64 | 6 (9.4%) | 58 (90.6%) | ||
| Pos./pos. | 249 | 34 (13.7%) | 215 (86.3%) | 209 | 14 (6.7%) | 195 (93.3%) | ||
| HCV D/R | 0.511 | 0.500 | ||||||
| Neg./neg. | 547 | 73 (13.3%) | 474 (86.7%) | 474 | 40 (8.4%) | 434 (91.6%) | ||
| Neg./pos. | 13 | 3 (22.1%) | 10 (76.9%) | 11 | 2 (18.2%) | 9 (81.8%) | ||
| Pos./neg. | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Pos./pos. | 2 | 0 | 2 (100.0%) | 1 | 0 | 1 (100.0%) | ||
| Cold ischemia time | 0.845 | 0.965 | ||||||
| N | 76 | 482 | 41 | 441 | ||||
| Median (h) | 12.0 | 12.2 | 11.6 | 12.4 | ||||
| IQR | (0, 35.5) | (0, 30.0) | (0, 24.0) | (0, 35.5) | ||||
N = total number of patients within category; n+ = number of patients with viruria; n− = number of patients without viruria; DM = diabetes mellitus; HLA = human leukocyte antigen; DGF = delayed graft function; CMV = cytomegalovirus serology; HCV = hepatitis C; D/R = donor / recipient; neg. = negative; pos. = positive.
p-values from chi-square tests, for cold ischemia time from Wilcoxon rank-sum tests.
BKV viremia rates increased in both treatment arms over the first 3 months, but then diverged as patients in the CsA-arm had a lower rate of viremia compared to patients in the Tac-arm, both at month 6 (10.6% vs. 16.3%, p = 0.048) and at month 12 (4.8% vs. 12.1%, p = 0.004; Figure 2D). The on-treatment analysis revealed no significant differences in the incidence rates compared to the results presented above, e.g. BKV viremia at months 6 and 12 was 10.9% and 4.4% for the CsA- and 15.3% and 11.7% Tac-arm, respectively. The rate of high-level viremia of more than 10 000 copies/mL (4 log10) was lower in patients randomized to the CsA- than to Tac-arm at month 12 (2.2% vs. 9.4%, p<0.001; Figure 2E). Moreover, median plasma BKV loads were 15-fold (1.2 log10/mL) lower in CsA-MPA than in Tac-MPA treated patients (p = 0.028; Figure 2F).
Other potential determinants of BKV replication were not significantly associated with BKV viruria or BKV viremia, but we noted a trend toward a higher rate of viremia at month 12 for male patients (male: 10.2%, female 4.8%, p = 0.052) and for higher rates of viruria and viremia in deceased donors (Table 1A).
Patients on CsA reached the C2 target range on average by day 15. Patients with viruria at month 6 had slightly lower CsA C2 values at month 3 (median 1053; interquartile range [IQR] 825, 1255) than those without viruria (median 1173; IQR 900, 1500; p = 0.041). Similarly, CsA C2 values at month 6 were lower in viruric patients (median 833, IQR 610, 1016) compared to nonviruric patients (median 934, IQR 697, 1204, p = 0.025). Patients on Tac reached the C0 target range on average by day 11. An association between Tac trough levels and the occurrence of viruria or viremia could not be identified (all p-values >0.05). Since MPA exposure as measured by AUC was not determined in this study, MPA dosing was analyzed. MPA dosing decreased posttransplant in both treatment arms, but the mean dose of MPA was higher in CsA- than in Tac-treated patients at all time points in line with current clinical practice to accommodate lower exposure in CsA-treated patients (p-values < 0.001; Figure 3). MPA-dosing was not different between patients with or without BKV viruria or viremia at month 6 (all p-values > 0.10). To exclude undefined effects of the different CNI–MPA interaction, MPA-dosing was examined separately in patients of either treatment arm. There was no significant difference of MPA dosing in months 4–6 among Tac-MPA treated patients with or without viruria or viremia at month 6.
Figure 3.
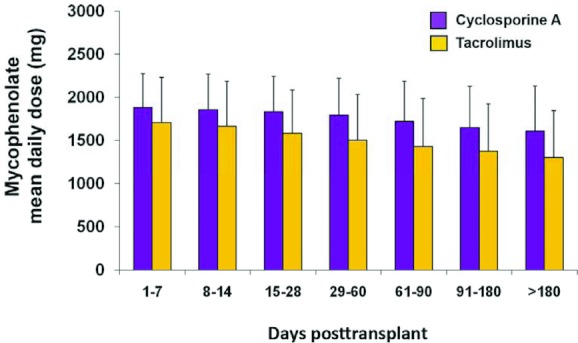
Mycophenolate dosing over time posttransplant by calcineurin inhibitor.
Examining the role of steroids, we found no association with BKV viruria, but BKV viremia was significantly associated with a higher cumulative steroid exposure until month 1 having a median of 1470 mg (IQR 995 mg, 1808 mg) compared to 1250 mg (IQR 870 mg, 1655 mg) for patients without viremia (p = 0.031; Table 2). This difference persisted up to month 6 suggesting that corticosteroid exposure was an important modulator of the risk of BKV replication early posttransplant.
Table 2.
Cumulative dose of corticosteroids in patients with or without BK viruria and viremia at month 6
| Viruria at month 6 | Viremia at month 6 | ||||||
|---|---|---|---|---|---|---|---|
| Period1 | Viruria | No viruria | p | Viremia | No viremia | p | |
| ≤Month 1 | Median | 1241 | 1274 | 0.572 | 1470 | 1250 | 0.031 |
| (Q1, Q3) | (930, 1689) | (888, 1678) | (995, 1808) | (870, 1655) | |||
| ≤Month 2 | Median | 1831 | 1795 | 0.409 | 1924 | 1770 | 0.017 |
| (Q1, Q3) | (1370, 2318) | (1330, 2205) | (1476, 2409) | (1313, 2203) | |||
| ≤Month 3 | Median | 2161 | 2112 | 0.403 | 2315 | 2100 | 0.013 |
| (Q1, Q3) | (1625, 2791) | (1616, 2642) | (1815, 3009) | (1606, 2643) | |||
| ≤Month 6 | Median | 3094 | 2898 | 0.404 | 3158 | 2897 | 0.021 |
| (Q1, Q3) | (2180, 3785) | (2160, 3593) | (2549, 4217) | (2145, 3585) | |||
Cumulative dose in mg determined from transplantation up to end of the respective month.
Q1 = first quartile; Q3 = third quartile.
p-values from Wilcoxon rank-sum tests.
In the multivariate logistic regression model, BKV viruria was not significantly associated with any of the variables (Table 3). Investigating high-level viruria, however, randomization to CsA-MPA treatment significantly decreased the risk compared to Tac-MPA at month 6 (OR = 0.56, 95% CI 0.31, 0.99; p = 0.047) and month 12 (OR = 0.21, 95% CI 0.08, 0.57; p = 0.002). The cumulative steroid dose was still significant at month 6 (per 1 g higher: OR = 1.26), but not at month 12 (Table 3). For BKV viremia at month 6, CsA-MPA remained an independent factor decreasing risk compared to Tac-MPA (OR = 0.60, p = 0.044), while higher cumulative steroid dose increased the risk (per 1 g higher: OR = 1.19 per 1 g; p = 0.017). For BKV viremia at month 12, CsA-MPA was associated with decreased risk (OR of 0.33; p = 0.003). BKV viremia was independently associated with male gender (OR = 2.49; p = 0.038) and increasing age (OR = 1.41 per 10 years; p = 0.013; Table 3). For high-level viremia at month 12, CsA-MPA remained significant (OR = 0.19; p = 0.001), whereas age, gender or steroids were not.
Table 3.
Results from logistic regression analyses for occurrence of BK viruria and viremia at months 6 and 12 posttransplant dependent on the CNI type and other potential risk factors
| Viruria (n = 558) | Viremia (n = 564) | |||||
|---|---|---|---|---|---|---|
| Month 6 | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| CNI (CsA vs. Tac) | 0.85 | (0.57, 1.24) | 0.394 | 0.60 | (0.36, 0.99) | 0.044 |
| Agea | 0.99 | (0.85, 1.16) | 0.928 | 1.14 | (0.94, 1.40) | 0.187 |
| Gender (male vs. female) | 1.00 | (0.66, 1.51) | 0.992 | 1.03 | (0.61, 1.74) | 0.920 |
| Race (white vs. nonwhite) | 1.04 | (0.60, 1.81) | 0.892 | 0.69 | (0.35, 1.34) | 0.272 |
| History of DM (yes vs. no) | 1.10 | (0.63, 1.92) | 0.724 | 1.32 | (0.64, 2.72) | 0.449 |
| HLA mismatches (>4 vs. < = 4) | 0.88 | (0.55, 1.40) | 0.581 | 1.21 | (0.66, 2.21) | 0.544 |
| DGF (yes vs. no) | 0.98 | (0.59, 1.64) | 0.947 | 1.62 | (0.79, 3.32) | 0.192 |
| Cumulative steroid dose2 | 1.09 | (0.96, 1.24) | 0.197 | 1.19 | (1.03, 1.38) | 0.017 |
| Viruria (n = 462) | Viremia (n = 487) | |||||
|---|---|---|---|---|---|---|
| Month 12 | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| CNI (CsA vs. Tac) | 0.64 | (0.40, 1.03) | 0.065 | 0.33 | (0.16, 0.68) | 0.003 |
| Age1 | 1.19 | (0.98, 1.44) | 0.076 | 1.41 | (1.07, 1.84) | 0.013 |
| Gender (male vs. female) | 1.12 | (0.67, 1.86) | 0.661 | 2.49 | (1.05, 5.89) | 0.038 |
| Race (white vs. nonwhite) | 0.91 | (0.46, 1.81) | 0.791 | 0.75 | (0.29, 1.96) | 0.556 |
| History of DM (yes vs. no) | 1.00 | (0.52, 1.92) | 0.994 | 2.52 | (0.83, 7.65) | 0.103 |
| HLA mismatches (>4 vs. < = 4) | 1.16 | (0.65, 2.05) | 0.618 | 1.49 | (0.64, 3.46) | 0.349 |
| DGF (yes vs. no) | 0.84 | (0.46, 1.54) | 0.579 | 0.80 | (0.34, 1.86) | 0.602 |
| Cumulative steroid dose2 | 1.08 | (0.93, 1.25) | 0.322 | 1.09 | (0.88, 1.35) | 0.424 |
Odds ratio represents an increment of 10 years.
Cumulative steroids dose up to month 3, odds ratio represents an increment of 1 g.
OR = odds ratio; CI = confidence interval; CNI = calcineurin inhibitor; CsA = cyclosporine; Tac = tacrolimus; DM = diabetes mellitus; HLA = human leukocyte antigen; DGF = delayed graft function defined as a decrease in serum creatinine ≤ 20% or the need for at least one dialysis in the first 3 days posttransplantation.
Discussion
This prospective, randomized, multicenter study provides the largest systematic analysis of BKV viruria and viremia after kidney transplantation using a predefined protocol of immunosuppression. The results demonstrate that reactivation of BKV replication is common with BKV viruria reaching a cumulative incidence of 39.5% (95% CI 35.4%, 43.5%) by 12 months posttransplant. One-fourth of KT patients developed high-level viruria of >7 log10 copies/mL, a molecular equivalent of urinary decoy cell shedding, as well as viremia, both biomarkers of an increasing risk of progression to PyVAN 9,24. Plasma BKV loads >4 log10 copies/mL were observed in 16% of KT patients, thereby fulfilling the working definition of presumptive PyVAN for which judicious reduction of immunosuppression is currently recommended 15,16. The rates are in line with a smaller prospective study of 78 patients by Hirsch et al. reporting Kaplan–Meier estimates of high-level viruria (decoy cells) of 30% (95% CI 20–40%) and viremia of 13% (95% CI 5–21%) 9, as well as with results by Brennan et al. 2005 11 and Ginveri et al. 2007 13.
Randomizing more than 600 patients 1:1 to either CsA or Tac on a common backbone of basiliximab induction, MPA and prednisone provided an unprecedented large sample size associating CsA-MPA with a significantly lower rate of viremia than Tac-MPA, both at month 6 (10.6% vs. 16.3%, p = 0.048) and at month 12 (4.8% vs. 12.1%, p = 0.004). Furthermore, median plasma BKV loads were 10-fold higher at month 12 in Tac-MPA compared to CsA-MPA treated patients and high-level BKV viremia of >4 log10 copies/mL was significantly more frequent. In the first 3 months, however, the rate of BKV replication did not significantly differ between patients randomized to CsA or Tac suggesting that differences between the CNIs did not play out early, but during the second half of the first year posttransplant, when most cases of PyVAN had been previously diagnosed 1,4,6. Multivariate analysis confirmed the reduced risk of CsA-MPA treated patients for BKV viremia at months 6 and 12 compared to Tac-MPA, and identified higher steroid exposure in the first 3 months as an independent cofactor for high-level viruria and viremia. This association is of interest since it provides a rationale for the early onset of BKV replication, independent of the choice of CNI. Indeed, pulse-steroids had been identified as independent risk factor for high-level viruria (decoy cells), viremia and PyVAN 9. Dadhania and colleagues reported that steroid maintenance therapy was associated with BKV replication 25. The BKV-promoting effect of steroids likely is the result of both, activating BKV early gene expression via glucocorticoid response elements in the viral noncoding control region 26 and its immunosuppressive effect. This synergy of virus activation and immunity inactivation is also documented in the poor outcome of polyomavirus-associated nephropathy almost a decade ago when the disease was erroneously treated as acute cellular rejection 1,2. The results of our study further suggest that following protocol-driven corticosteroid dose tapering from month 3 onward, the choice CNI exerted a greater influence on BKV replication rates. Of note, male gender and older recipient age were identified as independent risk factors for BKV viremia at month 12. Both patient determinants have been reported previously as being associated with PyVAN in some single-center studies 4,27 as well as in the large UNOS/OPTN registry analyses 7,8.
Brennan et al. 11 randomized 200 KT patients in a ratio of 2:1 to either Tac or CsA that was combined with either azathioprine or MPA 11. While the CNI per se was not found to influence the overall rates of BKV viruria and viremia in that study, there was a trend for an increased rate of sustained viremia in Tac- versus CsA-treated patients (p = 0.10). Similar to our results, BKV viruria was more frequent among Tac-MPA versus CsA-MPA treated patients (46% vs. 13%; p = 0.005). The rates of viremia in Tac-MPA versus CsA-MPA treated patients were similar to our study (13% vs. 4%), but without reaching statistical significance which may reflect their smaller sample size of 88 patients only in these comparator arms 11 compared to 629 patients reported here.
The difference between both Tac-MPA and CsA-MPA regarding BKV has previously been attributed to the potential influence of a higher overall immunosuppressive burden of Tac-containing regimens. In this study, CsA was targeted according C2 monitoring providing optimal exposure early posttransplant reaching median concentrations of 885 ng/mL at month 6, while tacrolimus was standard-dosed with predefined tapering reaching trough levels of 8 ng/mL at month 6. Under these conditions, the primary efficacy endpoints were found to be comparable including biopsy-proven acute rejection 22. The significant association of BKV viremia with patients randomized to Tac may also be influenced by MPA exposure, but frequent and possibly more precise pharmacokinetic measurements were not performed 28. On the other hand, representative time points posttransplant are not defined for comprehensive AUC measurements. This particularly concerns the question when to expect effects on BKV viremia, since pharmacokinetic measurements only provide data as a point prevalence that are not necessarily representative of concurrent, cumulative or subsequent effects. In our study, however, MPA dosing, decreased posttransplant and was, at all time points, significantly lower in patients randomized to Tac compared to CsA, in agreement with common routine practice to reduce differences in exposure (Figure 3). Of note, we found no difference in MPA doses between viruric or nonviruric, or between viremic and nonviremic patients within either CNI treatment group at month 6. Although screening was not yet widely recommended and practiced during the study period 15,29, we cannot exclude that treatment for BKV viremia might have occurred and thereby shortened viremia duration in some patients. However, even with proactive reduction of immunosuppression, the median duration of BKV viremia is long ranging from 2.9 to 8.8 months 14,30. Given this long duration, we consider it unlikely that potential screening and treatment of some centers might have significantly changed the results.
Agent-specific mechanisms may also play a role in the different clinical adverse event profile of Tac and CsA as evidenced by changes in glucose or lipid metabolism 22. Although Tac and CsA both inhibit the calcineurin phosphatase required for interleukin-2 expression in T-lymphocytes subsequent to T cell receptor activation, they have different molecular targets: CsA binds to cyclophilins while tacrolimus binds to FK-binding protein 12. Interestingly, in vitro studies indicate that CsA and MPA inhibit BKV replication 31–33, whereas Tac activates BKV replication via FK-binding protein 12 in primary human tubular epithelial cells 34. The “net state of immunosuppression” coined by Rubin and Fishman 35 may be used to also integrate net effects on virus replication by different drugs as well as quantitative and qualitative differences of virus-specific T cell repertoire in the individual transplant recipients 36. Clearly, at high doses of immunosuppressive drugs, BKV-inhibitory effects of CsA, mTOR inhibitors and MPA may not play out and immunosuppressive effects predominate. As dosing is lowered posttransplant, e.g. at 3 months onward, however, drug-specific differences may become apparent and BKV-activating effects as described for corticosteroids and Tac may increase the risk over drugs with BKV-inhibitory effects such as CsA, mTOR inhibitors and MPA, at an otherwise appropriate maintenance immunosuppression for a given patient–allograft combination. The role of differential direct activating and inhibiting viral effects of immunosuppressive drugs like tacrolimus versus cyclosporine, MPA and mTOR inhibitors are currently emerging, together with their differences in immunosuppressive action. The improved understanding direct drug mechanisms on infectious agents and the immune system will stimulate more specific clinical studies that allow to better evaluate the competing risks of rejection and infection in future personalized transplantation medicine.
In conclusion, CsA is associated with a significantly lower risk than Tac regarding BKV viremia at months 6 and 12 in de novo kidney transplant patients treated with basiliximab, MPA and steroids. Steroids appear as an independent risk factor for BKV viremia early posttransplant, whereas male gender and older age contribute later in the first year posttransplant, respectively. Together, these data support the hypothesis of a dynamic risk evolution across multiple factors including the choice of the CNI, thereby potentially influencing the screening after 6 months posttransplant and the management of patients at higher risk for BKV viremia and progression to nephropathy.
Acknowledgments
The DIRECT Study Group was designed, overseen and implemented, whereas the sponsor (Novartis Pharma) had not influence. The participants are as follows:
Juan Jose Almenabar, Hospital de Cruces, Barakaldo, Spain; Amado Andres, Hospital 12 de Octubre, Madrid, Spain; Lászió Asztaios, University of Debrecen, Debrecen, Hungary; William Bennett, Good Samaritan Hospital, Portland, USA; François Berthoux, Hôpital Nord, Saint Priest en Jarez, France; Roy Bloom, University of Pennsylvania Medical Center, Philadelphia, USA; Kenneth L. Brayman, University of Virginia Health System, Charlottesville, USA; Laura Buist, Glasgow Western Infirmary, Glasgow, UK; Jesus Bustamante, Hospital Universitario de Valladolid, Valladolid, Spain; Josep Campistol, Hospital Clinic de Barcelona, Barcelona, Spain; Carl Cardella, Toronto General Hospital, Toronto, Canada; M. Castagneto, Istituto di Clinica Chirurgica Centro Trapianti Policlinico Gemelli, Rome, Italy; Domingo del Castillo, Hospital Reina Sofia de Cordoba, Cordoba, Spain; Arun Chandrakantra, University of Alabama, Birmingham, Birmingham, USA; Dr. Cotterell, VCU Health Systems Medical College of Virginia, Richmond, USA; Mohammed El-Shahawy, University of Southern California Kidney Transplant Program, Los Angeles, USA; Josette Eris/Steve Chadban, Royal Prince Alfred Hospital, Camperdown, Australia; Pedro Errasti, Clinica Universitari de Navarra, Pamplona, Spain; A. Famulari, Centro Trapianti di Rene-Ospedale Civile S. Salvatore, Aquila, Italy; Styrbjörn Friman, Enheten för transplantation och leverkirurgi, Gothenburg, Sweden; Jorge Garces, Ochsner Clinic Foundation Kidney Transplant Program, New Orleans, USA; Reginald Gohh, Rhode Island Hospital, Providence, USA; Peter Gross, Medizinische Klinik III/Nephrologie, Dresden, Germany; Marcus Hart, University of California-San Diego, San Diego, USA; Andrew House, London Health Sciences Center, London, ON, Canada; Ashley Irish, Renal Unit Royal Perth Hospital, Perth, Australia; Jeno Jarai, Semmelweis University, Budapest, Hungary; Trond Jenssen, Rikshospitalet, Oslo, Norway; Dr. Johnston, University of Kentucky Transplant Center; Lexington, USA; Anil Kapoor, McMaster University, St. Joseph's Healthcare, Urology Institute, Hamilton, Canada; Marian Klinger, Klinika Nefrologii, Wroclaw, Poland; Gregory Knoll, The Ottowa Hospital, Ottowa, Canada; Ricardo Lauzurica, Hospital Universitari Trias I. Pujol, Badalona, Spain; Christophe Legendre, Hôpital Necker, Paris, France; Jimmy Light, Washington Hospital Center, Washington, USA; Arnost Martinek, Faculty Hospital Ostrava, Ostrava, Czech Republic; Robert Mendez, St. Vincents MC/NIT, Los Angeles, USA; Hans-H Neumayer, Medizinische Klinik mit Schwerpunkt Nephrologie, Berlin, Germany; Barbara Nonnast-Daniel, Medizinische Klinik IV, Erlangen, Germany; Leszek Paczek, Klinika Immunologii, Transplantologii I Chorob Wewnetrznych, Warsaw, Poland; Ravi Parasuraman, Henry Ford Hospital, Detroit, USA; Mark D. Pescovitz; Indiana University, Indianapolis, USA; Thomas Pearson, Emory University Hospital, Atlanta, USA; Lionel Rostaing, Hôpital Rangueil, Toulouse, France; Graeme R. Russ, The Queen Elizabeth Hospital, Woodville, Australia; Ernst Scheuermann, Medizinische Klinik IV, Frankfurt, Germany; G. Segoloni, Divisione di Nefrologia Dialisi e Trapianti Presidio Molinette, Torino, Italy; Craig Shadur, Iowa Methodist Medical Center, Iowa, USA; Jean Paul Soulilou, Hôpital Hôtel Dieu, Nantes, France; V. Sparacino, Unita Medica di Trapianto, Ospedale Civico G. di Cristina M. Ascoli, Palermo, Italy; Gunter Stein, Klinik für Innere Medizin III, Universitätsklinik Jena, Jena, Germany; Pal Szenohradszy, University of Szeged, Szeged, Hungary; Jean Tchervenkov, Royal Victoria Hospital, Montreal, Canada; Richard Thistlethwaite, University of Chicago, Chicago, USA; Murat Tuncer, Akdeniz U. Medical School, Antalya, Turkey; Aydin Turkmen, Istanbul Medical School, Istanbul, Turkey; Kazuharu Uchida, Nagoya Daini Red Cross Hospital, Nagoya City, Japan; Flavio Vincenti, UCSF Kidney Transplant Service, San Francisco, USA; Andrzej Wiecek, Klinika Nefrologii Immunologii Transplantologii I Chorob Wewnetrznych, Warsaw, Poland.
We are indebted to Dr. Mark D. Pescovitz who has participated in the design of this study as a member of the scientific advisory board. Dr. Pescovitz died in a tragic accident December 3, 2010, after he had been communicating his comments to this manuscript to H.H.H.
Glossary
- BKV
BK virus
- CsA
cyclosporine-A
- CNI
calcineurin inhibitor
- DIRECT
Diabetes Incidence after REnal Transplantation Cyclosporine C2 monitoring versus Tacrolimus
- GFR
glomerular filtration rate; HLA, human leukocyte antigen
- HCV
hepatitis C virus
- KT
kidney transplant
- MMF
myophenolate mofetil
- MPA
mycophenolic acid; PyVAN, polyomavirus-associated nephropathy
- Tac
tacrolimus
Disclosure
The DIRECT study was funded by Novartis Pharma AG. The manuscript was prepared by H. H. H. All coauthors contributed to the interpretation of the data and the final manuscript. The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation with regard to Novartis: F.C.—No conflicts to disclose for this manuscript; S.F.—Advisory boards for Novartis; H.H.H.—Advisory Boards for Novartis; M.K.—No conflicts to disclose for this manuscript; M.P.—past advisory Boards for Novartis; H.P.—Statistician, retired employee of Novartis Pharma; G.R.R.—Advisory Boards for Novartis; E.-H.S.—No conflicts to disclose for this manuscript; M.T.—Travel grants from Novartis Pharma; F.V.—Received grant/research from Pfizer, Astellas, Novartis, Bristol-Myers Squibb; A.W.— No conflicts to disclose for this manuscript.
References
- 1.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103–109. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 2.Binet I, Nickeleit V, Hirsch HH, et al. Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918–922. doi: 10.1097/00007890-199903270-00022. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: State of affairs. Transplantation. 2009;87:621–630. doi: 10.1097/TP.0b013e318197c17d. [DOI] [PubMed] [Google Scholar]
- 4.Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145–2151. doi: 10.1097/01.asn.0000023435.07320.81. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 6.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN) Am J Transplant. 2006;6(5 Pt 1):1025–1032. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 7.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–1026. doi: 10.1097/TP.0b013e31819cc383. [DOI] [PubMed] [Google Scholar]
- 8.Schold JD, Rehman S, Kayler LK, Magliocca J, Srinivas TR, Meier-Kriesche HU. Treatment for BK virus: Incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22:626–634. doi: 10.1111/j.1432-2277.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 10.Nickeleit V, Klimkait T, Binet IF, et al. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med. 2000;342:1309–1315. doi: 10.1056/NEJM200005043421802. [DOI] [PubMed] [Google Scholar]
- 11.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 12.Viscount HB, Eid AJ, Espy MJ, et al. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation. 2007;84:340–345. doi: 10.1097/01.tp.0000275205.41078.51. [DOI] [PubMed] [Google Scholar]
- 13.Ginevri F, Azzi A, Hirsch HH, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7:2727–2735. doi: 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 14.Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615–2623. doi: 10.1111/j.1600-6143.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasiske BLZM, Craig JC, Ekberg H, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch HH, Randhawa P. BK virus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S136–S146. doi: 10.1111/j.1600-6143.2009.02904.x. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 18.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 19.Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: Evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6(5 Pt 2):1111–1131. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 20.Manitpisitkul W, Drachenberg C, Ramos E, et al. Maintenance immunosuppressive agents as risk factors for BK virus nephropathy: A case–control study. Transplantation. 2009;88:83–88. doi: 10.1097/TP.0b013e3181aa8d93. [DOI] [PubMed] [Google Scholar]
- 21.Ramos E, Drachenberg CB, Portocarrero M, et al. BK virus nephropathy diagnosis and treatment: Experience at the University of Maryland Renal Transplant Program. Clin Transplant. 2002:143–153. [PubMed] [Google Scholar]
- 22.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch HH, Mohaupt M, Klimkait T. Prospective monitoring of BK virus load after discontinuing sirolimus treatment in a renal transplant patient with BK virus nephropathy. J Infect Dis. 2001;184:1494–1495. doi: 10.1086/324425. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 24.Funk GA, Gosert R, Comoli P, Ginevri F, Hirsch HH. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am J Transplant. 2008;8:2368–2377. doi: 10.1111/j.1600-6143.2008.02402.x. [DOI] [PubMed] [Google Scholar]
- 25.Dadhania D, Snopkowski C, Ding R, et al. Epidemiology of BK virus in renal allograft recipients: Independent risk factors for BK virus replication. Transplantation. 2008;86:521–528. doi: 10.1097/TP.0b013e31817c6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosert R, Rinaldo CH, Funk GA, et al. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J Exp Med. 2008;205:841–852. doi: 10.1084/jem.20072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamash HA, Wadei HM, Mahale AS, et al. Polyomavirus-associated nephropathy risk in kidney transplants: The influence of recipient age and donor gender. Kidney Int. 2007;71:1302–1309. doi: 10.1038/sj.ki.5002247. [DOI] [PubMed] [Google Scholar]
- 28.Arns W, Breuer S, Choudhury S, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant. 2005;19:199–206. doi: 10.1111/j.1399-0012.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 29.Koukoulaki M, Grispou E, Pistolas D, et al. Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis. 2009;11:1–10. doi: 10.1111/j.1399-3062.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 30.Funk GA, Steiger J, Hirsch HH. Rapid dynamics of polyomavirus type BK in renal transplant recipients. J Infect Dis. 2006;193:80–87. doi: 10.1086/498530. [DOI] [PubMed] [Google Scholar]
- 31.Li YJ, Weng CH, Lai WC, et al. A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation. 2010;89:299–306. doi: 10.1097/TP.0b013e3181c9b51c. [DOI] [PubMed] [Google Scholar]
- 32.Acott PD, O'Regan PA, Lee SH, Crocker JF. In vitro effect of cyclosporin A on primary and chronic BK polyoma virus infection in Vero E6 cells. Transpl Infect Dis. 2008;10:385–390. doi: 10.1111/j.1399-3062.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 33.Acott P, O'Regan PA, Crocker JF. Suppression of early and chronic BK polyoma virus replication by mycophenolic acid in Vero cells. Transpl Int. 2009;22:225–231. doi: 10.1111/j.1432-2277.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch HH, Min L, Wernli M. Polyomavirus BK (BKV) replication in renal tubular epithelial cells is inhibited by mTOR inhibitors, but activated by tacrolimus in a pathway involving the FKBP12 (Abstract #497; Accepted for Oral Presentation at the 11th ATC 2011) Am J Transplant. 2011:11–179. [Google Scholar]
- 35.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 36.Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant. 2008;13:569–574. doi: 10.1097/MOT.0b013e3283186b93. [DOI] [PubMed] [Google Scholar]