Abstract
Neuromodulatory systems such as noradrenaline (NE), acetylcholine (ACh) and serotonin (5HT) serve important functions in sensory perception. We use the olfactory bulb (OB) as a model system to study the roles of individual neuromodulators in sensory perception. Using a spontaneous, non-reward motivated detection task, as well as a reward-motivated task, we show that rats can easily respond to odorants at very low concentrations when motivated to do so in a food rewarded task, despite not showing spontaneous responses to these low concentration odorants. Using the same tasks paired with local bulbar infusions of noradrenergic and cholinergic drugs, we then show that rats engage their noradrenergic, but not their cholinergic system to better respond to near threshold odorants. These results suggest that while cholinergic modulation of OB function is mostly important for odor decorrelation and discrimination, noradrenergic modulation is important for signal-to-noise modulation.
Keywords: Olfaction, neuromodulation, olfactory bulb, noradrenaline, acetylcholine
Introduction
Neuromodulatory systems such as noradrenaline (NE), acetylcholine (ACh) and serotonin (5HT) serve important functions in sensory perception. Classically, each has been linked to specific functions such as improvement of neuronal signal to noise ratios, attentional processes, general arousal, learning and consolidation (reviewed in (Yu & Dayan, 2005). Each neuromodulator acts upon neurons in a variety of brain regions through a host of specific receptors with mechanisms including, but not limited to, membrane depolarization, modulation of network properties, changes in oscillatory dynamics, changes in synchronization, signal-to-noise ratio modulation, network excitability and plasticity. These effects have been linked to alterations in sensory response magnitudes via altered signal to noise ratios or changes in the temporal precision between afferent input and postsynaptic responses (for review see (Deco & Thiele, 2009; Hurley, Devilbiss, & Waterhouse, 2004; Noudoost & Moore, 2011). At the network level, neuromodulation of cellular properties leads to lowering of sensory thresholds, refinement of receptive fields, changes in oscillatory dynamics and synchronization, and increased plasticity. The overall effects of different modulators in a specific sensory system can be very similar and dissociating their respective functions is challenging.
We here use the olfactory bulb (OB) as a model system to study the roles of individual neuromodulators in sensory perception. The OB receives neuromodulatory inputs from all major systems except dopamine (DA), which is intrinsic to the OB. In adult rodents, noradrenergic inputs to the OB have been shown to be implicated in discrimination between perceptually similar odorants, as well as detection of very low concentration odorants (Doucette, Milder, & Restrepo, 2007; Escanilla, Arrellanos, Karnow, Ennis, & Linster, 2010; Guerin, Peace, Didier, Linster, & Cleland, 2008; Mandairon et al., 2008). Cholinergic modulation of OB processing has been shown to be involved in perceptual functions such as discrimination between similar odorants, short term memory, pro-active interference and perceptual habituation (Chaudhury, Escanilla, & Linster, 2009; De Rosa, Hasselmo, & Baxter, 2001; Linster & Cleland, 2002; Linster, Garcia, Hasselmo, & Baxter, 2001; Mandairon et al., 2006; Ravel, Elaagouby, & Gervais, 1994). At a neural level, both NE and ACh modulate cellular properties of OB mitral and granule cells (Castillo, Carleton, Vincent, & Lledo, 1999; Hayar, Heyward, Heinbockel, Shipley, & Ennis, 2001); in addition, ACh excites inhibitory interneurons in the glomerular layer of the OB (Castillo et al., 1999; Ravel, Akaoka, Gervais, & Chouvet, 1990). As a consequence, experimental data so far suggest very similar functions for both modulatory systems in odor perception, i.e. modulation of odor discrimination. Based on our previous results, we here show, for the first time, a dissociation of their respective functions using two olfactory behavioral tasks testing odorants near response threshold.
We first confirm that rats respond to odorants at very low concentrations when motivated to do so in a food rewarded task, despite not showing spontaneous responses to these low concentration odorants in a habituation/dishabituation task. We then show that activation of bulbar NE, but not ACh receptors modulates spontaneous responses to low concentration odorants. In addition, blockade of NE, but not ACh receptors decreases reward-motivated responses to these low concentration stimuli. These results, together with previous results, strongly suggest that while cholinergic modulation of OB function is mostly important for odor decorrelation and discrimination, noradrenergic modulation is important for signal-to-noise modulation.
Methods
Behavioral experiments
The behavioral experiments performed tested odor detection thresholds by using either an unrewarded habituation/cross habituation paradigm (Experiments 1a, 2; 4) or a rewarded go/no-go discrimination paradigm (Experiments 1b; 3). Behavioral experiments were performed in rats in which cannulae had been surgically introduced into the OBs to allow infusion of noradrenergic and cholinergic drugs during behavioral testing.
Subjects
A total of fifteen male Sprague-Dawley rats, 200–250- grams, obtained from Charles Rivers Laboratories (Wilmington, MA) were used. Rats were kept in standard laboratory cages (46x24 cm) on a 12 hr light/dark cycle at a constant temperature. Rats were tested during the last six hours of the dark period. Water was continuously available and rats were food deprived to keep them at approximately 85% of their ad lib weight. All experiments were carried out under a protocol approved by the Cornell University Institutional Animal Care and Use Committee in accordance with NIH guidelines. Experiments proceeded in the following order: Experiment 1a (n=15), Experiment 1b (n=14), Experiment 2a (n=7), Experiment 3a (n=13), Experiment 2b (n=10), Experiment 3b (n=9), Experiment 4 (n=9). In all experiments all rats used were run once under each treatment condition.
Cannulation
Rats were anesthetized with an intramuscular injection of 50 mg/kg ketamine and 7.5 mg/kg xylazine (in a volume of 1mL/kg) and secured in a stereotaxic instrument (David Kopf Instruments, Tujunga, California). Guide cannula (22- gauge; Plastics One, Roanoke, VA) were inserted bilaterally for infusions into both OBs at the following coordinates with respect to Bregma: AP: +8.0 mm; ML, +/−1.5 mm; DV, −4.5 mm. The tips of the guide cannula were positioned 1 mm dorsal to the target infusion site; consequently, infusion cannula extended 1 mm from the end of the guide cannula and were positioned to be in the middle of the OB. Four screws were drilled in the skull, and dental cement was used to secure the guide cannula and cover the incision area. Dummy infusion cannula were then placed into the guide cannula to prevent blockage or infection. Following surgery, rats were allowed to recover for 10 days.
Drug Administration
In Experiment 2, rats were infused with either 1 mM of norepinephrine (NE), 50 µM of the non-specific cholinergic agonist carbachol (CCh), or vehicle (0.9 % saline) to test how increasing noradrenergic or cholinergic modulation in the OB affects responses to low concentration odors. The chosen dosage of CCh, lower than dosages used for direct infusions in other brain areas, was the highest dose that did not evoke seizures in the rats. In experiment 3, rats were infused either with a combination the non-selective α receptor antagonist phentolamine (10 mM) and the non-selective β receptor antagonist alprenolol (120 mM), a combination of the nicotinic receptor antagonist methyllycaconitine citrate hydrate (MLA, 19.0 mM) and the muscarinic receptor antagonist scopolamine (38.0 mM) or saline, to test if blockade of noradrenergic or cholinergic bulbar receptors affects rewarded responses at low odor thresholds. Drugs were dissolved in 0.9% saline and prepared fresh every day; concentrations were based on results from previous experiments (Escanilla et al., 2010; Guerin et al., 2008; Mandairon et al., 2006; Mandairon et al., 2008). For drug administration, two infusion cannulae were fitted into the guide cannulae so that their tips protruded 1.0 mm beyond the end of the guide cannula into the center of each OB. Two 10 µL Hamilton syringes containing either drug solutions or vehicle (0.9% saline) were attached to the cannula with a polyethylene tube and driven with paired infusion pumps (YA-12 Genie pumps, Kent Scientific, Torrington, CT). Drugs were delivered bilaterally into awake rats at a rate of 2 µL/min for 3 minutes (6 µL total volume delivered per side). The 6 µl infusion volume was determined in previous studies to diffuse throughout the main and accessory OBs without spill over to neighboring brain areas (Chaudhury et al., 2009; Mandairon et al., 2006). The infusion cannulae remained in place for 1 additional minute after the infusion ended in order to minimize backflow. Behavioral testing was performed 20 minutes after drug administration was completed. Drugs were coded after dilution in order to blind the experimenters to the identity of the drugs.
Odor sets
To allow for repeated testing under all drug conditions without repeatedly testing rats on the same odorants, 15 odorants were used at four dilutions each, corresponding to vapor phase partial pressures of 10−5, 10−4 and 10−2 Pa. Because our focus here was the modulation of responses to very low concentration odorants, these odor concentrations are substantially lower than those used in previous experiments in our lab. Odors and their relative dilutions (% volume) to obtain 1 Pa vapor partial pressure were: Methyl salicylate (3.48%), Decanal (1.76%), +/−terpenine-4ol (6.63%), propionic acid (0.04%, +/− carvone (4.72%), ethyl acetate (0.002%), butanal (0.002%), octanoic acid (13.74%), hexanal (0.022%), neryl acetate (0.23%), acetic acid (0.008%), pentyl butyrate (0.57%), 1-nonanol (0.632%), butyl hexanoate (1.62%). In experiment 4, acetic acid (0.0078%), propionic acid (0.04%) and butyric acid (0.13%) were used. Odors were first diluted to obtain approximately 1 Pa vapor partial pressure, left to equilibrate for two days and then further diluted to the desired vapor partial pressures on day three. Vapor pressures of pure odorants were estimated using ACD ChemSketch software and variously diluted in mineral oil to concentrations theoretically emitting the same partial pressure over each odorant. A formula weight estimate of 335 g/mol for mineral oil (Jefo Nutrition, Inc.) was used for mole fraction calculations. Matching vapor partial pressure does not guarantee similar subjective intensities for odorants; however, given the limitations of these approaches in animal subjects, matching these in addition to counterbalancing odor presentations across animals and conditions is the best approach.
Behavioral testing
Unrewarded habituation/detection task
To test how odor response thresholds are affected by motivation and noradrenergic and cholinergic modulation, animals were subjected to a habituation experiment designed to test response thresholds in the absence of reward motivation as well as a rewarded forced choice discrimination task using the same odorants (Escanilla et al., 2010). The habituation task requires no prior shaping and was performed as follows (Figure 1A&B): rats were first subjected to three 50-s presentations of mineral oil (MO) at five-min intertrial intervals (ITIs), followed by a single 50-sec presentation of a test odorant (Odor) at a range of concentrations (10−2, 10−4 or 10−5 Pa). The amount of time that the rat spent actively sniffing within 1 cm of the odor source was measured using a stopwatch and regarded as active investigation. A significant increase in active investigation time to the presentation of odor indicates successful detection.
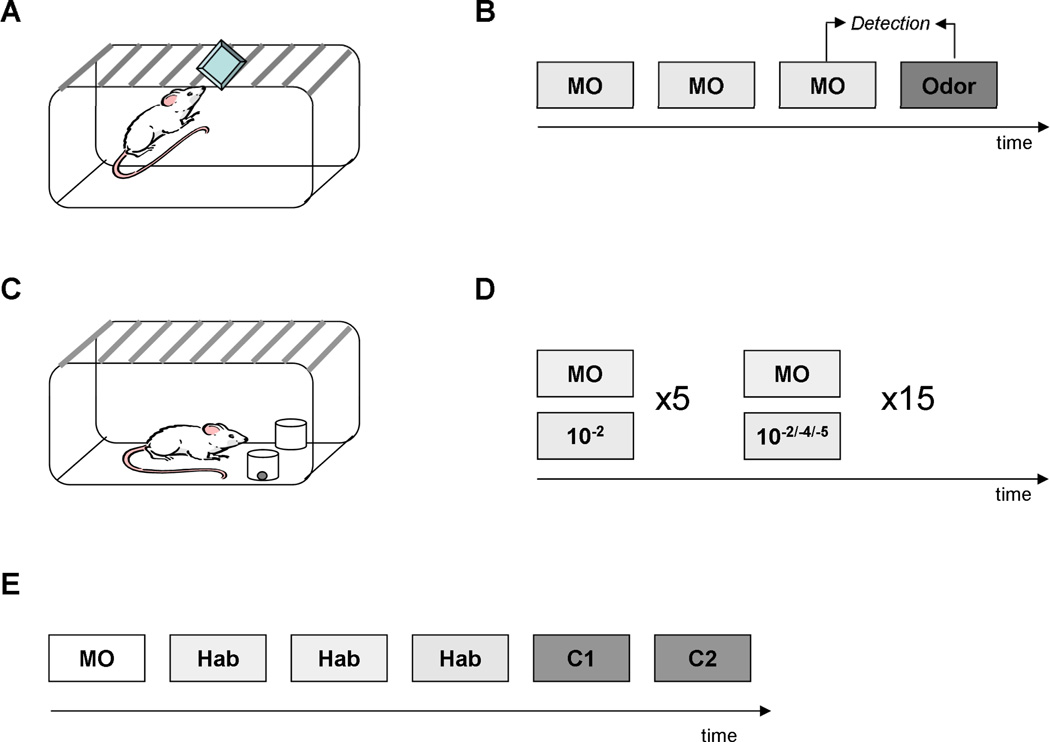
Figure 1. Behavioral methods.
A. Spontaneous detection task. Rats were presented with odorants in their homecage. A weighing dish with a scented filter paper was put on top of the cage and the time rats spent investigating the odor was recorded. B. Rats were presented with mineral oil (MO) during three trials separated by 5 min ITIs. During the 4th trial an odor diluted to 10−2, 10−4 or 10−5 Pa was presented. If rats investigated the odorant significantly more than the MO during the 3rd presentation, it was assumed that they were able to detect the odorant. C. Reward motivated odor detection. Rats were first shaped to retrieve a sucrose pellet from a dish filled with bedding. D. During detection testing, they were presented with a choice of a rewarded dish scented with an odorant at 10−2 Pa and an unrewarded, unscented dish, for five trials. During the remaining 15 trials, the scented odor was randomly presented at 10−2, 10−4 and 10−5 Pa. The dish in which the rat looked for the reward was recorded. E. CCh control experiment. Rats were habituated in a setup similar to A. They were first presented with a an unscented filter paper, then during three trials with a straight chain aliphatic odorant diluted to 1 Pa. During trials five and six, odorants of the same functional group differing by one or two carbons from the habituated odor were presented in random order. If the rats investigated the novel test odors significantly more than the habituated odor it was assumed that they discriminated between the two odorants.
All habituation experiments took place in the home cages of the test animals under red light (Figure 1A). All odors were diluted in mineral oil and stored at 5° C. Odors were presented by placing 60 µl of the odor stimulus onto a filter paper disc (Whatman #1) contained within a weighing dish that was placed on top of the wire cage lid. This procedure enabled the observer to change the odor stimulus without unduly disturbing the animal.
Rewarded forced-choice detection task
For the forced-choice detection task, rats were shaped to dig for a reward in a dish filled with bedding, as described previously (Cleland, Morse, Yue, & Linster, 2002; Linster & Hasselmo, 1999). During testing, rats were presented with an unrewarded, unscented dish as well as a rewarded, scented dish in a customized rat cage (Figure 1C, D). During the first five trials, rats learned to dig for the reward in the scented dish at an odor concentration known to be easily detected (10−2 Pa). During the next 15 trials, odors were presented at 10−2, 10−4 and 10−5 Pa in random sequence to test for responses to these low concentrations (Figure 1D). The dishes were evenly filled with 50 cm3 of bedding, after which 50 µl of diluted odorant was applied to the top of the bedding in the center of the dish. Another 50 cm3 of bedding was then added to bury the odorant within the bedding. During each trial, dishes were positioned randomly and the dish in which the rat dug first was recorded.
In Experiment 1, designed to test the effect of reward motivation on odor response thresholds, we first tested rats’ responses using the unrewarded habituation task and subsequently tested the same rats using the rewarded forced choice task. In the habituation task, rats were tested on odor concentrations equivalent to 10−2, 10−4 and 10−5 Pa; on each day a given rat was tested on a single odor concentration in increasing order (Experiment 1a). Three test odors (methyl salicylate, decanal, terpenine-4ol) were counterbalanced such that each rat was tested in a different order. To test how reward motivation affects the responses to these low concentration odorants, the reward motivated forced choice task was used (Experiment 1b) in the same rats, with only 10 trials at a fixed concentration. Each rat was tested once with a randomly chosen odorant from the list on Experiment 1a. Within a daily session they first trained to respond to 10−2 Pa of a given odor and the tested on 10−2, 10−4 and 10−5 Pa of that odor five times each in randomized order.
Experiment 2 tested if increasing cholinergic or noradrenergic modulation in the OB affects odor response thresholds. The noradrenergic effects repeated a subset of previously published experiments showing that introducing additional NE into the OB lowers odor detection thresholds. These experiments were repeated here to allow direct comparison to the effect of increasing cholinergic modulation. Rats were tested using the unrewarded habituation/detection task. In
In Experiment 2a, on each day, rats were infused with either vehicle (0.9 % saline) or NE (1mM ). In Experiment 2b, rats were infused with either vehicle (0.9% saline) or CCh (50 uM). In both experiments, each rat was tested on each drug condition and each odor concentration. A rat saw one of six odors on a given day in a counterbalanced order (hexanal, neryl acetate, acetic acid, pentyl butyrate, 1-nonanol, butyl hexanoate). Odor concentrations were presented in ascending order; for each concentration half the rats were tested on saline first and half the rats on the drug.
Experiment 3 was designed to test if rats engage their noradrenergic or cholinergic modulatory systems when motivated to respond to low odor concentrations in a food rewarded task. Rats were tested using the reward-motivated task; in Experiment 3a they were infused either with vehicle (0.9% saline) or a combination the non-selective α receptor antagonist phentolamine (10 mM) and the non-selective β receptor antagonist alprenolol (120 mM); in Experiment 3b they were infused with either vehicle (0.9% saline) or a combination of the nicotinic receptor antagonist methyllycaconitine citrate hydrate (MLA, 19.0 mM) and the muscarinic receptor antagonist scopolamine (38.0 mM). Rats were tested on a specific odor/drug combination on each day and the order of drug and odors was randomized among rats. No rat was tested on a given odor more than once for this experiment. Because each rat was tested twice for each experiment (Saline or antagonist), two odors were used in these experiments (carvone, ethyl acetate). The two experiments were separated in time to prevent interference with previous learning.
Experiment 4 served as a positive control for the effect of CCh in the OB. Rats were tested in a task previously shown to be modulated by increasing cholinergic modulation by using the acetylcholinesterase inhibitor neostigmine. A modified version of the habituation/detection task was used in which rats were first habituated to MO during a single trial, then habituated to a straight chain aliphatic odorant (acetic acid) during four trials separated by five min ITIs and then tested on novel odorants either one carbon (propionic acid) or two carbons (butyric acid) removed from the habituated odorant (Figure 1E). Because this task replicated a previous task (Chaudhury et al., 2009), structurally related odorants were used to test effects on perception of similar odorants, whereas in Experiments 1–3 structurally unrelated odorants were used to prevent interference between odorsets. Rats were infused with either vehicle (0.9 % saline) or CCh (50 uM), half the rats were tested under saline first and half under CCh.
Data analysis
Data analyses were performed using SPSS statistical software (SPSS, Chicago, IL) with the time spent investigating the odor stimuli during presentation trials or numbers of correct trials as the dependent variables. Outlier trials that deviated from the mean by more than two standard deviations were excluded from analysis in order to exclude trials during which rats were distracted by other sensory stimuli. Less than 5% of the total trials were excluded as outliers. For non-rewarded habituation/detection, analyses of variance (ANOVA) were performed with trial number (detection: last MO4 and O) as the within-subjects factor and drug treatment and odor concentration as a between-subjects factor to assess detection. For rewarded forced-choice detection, analysis of variance with experimental group as main effect was performed on the number of correct trials per session.
Results
Rats respond to odorants at concentrations lower than their spontaneous response threshold when motivated (Experiment1; Figure 2).
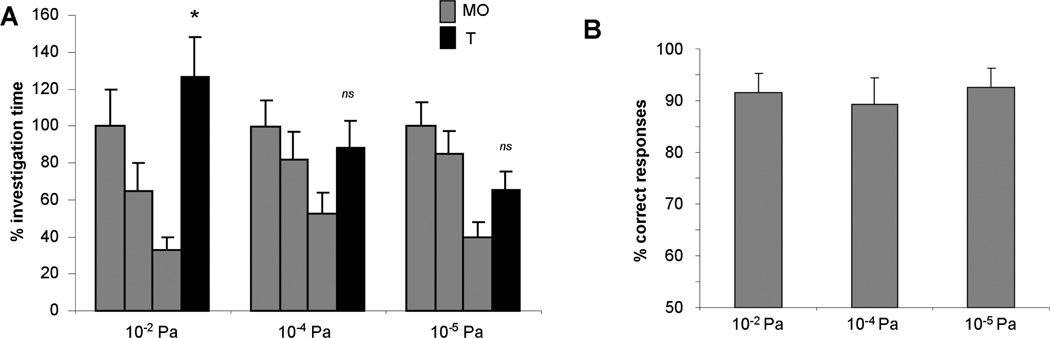
Figure 2. Spontaneous versus rewarded detection of low concentration odorants.
A. Spontaneous detection. Rats habituated to mineral oil only investigated odorants at 10−2, but not 10−4 or 10−5 Pa significantly more than the last presentation of mineral oil. Asterisks indicate a significant increase as compared to the last habituation trial. B. When motivated to find a reward in low concentration odorants, rats performed equally well on all concentrations used, showing that they are capable of responding to these low concentration odorants. The graphs show the average percent of investigation time as compared to the first habituation trial for all three concentrations (A) as well as the average percentage of correct choices made in the reward motivated detection task for all three concentrations (B).
Experiment 1a
We first tested rats’ spontaneous, non-motivated, odor response thresholds using a habituation-dishabituation paradigm. Rats were first presented with mineral only for three trials separated by five minute ITIs followed by a single presentation of an odorant diluted to approximately 10−2, 10−4 or 10−5 vapor partial pressure. As in our previous experiments (Escanilla et al., 2010), rats responded to odors diluted to 10−2, but not 10−4 or 10−5 Pa. ANOVA showed a significant effect of trial (F(4, 39) = 22.344, p < 0.001), as well as a significant interaction between trial and concentration (F(8, 80) = 2.571, p = 0.015) indicating that the response to the test odor significantly depended on odor concentration. Further posthoc testing (student t-test) showed that rats responded significantly more to the odor presentation than to the last habituation trial only at the highest dilution, 10−2 Pa (p < 0.02). This shows that at 10−4 and 10−5 Pa dilutions, rats do not spontaneously indicate detection of odorants by change in investigation time; these results are in agreement with our previous findings (Figure 2A).
Experiment 1b
We tested if rats can respond to these low odor concentrations when motivated to do so in a forced choice task. Rats could easily respond to odorants at all three dilutions. ANOVA using the number of correct trials as measurement showed no effect of concentration (F (2, 35) = 0.137, p = 0.872); rats performed at around 90% correct on all three concentrations. These data show that when motivated, rats are easily able to discriminate odors from a non-scented dish at these low odor concentrations. (Figure 2B).
Additional activation of noradrenergic, but not cholinergic bulbar receptors lowers spontaneous response thresholds (Experiment 2; Figure 3). Previous experiments showed that spontaneous response thresholds can be significantly lowered by activation of NE receptors in the OB (Escanilla et al., 2010). Because this effect could simply be due to excitation of mitral cells, and hence should be possible by multiple means, we here tested if activation of cholinergic receptors, via infusions of the cholinergic agonist carbachol (CCh) would also lower spontaneous odor response thresholds. As shown previously, infusions of NE (1 mM) bilaterally into the OB increased odor responses to low concentration stimuli as compared to saline infused control rats.
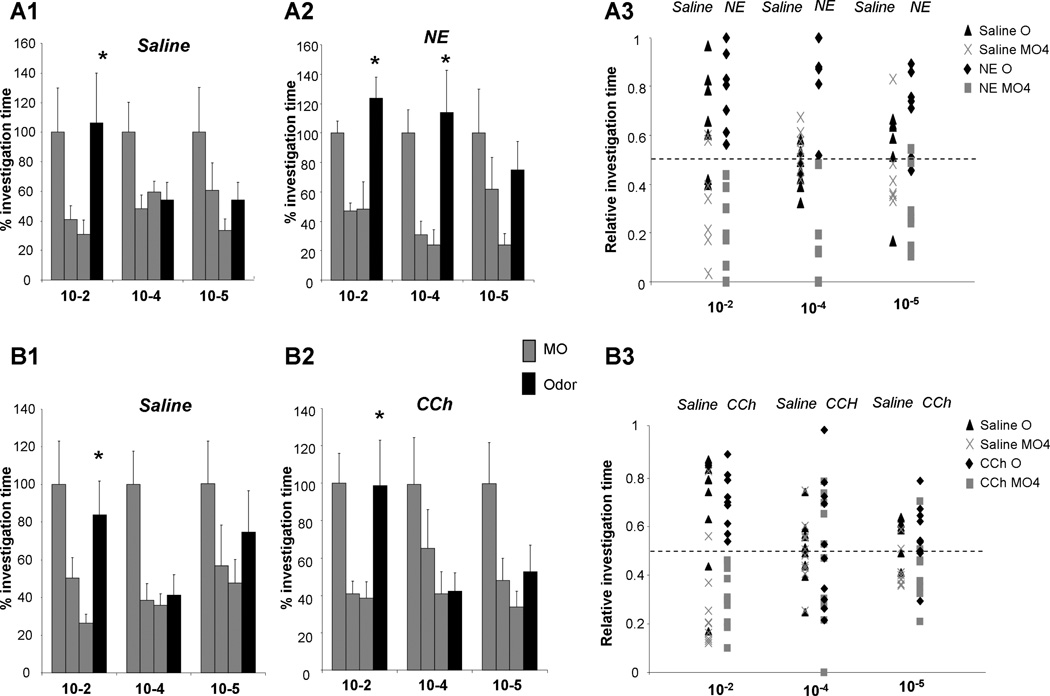
Figure 3. Infusion of bulbar NE but not ACh modulates responses to low concentration odorants.
A1. Saline infused rats significantly responded to odorants at 10−2 Pa dilution only. A2. Rats with 1mM NE infused into the OB responded to odorants diluted to 10−2 and 10−4 Pa. B1. Saline infused control rats significantly responded to odorants at 10−2 Pa dilution only. B2. Similarly, rats infused with CCh responded to odorants at 10−2 Pa dilution only. The graphs show the percent of investigation time as compared to the first habituation trial during habituation to mineral oil (MO) and novel odor detection (Odor), for each odor concentration and drug group. Asterisks indicate a significant increase in response level as compared to the last habituation trial. A3&B3. The graphs show the relative investigation time of the odor (O; O/(O+MO4) or the last mineral oil trial (MO4; MO4/(MO4+O) for all three odor concentrations, treatment groups and individual rats. Relative investigation times around 0.5 indicate that rats investigated the odor to the same degree than the MO, odor investigation times clustered above 0.5 indicate that rats investigated the odor more strongly than MO.
Experiment 2a (NE infusion)
ANOVA testing showed a significant overall effect of trial (F(3, 32) = 23.666; p <0.001), as well as a significant interaction between trial and odor concentration (F(6, 64) = 2.894; p = 0.015) and a significant interaction between trial and drug treatment (saline or NE; (F(3, 32) = 5.723; p = 0.03). This shows that NE treatment changed rats’ spontaneous responses to low concentration odorants. Specifically, saline treated rats responded significantly more to the test odor than to the last MO presentation only at the highest concentration tested (p < 0.02), whereas NE treated rats responded significantly at the highest and second highest concentration tested (p < 0.001 and p < 0.02). Habituation per se, when analyzed alone without the odor response trial, was not affected by drug (Fgroup*trial (2, 119) = 2.985; p > 0.05).
Experiment 2b (CCh infusion)
ANOVA shows a significant effect of trial (F(3, 51) = 25.145; p < 0.001) as well as a significant interaction of trial with concentration (F(6, 102) = 2.556; p = 0.024), but not drug (saline, CCh; F(3, 51) = 0.094; p > 0.9). This shows that rats’ responses to the test odor were significantly influenced by odor concentration but not by drug treatment, unlike in the case of NE treatment. Both saline and CCh treated rats responded significantly more to the test odor at the lowest concentration only (p < 0.01 and p < 0.05). Habituation alone, analyzed without the odor response trial, was not significantly affected by CCh infusions (Fgroup*trial(2, 173) = 0.088; p > 0.8). These data show that not every class of bulbar neuromodulatory transmitters modulates spontaneous responses to low concentration stimuli.
Motivated response to low concentration odorants is impaired when NE, but not ACh receptors in the OB are blocked (Experiment 3; Figure 4). We then asked if rats engage their NE (Experiment 3a) or ACh (Experiment 3b) systems when motivated to detect low concentration odorants by blocking receptors in the OB during the rewarded detection task. Rats infused with noradrenergic antagonists were impaired in this task, especially at the lower odor concentration (Figure 3a).
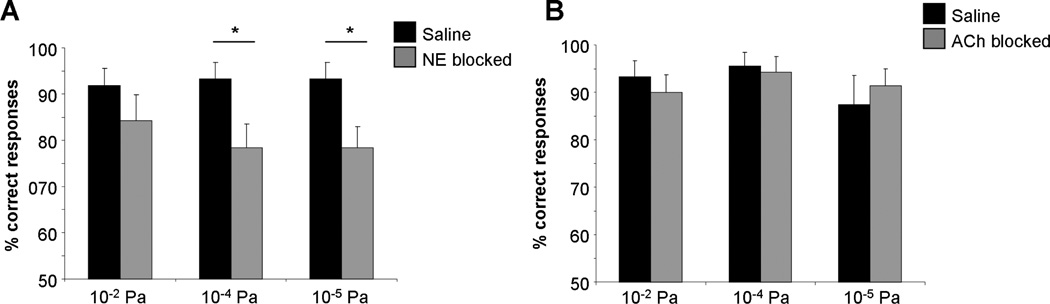
Figure 4. Blockade of bulbar NE (A), but not ACh (B) receptors decreases reward motivated odor responses.
A. Rats with NE receptors blocked are significantly impaired at responding to odors diluted to 10−4 and 10−5 Pa. B. Rats with ACh receptors blocked behave similarly to saline infused rats. The graphs show the average percent of correct responses to each odor concentration for each drug group. * indicates a response that is significantly different from saline.
Experiment 3a
Saline infused control rats and rats infused with NE blockers performed similarly during the first five training trials using 10−2 Pa odors (F(1, 25) = 0.802; p > 0.3), indicating that NE blockers did not impair learning of the task. For the remaining 15 trials, during which odors were presented at 10−2, 10−4 and 10−5 Pa in randomized order, ANOVA showed a significant effect of drug (F (1,71) = 10.8, p = 0.002) but not concentration (F(2, 71) = 0.155, p > 0.8). Rats with NE receptors blocked were significantly impaired at 10−4Pa (p = 0.034) and 10−5 Pa (p =0.024) but not 10−2 Pa (p > 0.2) as compared to saline infused rats. These data show that rats use NE modulation when motivated to respond to very low concentration odorants.
Experiment 3b
Saline infused control rats and rats infused with ACh blockers performed similarly during the first five training trials using 10−2 Pa odors (F(1, 18) = 0.160; p > 0.6), indicating that ACh blockers did not impair learning of the task. For the remaining 15 trials, during which odors were presented at 10−2, 10−4 and 10−5 Pa in randomized order ANOVA showed no effect of drug (F(1, 40) = 0.004; p > 0.9) or concentration (F(2, 39) = 0.823; p > 0.4), showing that blockade of cholinergic receptors did not affect rats’ motivated responses to low concentration odorants.
Cholinergic receptor activation can modulate olfactory bulb processing. As a positive control for the effects of CCh on OB processing, we infused CCh during a task in which we previously measured an effect of increasing ACh by using the acetylcholinesterase inhibitor neostigmine (Experiment 4; Figure 5).
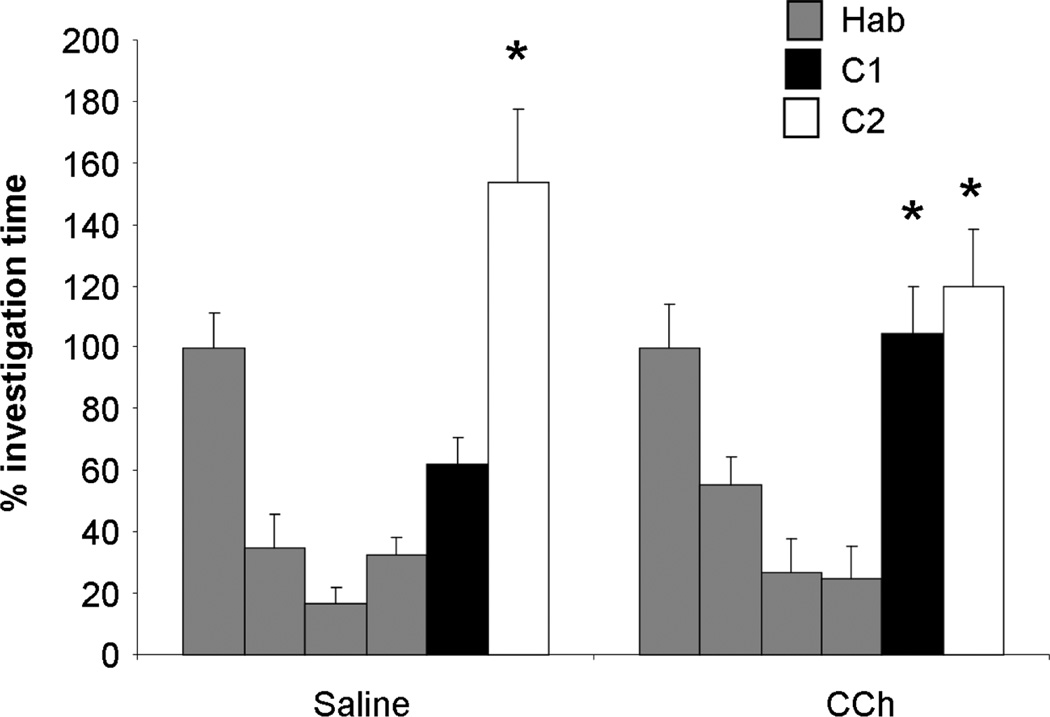
Figure 5. Positive control for the effect of CCh.
The graphs show the average percent investigation time as compared to the first habituation trial. Saline infused control rats respond significantly more to C2 (two carbon difference to habituation odor) than to the last habituation trial. In contrast, CCh infused rats respond significantly more to both C1 (one carbon difference to habituation odor) and C2. These data compare to those previously obtained with neostigmine (Chaudhury et al., 2009) and show that the dosage of CCh used here is effective in the OB. Asterisks indicate a significant increase in response level compared to the last habituation trial.
Experiment 4
In this task, rats are first habituated to a conditioned odor and their responses to chemically similar test odors are tested. Saline infused rats can discriminate odorants two carbons removed from the habituation odor, whereas rats infused with CCh also discriminated odorants a single carbon removed from the habituation odor, a result consistent with that obtained using neostigmine (Chaudhury et al., 2009). ANOVA showed a significant effect of trial (F(5, 6) = 11.382; p = 0.005) as well as a significant interaction between trial and drug treatment (F(5, 6) = 5.688; p = 0.028). Posthoc testing shows that while saline infused rats differentiate only odor C2 from the habituation odor (p < 0.01), CCh infused rats differentiated both test odors from the habituated odor (p < 0.01). This control shows that CCh, at the concentration used here, is effectively modulating bulbar processing.
Discussion
Our experiments show differential effects of OB cholinergic and noradrenergic modulation in the processing of very low concentration odorants. When motivated, rats respond to odorants which they do not spontaneously respond to in a non-rewarded task. Responses to low concentration odorants can be enhanced by local OB infusions of noradrenaline but not carbachol, a non-specific cholinergic agonist. In agreement with this result, motivated responses to low concentration odorants were impaired by blockade of noradrenergic, but not cholinergic receptors in the OB. Activation of cholinergic receptors, by contrast, improved discrimination between structurally similar odors, consistent with previous effects observed by increases in endogenous ACh level by OB acetylcholinesterase inhibition (Chaudhury et al., 2009; Mandairon et al., 2006). This shows that at the same dosage, the non-specific cholinergic agonist CCh modulates the discrimination of perceptually similar odorants at a relatively high odor concentration (1 Pa), but not the discrimination of very low concentration odorants from MO, suggesting a specific role for odor quality processing. Given that we used a relatively low dose of this drug to prevent seizures, it is possible that a modulation of response thresholds could be obtained with higher cholinergic modulation in the OB.
The present data cannot distinguish between an effect on sensory processing, i.e. a change in neural activity within the olfactory bulb, or a modulation of general arousal or attentional state (Yu & Dayan, 2005). Overall, the responses during habituation to mineral oil were not affected by drug infusions, suggesting that arousal or levels of activity were not affected by bulbar NE or ACh. In the forced choice discrimination task, the number of correct choices during the first five training trials was similar in all experimental groups, suggesting that learning of the discrimination task itself was not affected. Nevertheless, behavioral data cannot differentiate between changes in sensory representations and changes in other processes that may affect the behavioral responses measured.
Previous behavioral experiments have suggested very similar perceptual roles for cholinergic and noradrenergic bulbar modulation: both have been shown to modulate the discrimination of perceptually similar odorants in spontaneous and reward-motivated tasks (Doucette et al., 2007; Mandairon et al., 2006; Mandairon et al., 2008). Here, we show for the first time a specific behavioral situation in which one of these neuromodulatory transmitters seems implicated but not the other. Specifically, a comparison of discrimination of perceptually similar odorants and detection of low concentration odorants in the habituation/dishabituation task shows an effect of increasing ACh modulation on one but not the other. In contrast, increasing NE in the bulb modulates both detection of low concentration odorants and discrimination of perceptually similar odorants in this task (Escanilla et al., 2010).
The known cellular effects of NE and ACh in the OB overlap substantially. NE increases the responsiveness of mitral cells to weak or subthreshold inputs (Hayar et al., 2001; Jiang, Griff, Ennis, Zimmer, & Shipley, 1996) while simultaneously modulating the degree of inhibition mitral cells receive from granule cells (Nai, Dong, Hayar, Linster, & Ennis, 2009; Nai, Dong, Linster, & Ennis, 2010). The combined effect enhances the responses to low concentration odorants while preserving discrimination capabilities (Escanilla et al., 2010; Linster, Nai, & Ennis, 2011). Computational modeling suggested that the balance of excitation and inhibition created by NE modulation affects signal-to-noise ratios of odor responses by changing mitral cell response sensitivity as well as oscillatory dynamics and synchronization (Escanilla et al., 2010; Linster et al., 2011). ACh directly depolarizes glomerular layer periglomerular interneurons thought to be mainly responsible for contrast between chemically similar odorants (Castillo et al., 1999; Ravel et al., 1990) (Cleland & Sethupathy, 2006). Additionally, ACh depolarizes mitral cells, boosting the activation in those cells that are strongly activated by odorants (Castillo et al., 1999) (Mandairon et al., 2006). ACh modulates granule cell responsiveness to cortical inputs by increasing afterdepolarizations and hence modulates bulbar sensitivity to top down influences (Pressler, Inoue, & Strowbridge, 2007). The present data, in agreement with previous data and computational modeling, suggest, that while both modulators are involved in regulating olfactory discrimination, the noradrenergic system is more specifically activated in behavioral situation in which low signal-to-noise signals need to be processed. The cholinergic system, in contrast would be activated in behavioral situations in which highly overlapping odor representations need to be separated.
The present experiments show that behaviorally measured sensory responses can strongly depend on the behavioral task used to probe the animal: rats can easily learn to discriminate a very low concentration odor from mineral oil when rewarded, even if they do not spontaneously discriminate between those two stimuli. In the present experiment we used stimulus concentration to manipulate the difficulty of the task. We have previously used odor quality to manipulate the difficulty of the task; for example, rats could easily learn to discriminate between the enantiomers of limonene when motivated by reward, but did not spontaneously discriminate between these in a habituation/dishabituation task (Linster, Johnson, Morse, Yue, & Leon, 2002; Linster, Johnson et al., 2001). These data show that the neural representations of these stimuli are sufficiently detailed to allow for discrimination when the animal is motivated to do so, yet sufficiently similar to have the animals confuse these stimuli when not motivated to discriminate. Neuromodulators such as NE and ACh may be differentially involved in sensory processing with respect to task difficulty as well as with respect to a learned versus a spontaneous behavior. The effect of these neuromodulators may be at the level of the neural representation of the stimulus (as proposed in (Linster & Cleland, 2002; Linster et al., 2011)), or at the level of the interpretation of this representation according to the behavioral needs of the animal (Yu & Dayan, 2005). As a consequence, any behavioral study asking a specific perceptual question should use more than one type of behavioral measure.
Acknowledgements
This work was supported by NIH/NIDCD grants DC008702 to CL and ME and DC009948 (CL co-PI).
References
- Castillo PE, Carleton A, Vincent JD, Lledo PM. Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J Neurosci. 1999;19(21):9180–9191. doi: 10.1523/JNEUROSCI.19-21-09180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci. 2009;29(1):52–60. doi: 10.1523/JNEUROSCI.4036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 2006;7:7. doi: 10.1186/1471-2202-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Hasselmo ME, Baxter MG. Contribution of the cholinergic basal forebrain to proactive interference from stored odor memories during associative learning in rats. Behav Neurosci. 2001;115(2):314–327. [PubMed] [Google Scholar]
- Deco G, Thiele A. Attention: oscillations and neuropharmacology. Eur J Neurosci. 2009;30(3):347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14(8):539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanilla O, Arrellanos A, Karnow A, Ennis M, Linster C. Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur J Neurosci. 2010;32(3):458–468. doi: 10.1111/j.1460-9568.2010.07297.x. [DOI] [PubMed] [Google Scholar]
- Guerin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci. 2008;122(4):816–826. doi: 10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol. 2001;86(5):2173–2182. doi: 10.1152/jn.2001.86.5.2173. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14(4):488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci. 1996;16(19):6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw. 2002;15(4-6):709–717. doi: 10.1016/s0893-6080(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001;115(4):826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 1999;66(3):497–502. doi: 10.1016/s0031-9384(98)00324-2. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22(16):6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21(24):9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Nai Q, Ennis M. Nonlinear effects of noradrenergic modulation of olfactory bulb function in adult rodents. J Neurophysiol. 2011;105(4):1432–1443. doi: 10.1152/jn.00960.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24(11):3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci. 2008;27(5):1210–1219. doi: 10.1111/j.1460-9568.2008.06101.x. [DOI] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Hayar A, Linster C, Ennis M. Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J Neurophysiol. 2009;101(5):2472–2484. doi: 10.1152/jn.91187.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Linster C, Ennis M. Activation of alpha1 and alpha2 noradrenergic receptors exert opposing effects on excitability of main olfactory bulb granule cells. Neuroscience. 2010;169(2):882–892. doi: 10.1016/j.neuroscience.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T. The role of neuromodulators in selective attention. Trends Cogn Sci. 2011;15(12):585–591. doi: 10.1016/j.tics.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, Inoue T, Strowbridge BW. Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci. 2007;27(41):10969–10981. doi: 10.1523/JNEUROSCI.2961-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel N, Akaoka H, Gervais R, Chouvet G. The effect of acetylcholine on rat olfactory bulb unit activity. Brain Res Bull. 1990;24(2):151–155. doi: 10.1016/0361-9230(90)90199-a. [DOI] [PubMed] [Google Scholar]
- Ravel N, Elaagouby A, Gervais R. Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behav Neurosci. 1994;108(2):317–324. doi: 10.1037//0735-7044.108.2.317. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46(4):681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]