Abstract
Elevated red blood cell (RBC) aggregation increases low-shear blood viscosity and is closely related to several pathophysiological diseases such as atherosclerosis, thrombosis, diabetes, hypertension, cancer, and hereditary chronic hemolytic conditions. Non-ionic linear polymers such as poly(ethylene glycol) (PEG) and Pluronic F68 have shown inhibitory effects against RBC aggregation. However, hypersensitivity reactions in some individuals, attributed to a diblock component of Pluronic F68, have been reported. Therefore, we investigated the use of an amphiphilic star-shaped PEG polymer based on a cholic acid core as a substitute for Pluronics to reduce RBC aggregation. Cholic acid is a natural bile acid produced in the human liver and therefore should assure biocompatibility. Cholic acid based PEG polymers, termed CA(PEG)4, were synthesized by anionic polymerization. Size exclusion chromatography indicated narrow mass distributions and hydrodynamic radii less than 2 nm were calculated. The effects of CA(PEG)4 on human RBC aggregation and blood viscosity were investigated and compared to linear PEGs by light transmission aggregometry. Results showed optimal reduction of RBC aggregation for molar masses between 10–16 kDa of star-shaped CA(PEG)4 polymers. Cholic acid based PEG polymers affect the rheology of erythrocytes and may find applications as alternatives to linear PEG or Pluronics to improve blood fluidity.
Keywords: Star-shaped poly(ethylene glycol), erythrocytes, aggregation, cholic acid, blood rheology, blood viscosity
1. Introduction
Organ and tissue perfusion strongly depends on adequate blood supply to the microcirculation, and subtle disturbances of microcirculatory flow can lead to clinical disorders including tissue dysfunction or ischemia [1]. The hyper viscosity syndrome (i.e., greatly elevated blood viscosity) is a condition associated with enhanced red blood cell (RBC) aggregation that can reduce blood flow and lead to localized stagnation. Increased RBC aggregation has been observed in this condition and is implicated in the pathophysiology of numerous diseases with circulatory disorders such as cardiovascular diseases, chronic and acute inflammatory diseases, diabetes, cancers, sickle cell disease, thalassemia and trauma [2–4]. Moreover, epidemiological studies have recognized RBC aggregation as a strong cardiovascular risk factor [5].
Over the past several decades, there has been a strong interest in possible therapeutic agents that can counter RBC hyper-aggregation. For clinical therapy, polymers added to blood to improve rheology by reducing RBC aggregation were proposed [6, 7]. Therapeutic approaches have included infusion of selected polymers or their covalent linkage to the RBC membrane. These polymers include linear poly(ethylene glycol) (PEG), and an amphiphilic block copolymer of poly(propylene glycol) (PPG) and PEG (generic name poloxamers, trademark Pluronics and Lutrol); the block copolymers are comprised of a central hydrophobic moiety core of PPG flanked by two equal PEG chains, PEG-PPG-PEG [8–12]. Additionally, covalently linking of PEG to the RBC surface can mask antigenic sites, thus offering the potential for a universal donor RBC and for improved drug delivery systems [13, 14]. Though Pluronic F68 has shown promising results for the treatment of some hyper-viscosity disorders (e.g., sickle cell disease and myocardial infarction) [7, 15], adverse reactions attributed to unsaturated chains of a diblock PPG-PEG component of the copolymer have been observed in some patients [16].
As an alternative to linear PEG-containing polymers, we propose the use of an amphiphilic star-shaped PEG polymer based on cholic acid core or CA(PEG)4. Star-shaped PEG polymers may show particular promise for improving blood circulation in chronic disorders because the star conformation provides a smaller hydrodynamic radius and lower viscosity than a linear PEG of the same molecular mass [18, 19]. Cholic acid, which is a natural bile acid produced in the human liver, is currently used for biomedical and supra-molecular applications [20–22]. A series of cholic acid polymer derivatives have been used for drug delivery systems, molecular recognition, dental fillings, and bone repairing materials [23–25]. It is now recognized that the incorporation of a bio-compound such as cholic acid into polymers can improve biological compatibility, activity and safety for biomedical applications [26, 27]. We thus hypothesize that such amphiphilic star-shaped CA(PEG)4 polymers may be useful for coating RBC before blood transfusion or for intravenous injection in the treatment of elevated RBC aggregation, thus providing rheological benefits similar to Pluronic F68 and PEG. The CA(PEG)4 polymers consist of a hydrophobic core of bile acid and four hydrophilic PEG chains on the periphery located on the concave side of cholic acid.
Although prior studies have described several aspects of RBC adsorption and grafting with polymers having similar structural conformations (i.e., amphiphilic hyperbranched polyglycerol) [28, 29], they have not investigated their effects on RBC aggregation. In the present study, we examined the influence of star-shaped CA(PEG)4 polymers in an attempt to better understand how these polymers inhibit RBC aggregation and may thus be of value for therapeutic use.
2. Materials and methods
2.1. Materials
Star-shaped PEG with a cholane core, abbreviated as CA(PEG)4, were synthesized by a previously reported method [30] and were characterized by 1H NMR spectroscopy and MALDI-TOF mass spectrometry. Linear PEGs of 2, 5, 7 and 12 kDa were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA), whereas the 22.8 kDa linear PEG was obtained from Polymer Laboratories (Church Stretton, UK).
2.2. Methods
The molar masses of the various CA(PEG)4 listed in Table 1 were measured by size exclusion chromatography (SEC) coupled to a multi-angle laser light scattering detector (MALLS). The chromatographic system was equipped with a Waters 510 HPLC pump, pre-guard and two columns (PLAquagel-OH-30 8 μm, 103 Å and 105 Å), a Dawn EOS multi-angle light scattering detector (λ = 690 nm) coupled to a Optilab Rex refractive index detector, and a Wyatt quasi-elastic light scattering detector, all from Wyatt Technology Corporation (Santa Barbara, CA, USA). Data were collected and analyzed by the ASTRA software (version 5.3.4.18) also from Wyatt Technology Corporation. The dn/dc (differential refractive index increment) was determined online assuming a total mass recovery or offline by assessing the refractive index at several concentrations.
Table 1.
Molar masses for CA(PEG)4 polymers in water and in PBS obtained by SEC-MALLS measurements.
| CA(PEG)4 polymers | intrinsic viscosity [η] ± Δb (mL/g) | hydrodynamic radius Rh ± Δh (nm) | SEC in water
|
SEC in PBS
|
||
|---|---|---|---|---|---|---|
| Mw (Da) | PDI | Mw (Da) | PDI | |||
| 1 | 2.26 ± 0.89 | 1.90 ± 0.76 | 22 540 | 1.12 | 28 130 | 1.47 |
| 2 | 1.81 ± 0.70 | 1.64 ± 0.63 | 21 500 | 1.22 | 20 370 | 1.32 |
| 3 | 1.21 ± 0.16 | 1.34 ± 0.14 | 15 350 | 1.11 | 15 840 | 1.30 |
| 4 | 1.06 ± 0.23 | 1.18 ± 0.66 | 13 800 | 1.04 | 13 430 | 1.25 |
| 5 | - | - | 9 980 | 1.05 | 10 460 | 1.07 |
| 6 | 0.35 ± 0.09 | 0.62 ± 0.17 | 4 840 | 1.05 | 5 780 | 1.04 |
Mw is weight-average molar mass determined by size exclusion chromatography (SEC) coupled to multi-angle laser light scattering in distilled water.
Δb is the confidence limits for the intercept b for the linear regression y = mx+b for n = 5 at 95% confidence interval.
Δh is the standard deviation of Rh.
The SEC technique coupled with MALLS enabled molar mass determinations independent of any calibration or reference standards. Distilled water and an in-house 10 mM phosphate buffer solution (PBS) with a pH of 7.4 were used as the mobile phase at a flow rate of 1 mL/min. A concentration of 0.05% of sodium azide (Sigma Aldrich) was added to prevent bacteria formation. The normalization and the alignment of the instrument were carried out with PEG standards, Pullulan-5 and Pullulan-100 (Shodex, F8400000) from Showa Denko America Inc. (New York, NY, USA) using rms radii of 2 nm and 10 nm, respectively. About 10 mg of polymer was dissolved in 1 mL of PBS to obtain a good signal-to-noise ratio from the light scattering detector. The molar masses of the polymers indicated in this paper are all weight-average molar masses (Mw); note that since the polydispersity of the polymers are generally very low, they are very close to their number-average molar masses (Mn) (Table 1).
2.3. Determination of the hydrodynamic radius, Rh
The hydrodynamic radii (Rh) of synthesized CA(PEG)4 polymers were derived from intrinsic viscosity measurements (Cambridge Applied Systems, VISCOlab 3000, Medford, MA, USA) at 25°C. Solutions of 1, 3, 5, 7, 13, 17 and 23 mM of CA(PEG)4 polymers in double distilled water were prepared and left standing at room temperature for 24 hours in a sealed vial; all solutions were clear and without evidence of precipitation. Viscosities were measured five times for each solution and the Rh values were calculated by
| (1) |
where [η] is the intrinsic viscosity, M is the molar mass of the polymer, and N is Avogadro’s number.
2.4. Polymer viscosity
Viscosity measurements of polymer solutions were made between 15 and 300 s−1 with an AR2000 rheometer (TA Instruments, Grimsby, ON, Canada) using a Couette measuring cell. Solutions of star-shaped CA(PEG)4 polymers in water and in PBS (10 mL) at different molar masses were prepared to study the effect of shear rate at 25 and 37 °C.
2.5. Blood samples
The experimental protocol was approved by the University of Southern California Institutional Review Board. After informed verbal consent was obtained, freshly drawn blood from three healthy human donors was anticoagulated with EDTA (1.5 mg/mL). Hematocrit (Ht) was adjusted to 42% by addition of autologous plasma or autologous packed RBC. Stock solutions of the CA(PEG)4 polymers and PEG standards listed in Table 1 were prepared in PBS to obtain a concentration of 100 mg/mL. The stock polymer was added to each blood sample yielding final polymer concentrations of 1.3, 4.0 and 6.7 mg/mL (the plasma dilution was the same for all concentrations, balance = PBS) and a final Ht of 40%.
2.6. RBC aggregation
Two methods were used to determine the extent of RBC aggregation. Apparent viscosities of 40% Ht RBC suspensions containing CA(PEG)4 at a concentration of 6.7 mg/mL were measured at 25 °C using a Contraves LS-30 Couette viscometer (Contraves AG, Zürich, Switzerland). The ratio of apparent viscosities at 0.15 and 94 s−1 was computed and normalized to that of the control solution (i.e., RBC without added polymer) to determine inhibition of RBC aggregation; the lower the ratio with respect to unity, the higher is the reduction of the aggregation. RBC aggregation was also quantified using a photometric rheoscope (model MA-1 aggregometer, Myrenne GmbH, Roentgen, Germany), which yields dimensionless indices of aggregation at stasis (M) and at a low shear rate of 3 s−1 (M1); both indices decrease with reduced aggregation [31]. Normalized Myrenne aggregation data (M and M1) were obtained by dividing M and M1 by M or M1 for RBC without added polymer. Normalized M and M1 values less than unity indicate reduced aggregation. The effects of CA(PEG)4 molar mass on the Myrenne aggregation indices for RBC suspended in plasma were evaluated at polymer concentrations of 1.3, 4.0 and 6.7 mg/mL. Comparison of Myrenne results for linear PEG added to blood was performed at a polymer concentration of 6.7 mg/mL. All viscometry and Myrenne measurements were done in duplicate and averaged.
2.7. Optical microscopy
The morphology of CA(PEG)4-RBC and of control RBC (no polymer added) were studied and photographed using bright-field light microscopy (Olympus BX-40 with 40x objective). Wet mount preparations were prepared by diluting 50 μL of a 40% Ht suspension with 250 μL of plasma and mixed; this low Ht suspension was placed between a glass slide and coverslip and allowed to stand at room temperature for 10 minutes.
2.8. Statistical methods
Data are presented as mean ± standard deviation (SD). Comparisons between groups were carried out pair-wise using analyses of variance (ANOVA) with the Bonferroni test for multiple comparisons (OriginPro 8 software, OriginLab, MA, USA). The Kruskal-Wallis ANOVA test was used if the normality test failed. Associations between variables were assessed by least-square linear regressions.
3. Results
3.1. Synthesis and characterization of CA(PEG)4
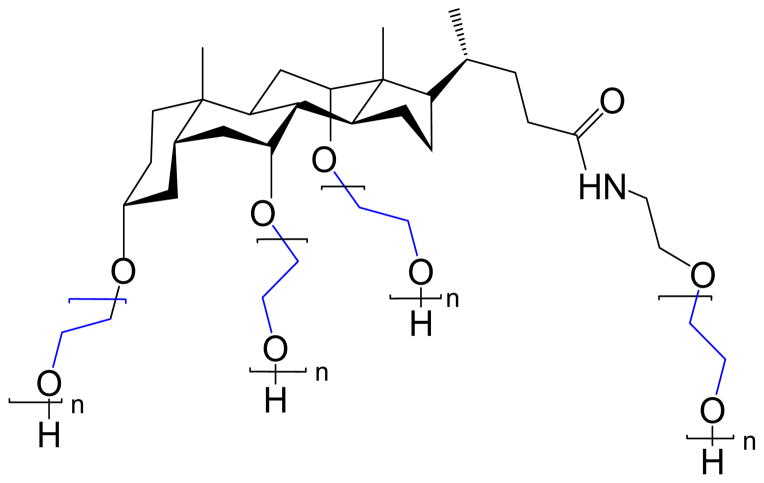
The CA(PEG)4 polymers with a molar mass > 7 kDa and with narrow polydispersity that were prepared in this study were characterized by SEC-MALLS in water and in PBS. Their weight- average molar masse (Mw) and polydispersity index (PDI) are given in Table 1, and show lower PDI (<1.22) in water than in PBS (PDI <1.47), especially for higher Mw polymers. Also, Mw values obtained in PBS are 1 – 1.2 times greater than in water. A series of CA(PEG)4 polymers were synthesized by controlled anionic polymerization and their chemical structure was confirmed by MALDI-TOF. It showed the repeating unit of ethylene oxide in the PEG chain with corresponding mass of 44.05 Da, and the residual mass of 451.65 Da corresponding to the cholic acid derivative residual mass. A chemical structure of CA(PEG)4 is shown in Figure 1. Methylene protons of PEG were present at about 3.4 – 3.5 ppm, and only a few of the cholane backbone chemical shifts are visible since they are smaller than the PEG chains.
Figure 1.
Structure of the star-shaped PEG with a cholane core CA(PEG)4. Methylene protons of PEG were present at about 3.4 – 3.5 ppm.
3.2. Determination of Rh and Mark Houwink constants
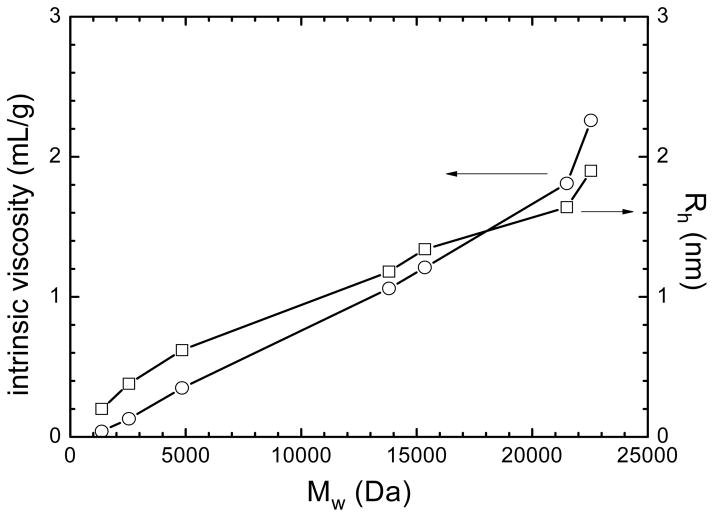
A detailed characterization of the self-diffusion of low molar mass CA(PEG)4 by NMR was presented earlier [32]. Here, we report solution properties of CA(PEG)4 in water as measured by viscometry. Figure 2 shows that the intrinsic viscosity [η] and hydrodynamic radius Rh increase with increasing molar masses. As expected, CA(PEG)4 polymers had a compact structure with Rh values less than about 2 nm and small [η] values below about 2 mL/g.
Figure 2.
The intrinsic viscosity [η] (circles) and hydrodynamic radius (Rh) (squares) against the number-average molar mass (Mw) for a series of solutions of CA(PEG)4 polymers in water at 25 °C. CA(PEG)4 polymers had a compact structure with Rh values less than about 2 nm and small [η] values below about 2 mL/g.
Within a series of polymer homologues, [η] increases with Mw as described by the Mark Houwink equation:
| (2) |
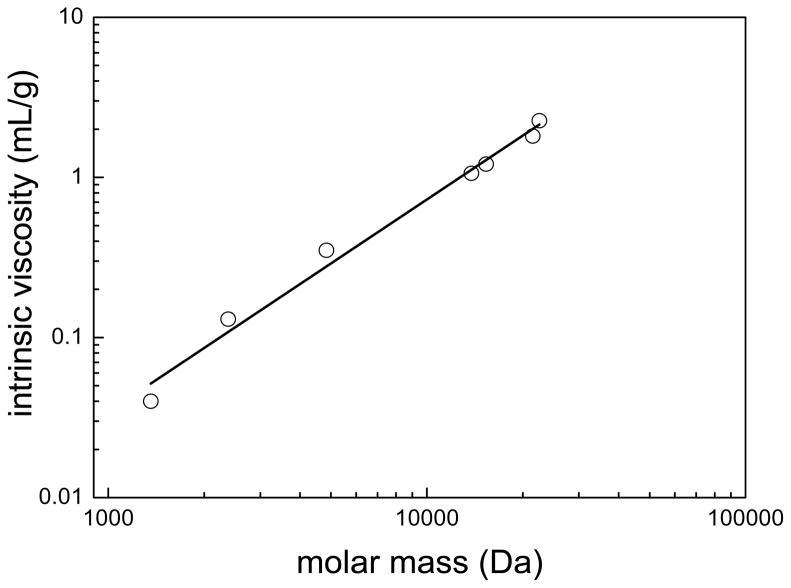
where K and a are empirical constants obtained from the slope and intercept of the logarithmic plot shown in Figure 3. The values of the constants were K = −5.44 and a = 1.3 with an adjusted R2 value of 0.985. The constant a is related to the way chains are added to the backbone of the molecule: 1) spherical structures have a slope of 0; 2) rod-like molecules have a slope of 2; and 3) random coiled molecules have a slope of 0.5 – 0.8. The a value of 1.3 for CA(PEG)4 indicates molecules that are close to rigid random coils, probably as a result of the cholic acid core. Based upon these experimental results, the scaling relations between radii and molecular masses (Rh ~ Mv) were established, where v ranges from 0.5–0.6 depending on whether the polymer is in a theta solvent or good solvent [33, 34]. Essentially, the scaling constant for the CA(PEG)4 polymers were within the range of a random coil conformation, as for linear polymers [32].
Figure 3.
Logarithmic plot of the intrinsic viscosity as a function of the molar mass of CA(PEG)4 polymers in double distilled water at 25°C. The solid line shows the fit to the Mark Houwink equation, where K = −5.445 and a = 1.3 with adjusted R2 = 0.985.
3.3. Rheological properties
Polymer solutions
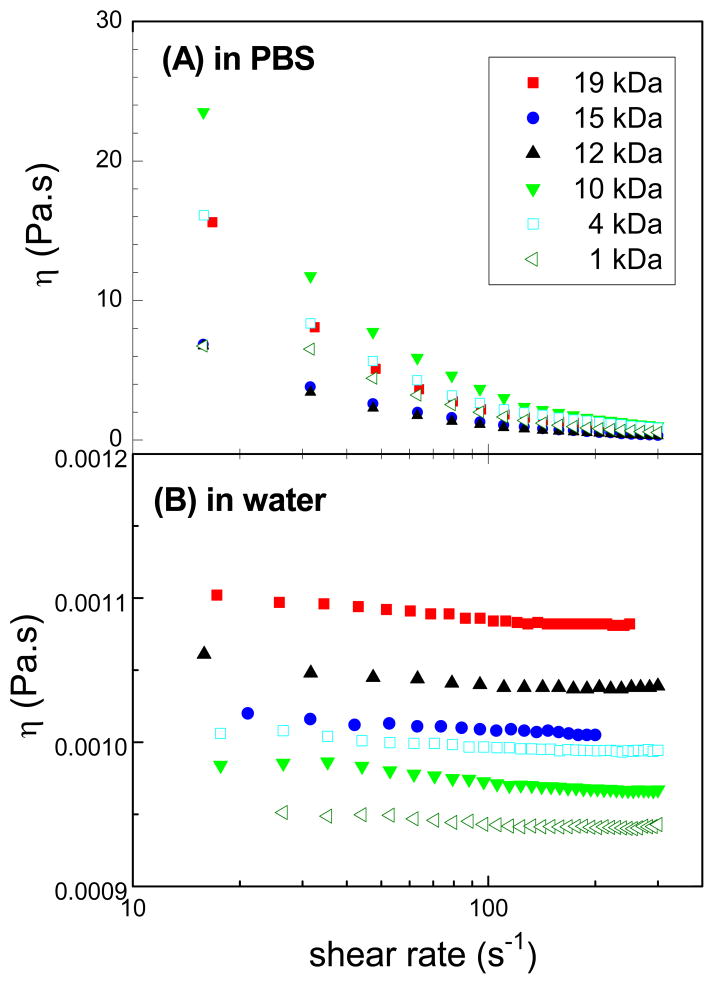
Viscosity profiles of different molar masses of star-shaped polymers were studied in water and in isotonic phosphate buffered saline (PBS); PBS was selected as a relevant buffer as this is commonly used in biological applications [35]. There was no significant difference between the viscosity profiles at 25 and 37 °C (p > 0.05). Flow viscosity curves in Figure 4 show that CA(PEG)4 in PBS exhibits pronounced shear thinning behavior, especially at high molar mass fractions, whereas shear thinning is less pronounced in water. Viscosities in PBS were several orders of magnitude higher than those for the same polymer in water.
Figure 4.
Semi-logarithmic plot of the dependence the flow curves on the molar mass of the star-shaped CA(PEG)4 polymers at 25 °C in (A) phosphate buffer saline solution (PBS) at 0.3% (w/v) and (B) in water. Flow viscosity curves show that CA(PEG)4 in PBS exhibits pronounced shear thinning behavior, especially at high molar mass fractions, whereas shear thinning is less pronounced in water.
Star-shaped CA(PEG)4 mixed with RBC
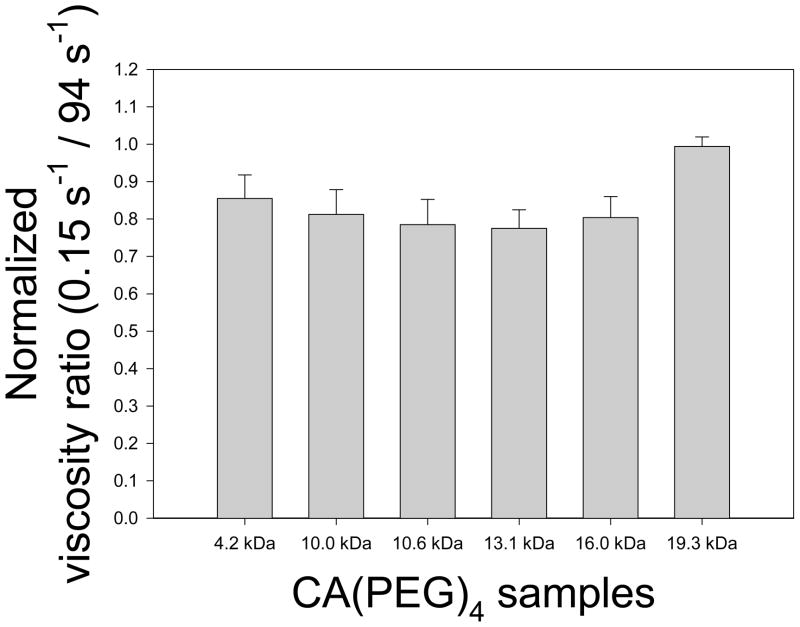
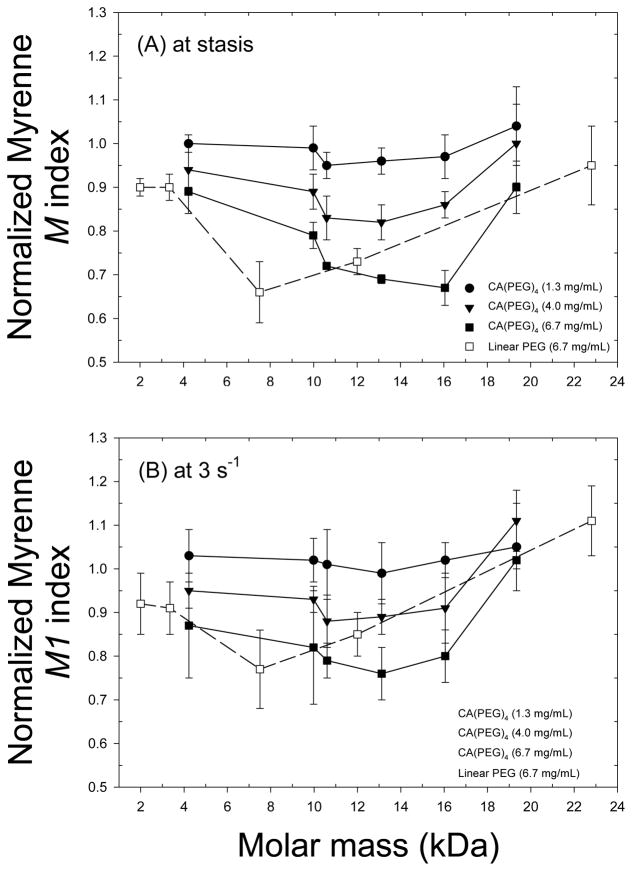
Normalized relative viscosity measurements of 40% Ht RBC-plasma suspensions containing various CA(PEG)4 polymers with molar masses between 4.2 – 19.3 kDa and a fixed concentration of 6.7 mg/mL are displayed in Figure 5. Compared to unity corresponding to the polymer-free suspension (control), inhibition of RBC aggregation was close to significance (p = 0.08). Figure 6 presents Myrenne rheoscope data; M and M1 values are shown relative to control and hence values less than unity also indicate reduced aggregation. These results clearly demonstrate that at concentrations of 4.0 and 6.7 mg/mL, star-shaped CA(PEG)4 having a fairly wide range of molecular masses are able to reduce RBC aggregation (p < 0.01). The concentration and molecular mass dependency indicated in Figure 6 are in agreement with prior studies, using other polymers, which describe an optimum effect at a specific concentration [36].
Figure 5.
Viscosity ratio (apparent viscosity at 0.15 s−1 divided by that at 94 s−1) of human RBC suspended in autologous plasma at 25°C with CA(PEG)4 solution (6.7 mg/mL) normalized to control (0 mg/mL) and presented as mean ± SD. Measurements were in duplicates for each donor (n = 3). Compared to unity corresponding to the polymer-free suspension (control), inhibition of RBC aggregation was close to significance (p = 0.08).
Figure 6.
Aggregation of RBC suspended in autologous plasma measured using a Myrenne aggregometer. Aggregation indices are M at stasis (A) and M1 at 3 s−1 (B) normalized to control (i.e., buffer added without polymer) and presented as mean ± SD for CA(PEG)4 at different concentrations (1.3, 4.0 and 6.7 mg/mL) and linear PEG at a concentration of 6.7 mg/mL. Values for M and M1 < 1 indicate inhibition of RBC aggregation. Measurements were in duplicates for each donor (n = 3). Results demonstrate that at concentrations of 4.0 and 6.7 mg/mL, star-shaped CA(PEG)4 are able to reduce RBC aggregation (p < 0.01).
Effects of CA(PEG)4 versus linear PEG on RBC aggregation
Figure 6 also presents normalized Myrenne M and M1 vs. molar mass for RBC suspended in plasma and mixed with linear PEG at a constant polymer concentration of 6.7 mg/mL. Star-shaped CA(PEG)4 yielded similar inhibition of RBC aggregation than linear PEG for a given molar mass. The maximum inhibition was observed with the 16 kDa CA(PEG)4, whereas the 7.5 kDa linear PEG provided a similar reduction of RBC aggregation.
3.4. Microscopic observations
Photographic microscope images for normal RBC suspended in native plasma (control) and normal RBC in plasma containing 6.7 mg/mL of 4 kDa CA(PEG)4 are shown in Figure 7. Normal discocytic RBC morphology was preserved in both samples.
Figure 7.
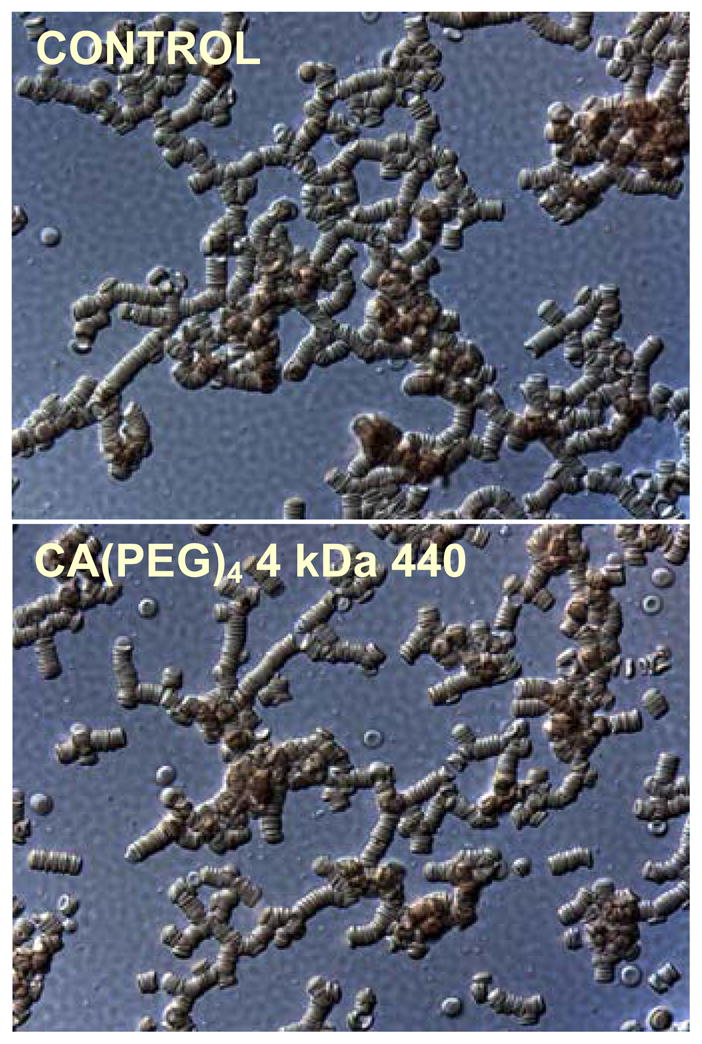
Photomicrograph images of RBC in plasma at 40x magnification. The CA(PEG)4 sample was obtained by adding the polymer at a concentration of 6.7 mg/mL to RBC and plasma. Normal discocytic RBC morphology is seen in both samples.
4. Discussion
Elevated RBC aggregation and the associated increase in blood viscosity are recognized risk factors for cardiovascular diseases [37]. Although Pluronic F68 (poloxamer 188), which is a PEG-PPG-PEG triblock copolymer, can reduce RBC aggregation, it has been shown to induce adverse reactions in some patients [16, 38] that has been attributed to the presence of unsaturation associated with a diblock PEG-PPG component of the copolymer. Synthesis of star polymers using cholic acid as the core group eliminates the possibility of an unsaturated diblock component and may be a viable approach to address the adverse reactions previously observed [16, 38].
The structural asymmetry of the star polymer CA(PEG)4 confers amphiphilic properties and such polymers are of growing interest for biomedical, pharmaceutical and biotechnology applications because they can behave as unimolecular micelles or be designed to exhibit a very low critical aggregation concentration (CAC) [39, 40]. Specifically, our results for CA(PEG)4 solution properties (Figures 2 and 3) show that they have a compact structure with a small hydrodynamic radius (< 2 nm) and a low intrinsic viscosity (~ 2 mL/g) due to the star conformation [41]. Also, constants obtained by fitting our data to the Mark-Houwink model and the scaling relation Rh ~ Mv suggest that CA(PEG)4 polymers have star conformations with values similar to those reported for star-shaped polymers [42].
Intrinsic viscosity [η] versus Mw results (Table 1, Figure 2) indicated two distinct linear regions with slopes differing by approximately 1.8. The difference can be explained by two competitive effects in polymer solutions: free volume and entanglements [43]. Indeed, at low molar masses, star polymers have relatively shorter and stiffer arms due to their greater proximity to the cholane core giving free movement and less entanglement. On the other hand, at higher molar masses, the polymer has many entanglements and limited motion [44]. Solution viscosity of CA(PEG)4 polymers showed interesting behavior; in PBS the polymer exhibited a shear thinning behavior, whereas in water there was minimal or no effect of shear (Figure 4). The shear-thinning results from the coil-like behavior of most polymer fluids [43] since under shear, coils rotate causing disentanglement/entanglement with their neighbors, thereby causing high viscosity at low shear rates. As the shear rate increases, coils rotate too fast to re-entangle and the viscosity decreases. The higher viscosity of CA(PEG)4 in PBS than in water can be explained by micelle formation; the CAC is lower in PBS than in water for some polymers (it was reported between 9 – 19 mM in water for CA(PEG)4 [30]). The onset of micelle formation for bile acids is further lowered by the addition of cations [45].
Results of Figure 6 indicate that the extent of inhibition of RBC aggregation with CA(PEGs)4 shows a dependence on both polymer concentration and Mw, with masses between 10 – 16 kDa most efficient in reducing RBC aggregation; these concentration and Mw findings are in agreement with other studies [7]. RBC aggregability (i.e., the intrinsic tendency for RBC to aggregate) is determined by cellular factors and varies between individuals. When RBC are suspended in the same aggregating medium (e.g., plasma or dextran solutions), different human donors can exhibit two-fold variations in RBC aggregation and hence in the aggregability of their red cells [7]. Interpretation of such findings in terms of physicochemical mechanisms is of importance, but is problematic due the current lack of information regarding details involved. Currently, two conflicting models are used to describe RBC aggregation: 1) the bridging model, which predicts increased aggregation resulting from an increased polymer concentration at the RBC surface; 2) the depletion model, which predicts that decreased polymer concentration near the RBC membrane favors aggregation [46]. The depletion model does not require the absence of polymer adsorption onto the RBC but only necessitates that the concentration of the polymer in the depletion layer be less than the bulk phase in order to cause aggregation. Given these two models, it is possible that star shaped CA(PEG)4 decrease RBC aggregation via either decreasing (bridging model) or increasing (depletion model) the concentration of the aggregating polymer near the RBC surface. However, there is increasing evidence to support the depletion model for polymer-induced RBC aggregation [8, 12, 47]. Therefore, if the depletion model is to be applied, it would seem most likely that CA(PEG)4 reduced RBC aggregation via reducing the depletion effect near the RBC surface (i.e., it’s glycocalyx). Considering the small size of CA(PEG)4 molecules, it is possible that they enter the depletion layer formed by larger pro-aggregating macromolecules (e.g., fibrinogen) such that they are able to effectively reduce the osmotic gradient between the intercellular gap and the bulk phase [8].
Ideally, comparing the effects of star-shaped CA(PEG)4 with those of linear PEGs on RBC-RBC interactions should be done on the basis of their hydrated size. As an approximation, this condition requires comparing polymers at an equivalent suspending phase viscosity. Since the relationship between molar mass and inhibition of RBC aggregation was similar between star-shaped CA(PEG)4 and linear PEG (Figure 6), this indicates the lack of interactions between the cholane acid core and the RBC surface and hence favors the depletion model. It is possible that CA(PEG)4 with smaller radii infiltrated the gap between RBC and/or RBC glycocalyx more efficiently than linear PEG. This possibility has been suggested previously for low molar masses of polysaccharides which reduce RBC aggregation [7].
5. Conclusion
A series of star-shaped CA(PEG)4 polymers with different molar masses and narrow molar mass distributions were synthesized by anionic polymerization. The hydrodynamic radius of these polymers, as determined by intrinsic viscosity measurements, clearly indicated the small and compact structure of star shaped polymers (< 2 nm). The polymers also demonstrated shear-thinning behavior in isotonic phosphate buffer (PBS). The extent of inhibition of RBC aggregation by star-shaped CA(PEG)4, as determined by a light transmission method (i.e., Myrenne aggregometer), was dependent on the concentration and molar mass of the polymers. Under the conditions used in this study, CA(PEG)4 and linear PEGs had similar effects on RBC aggregation. Additional studies should include: 1) testing with large control groups and patients with abnormal RBC aggregation; 2) covalently binding CA(PEG)4 to the RBC membrane by means of reactive groups such as succinimidyl propionate; and 3) testing the effects of polymers based on other bile acids and related bio-compounds.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CMI-72323), Heart and Stroke Foundation of Canada (PG-05-0313), and by the National Institutes of Health of USA (RO1 HL078655).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rad S, Neu B. Impact of cellular properties on red cell-red cell affinity in plasma-like suspensions. European Physical Journal E. 2009;30(2):135–40. doi: 10.1140/epje/i2009-10499-1. [DOI] [PubMed] [Google Scholar]
- 2.MacRury S, Lennie S, McColl P, Balendra R, MacCuish A, Lowe G. Increased red cell aggregation in diabetes mellitus: Association with cardiovascular risk factors. Diabet Med. 1993;10(1):21–26. doi: 10.1111/j.1464-5491.1993.tb01991.x. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler O, Guerci B, Muller S, Candiloros H, Méjean L, Donner M, et al. Increased erythrocyte aggregation in insulin-dependent diabetes mellitus and its relationship to plasma factors: A multivariate analysis. Metabolism. 1994;43(9):1182–86. doi: 10.1016/0026-0495(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 4.Shiga T, Maeda N, Kon K. Erythrocyte rheology. Crit Rev Oncol Hematol. 1990;10:9–48. doi: 10.1016/1040-8428(90)90020-s. [DOI] [PubMed] [Google Scholar]
- 5.Somer T, Meiselman H. Disorders of blood viscosity. Annals of Medicine. 1993;25:31–39. doi: 10.3109/07853899309147854. [DOI] [PubMed] [Google Scholar]
- 6.Chien S, Jan K. Red cell aggregation by macromolecules: Roles of surface adsorption and electrostatic repulsion. J Supramol Structure. 1973;1:385–409. doi: 10.1002/jss.400010418. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong JK, Wenby RB, Meiselman HJ, Fisher TC. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophysical Journal. 2004;87:4259–70. doi: 10.1529/biophysj.104.047746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toth K, Wenby RB, Meiselman H. Inhibition of polymer-induced red blood cell aggregation by poloxamer 188. Biorheology. 2000;37:301–12. [PubMed] [Google Scholar]
- 9.Armstrong J, Meiselman H, Fisher T. Inhibition of red blood cell-induces platelet-aggregation in whole blood by nonionic surfactant, poloxamer 188 (rheothr®) injection. Thrombosis Research. 1995;79(5–6):437–50. doi: 10.1016/0049-3848(95)00134-d. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong J, Meiselman H, Fisher T. Covalent binding of poly(ethylene glycol) (peg) to the surface of red blood cells inhibits aggregation and reduces low shear blood viscosity. Am J of Hematol. 1997;56(1):26–28. doi: 10.1002/(sici)1096-8652(199709)56:1<26::aid-ajh5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Hashemi-Najafabadi S, Vasheghani-Farahani E, Shojaosadati SA, Rasaee MJ, Armstrong JK, Moin M, et al. A method to optimize peg-coating of red blood cells. Bioconjugate Chemistry. 2006;17(5):1288–93. doi: 10.1021/bc060057w. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong JK, Meiselman HJ, Wenby RB, Fisher TC. Modulation of red blood cell aggregation and blood viscosity by the covalent attachment of pluronic copolymers. Biorheology. 2001;38(2–3):239–47. [PubMed] [Google Scholar]
- 13.Garratty G. Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sanguinis. 2008;94(2):87–95. doi: 10.1111/j.1423-0410.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 14.Bradley AJ, Murad KL, Regan KL, Scott MD. Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: Size does matter. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2002;1561(2):147–58. doi: 10.1016/s0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- 15.Orringer EP, Casella JF, Ataga KI, Koshy M, Adams-Graves P, Luchtman-Jones L, Wun T, Watanabe M, Shafer F, Kutlar A, Abboud M, Steinberg M, Adler B, Swerdlow P, Terregino C, Saccente S, Files B, Ballas S, Brown R, Wojtowicz-Praga S, Grindel JM. Purified poloxamer 188 for treatment of acute vaso-occlusive crisis of sickle cell disease: A randomized controlled trial. JAMA. 2001;286(17):2099–2106. doi: 10.1001/jama.286.17.2099. [DOI] [PubMed] [Google Scholar]
- 16.Moghimi S, Hunter A, Dadswell C, Savay S, Alving C, Szebeni J. Causative factors behind poloxamer 188 (pluronic f68, flocor (tm))-induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. Biochemica et Biophysica Acta-Molecular Basis of Disease I. 2004;1689(2):103–13. doi: 10.1016/j.bbadis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, et al. Antibody against poly(ethylene glycol) adversely affects peg-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110(1):103–11. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Zhang A. Synthesis and characterization of star-shaped poly(d,l-lactide)-block-poly(ethylene glycol) copolymers. Polymer Bulletin. 2010;65(9):883–92. [Google Scholar]
- 19.Lapienis G. Star-shaped polymers having peo arms. Progress in Polymer Science. 2009;34(9):852–92. [Google Scholar]
- 20.Hofmann AF. Bile-acids as drugs - principles, mechanisms of action and formations. Italian Journal of Gastroenterology. 1995 Mar;27(2):106–13. [PubMed] [Google Scholar]
- 21.Tamminen J, Kolehmainen E. Bile acids as building blocks of supramolecular hosts. Molecules. 2001;6:21–46. [Google Scholar]
- 22.Virtanen E, Kolehmaien E. Use of bile acids in pharmacological and supramolecular application. Eur J Org Chem. 2004:3385–99. [Google Scholar]
- 23.Enhsen A, Kramer W, Wess G. Bile acids in drug discovery. Drug Discovery Today. 1998;3(9):409–18. [Google Scholar]
- 24.Albert D, Feigel M. A steroidal cyclopeptide, synthesis and shape of the cavity. Tetrahedron Lett. 1994;35(4):565–568. [Google Scholar]
- 25.Zhu XX, Nichifor M. Polymeric materials containing bile acids. Acc Chem Res. 2002;35(7):539–46. doi: 10.1021/ar0101180. [DOI] [PubMed] [Google Scholar]
- 26.Benrebouh A, Zhang YH, Zhu XX. Hydrophilic polymethacrylates containing cholic acid-ethylene glycol derivatives as pendant groups. Macromolecular Rapid Communications. 2000;21(10):685–90. [Google Scholar]
- 27.Denike JK, Zhu XX. Preparation of new polymers from bile-acid derivatives. Macromolecular Rapid Communications. 1994;15(6):459–65. [Google Scholar]
- 28.Liu ZH, Janzen J, Brooks DE. Adsorption of amphiphilic hyperbranched polyglycerol derivatives onto human red blood cells. Biomaterials. 2010;31(12):3364–73. doi: 10.1016/j.biomaterials.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Rossi NAA, Constantinescu I, Kainthan RK, Brooks DE, Scott MD, Kizhakkedathu JN. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials. 2010;31(14):4167–78. doi: 10.1016/j.biomaterials.2010.01.137. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Giguere G, Zhu XX. Asymmetric poly(ethylene glycol) star polymers with a cholic acid core and their aggregation properties. Biomacromolecules. 2009;10(4):900–06. doi: 10.1021/bm801423p. [DOI] [PubMed] [Google Scholar]
- 31.Klose H, Volger E, Brechtelsbauer H, Schmid-Schonbein LaH. Microrheology and light transmission of blood. Pfluegers Arch. 1972;333:126–39. doi: 10.1007/BF00586912. [DOI] [PubMed] [Google Scholar]
- 32.Wang YJ, Therien-Aubin H, Baille WE, Luo JT, Zhu XX. Effect of molecular architecture on the self-diffusion of polymers in aqueous systems: A comparison of linear, star, and dendritic poly(ethylene glycol)s. Polymer. 2010;51(11):2345–50. [Google Scholar]
- 33.Willis SA, Dennis GR, Zheng G, Price WS. Hydrodynamic size and scaling relations for linear and 4 arm star pvac studied using pgse nmr. J Mol Liq. 2010;156(1):45–51. [Google Scholar]
- 34.Waggoner RA, Blum FD, Lang JC. Diffusion in aqueous solutions of poly(ethylene glycol) at low concentrations. Macromolecules. 1995;28(8):2658–64. [Google Scholar]
- 35.Albertsson P. Partition of cell particles and macromolecules. New York: Wiley; 1971. [Google Scholar]
- 36.Bauersachs R, Wenby R, Meiselman H. Determination of specific red blood cell aggregation indices via an automated system. Clin Hemorheol. 1989;9:1–25. [Google Scholar]
- 37.Lowe GDO, Lee AJ, Rumley A, Price JF, Fowkes FGR. Blood viscosity and risk of cardiovascular events: The edinburgh artery study. British Journal of Haematology. 1997;96(1):168–73. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- 38.Szebeni J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology. 2005;216(2–3):106–21. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Lele BS, Leroux JC. Synthesis of novel amphiphilic star-shaped poly([var epsilon]-caprolactone)-block-poly(n-(2-hydroxypropyl)methacrylamide) by combination of ring-opening and chain transfer polymerization. Polymer. 2002;43(21):5595–606. [Google Scholar]
- 40.Nichifor M, Stanciu MC, Zhu XX. Bile acids covalently bound to polysaccharides 2. Dextran with pendant cholic acid groups. Reactive & Functional Polymers. 2004;59(2):141–48. [Google Scholar]
- 41.Grest GS, Fetters LJ, Huang JS, Richter D. Advances in chemical physics. John Wiley & Sons, Inc; 2007. Star polymers: Experiment, theory, and simulation; pp. 67–163. [Google Scholar]
- 42.Vagberg LJM, Cogan KA, Gast AP. Light-scattering study of starlike polymeric micelles. Macromolecules. 1991;24(7):1670–77. [Google Scholar]
- 43.Allison C, Peacock A. Polymer chemisty: Properties and applications. Munich: Hanser Verlag; 2006. p. 106. [Google Scholar]
- 44.Huber K, Bantle S, Burchard W, Fetters LJ. Semidilute solutions of star branched polystyrene: A light and neutron scattering study. Macromolecules. 1986;19(5):1404–11. [Google Scholar]
- 45.Hofmann AF, Mysels KJ. Bile acid solubility and precipitation in vitro and in vivo: The role of conjugation, ph, and ca2+ ions. Journal of Lipid Research 1992. 1992 May 1;33(5):617–26. [PubMed] [Google Scholar]
- 46.Baskurt O, Meiselman H. Blood rheology and hemodynamics. Seminars in thrombosis and hemostatis. 2003;29(5):435–50. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 47.Neu B, Armstrong J, Fisher T, Bäumler H, Meiselman H. Electrophoretic mobility of human red blood cells coated with poly(ethylene glycol) Biorheology. 2001;38:389–403. [PubMed] [Google Scholar]