Abstract
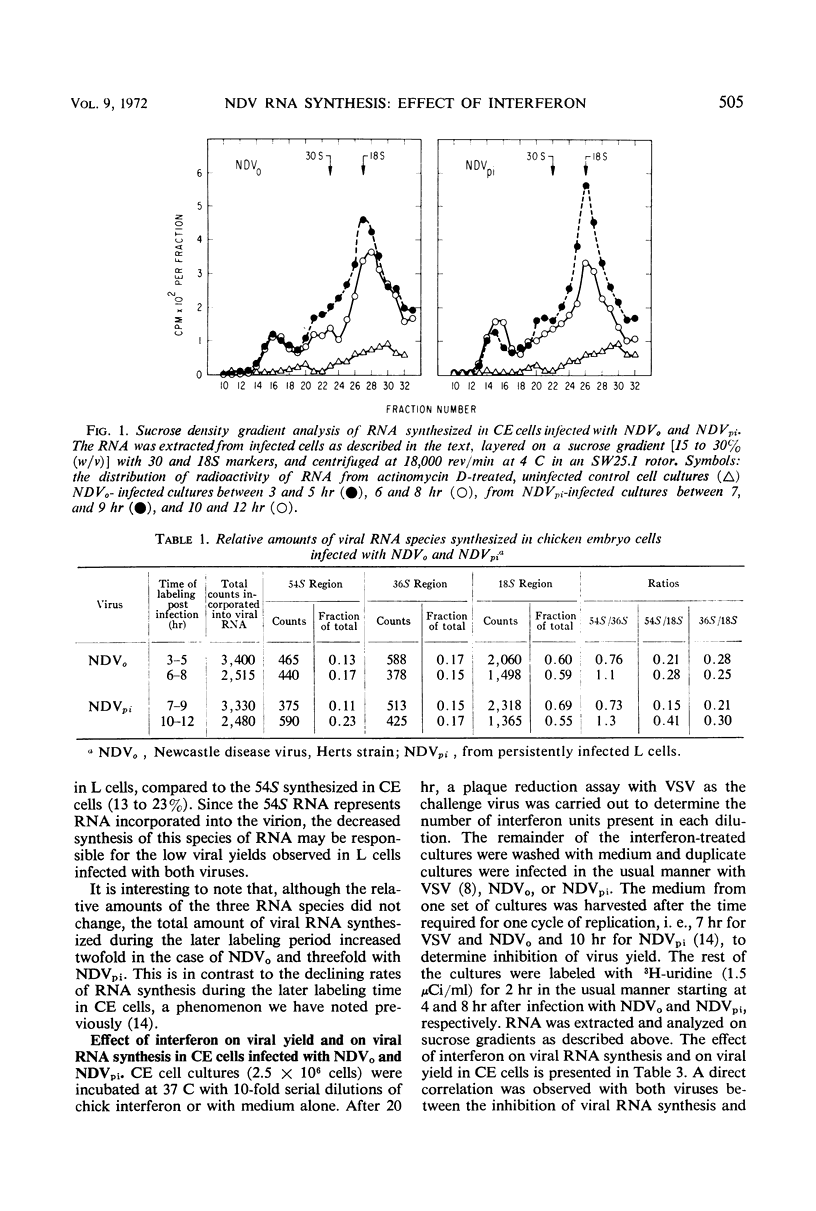
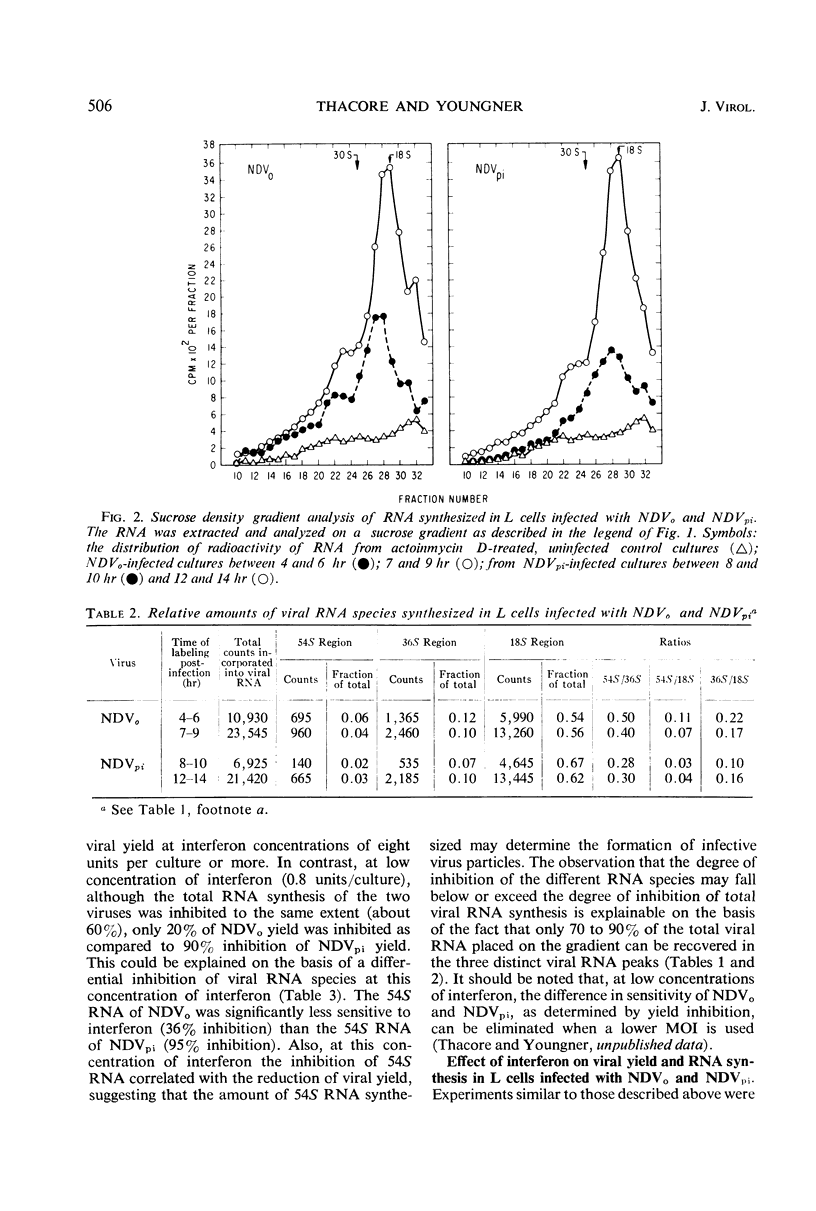
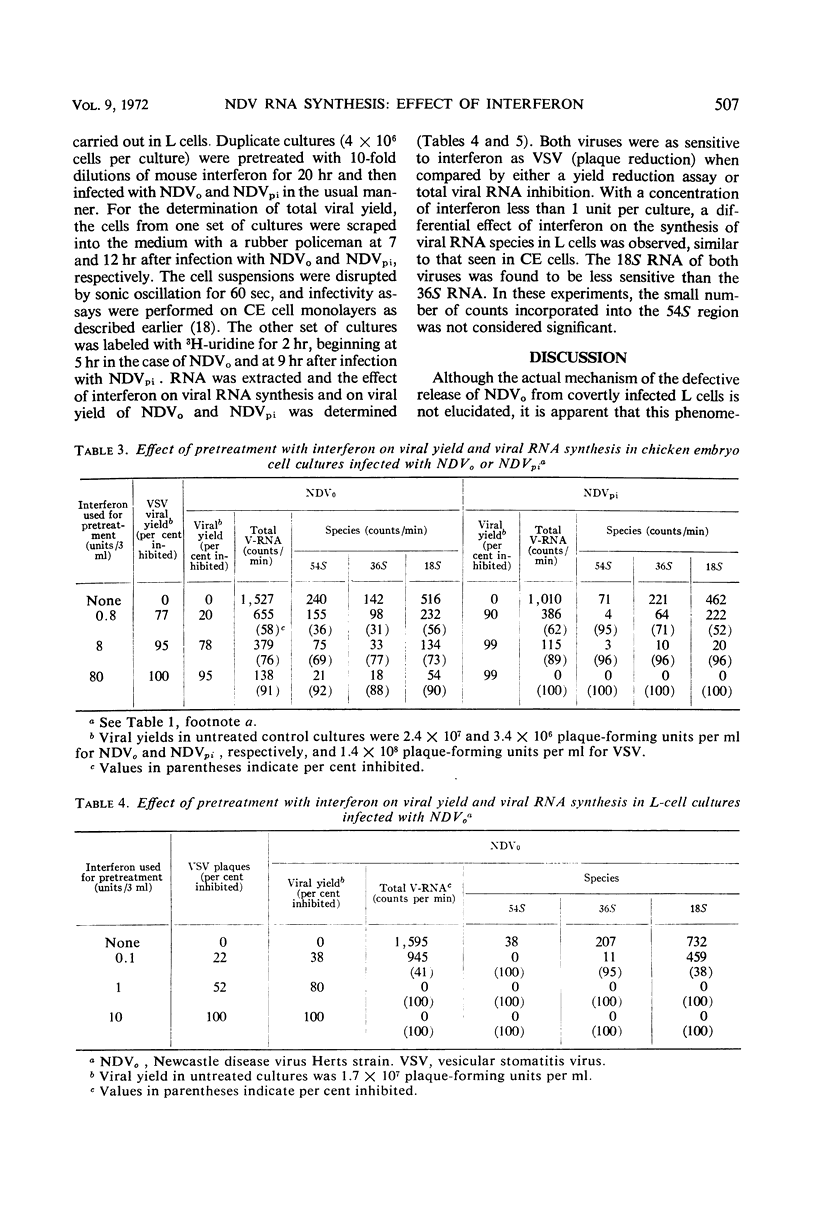
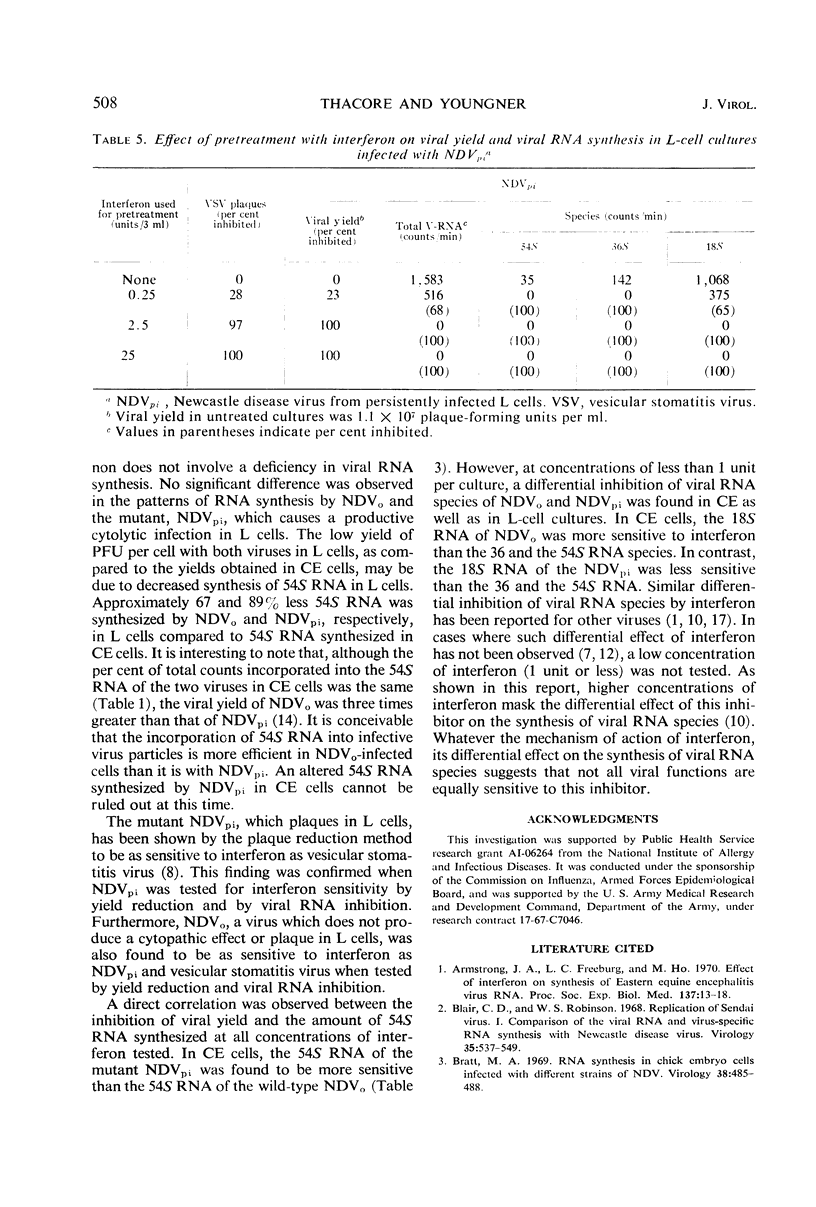
The synthesis of different viral ribonucleic acid (RNA) species was studied in chick embryo (CE) and mouse L-cell cultures infected with the Herts strain of Newcastle disease virus (NDVo) and a mutant isolated from persistently infected L cells (NDVpi). In CE cell cultures, both viruses synthesized significant amounts of 54, 36, and 18S RNA. However, in L cells, synthesis of 54S virion RNA was markedly reduced. From these results, it seems likely that the low yield of infective virus in L cells is due to a deficient synthesis of 54S RNA in this host. On this basis, however, it is apparent that the “covert” replication of NDVo in L cells is due to factors other than viral RNA synthesis. When low concentrations of interferon were used to pretreat CE cells, a differential effect on the synthesis of various RNA species was observed. The 18S RNA of NDVo was more sensitive to interferon action than the 36 and the 54S RNA species. In contrast, the 18S RNA of NDVpi was less sensitive than the 36S and the 54S RNA. The inhibition of 54S RNA synthesis correlated with the reduction of viral yield and explained the greater sensitivity of NDVpi to interferon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Freeburg L. C., Ho M. Effect of interferon on synthesis of Eastern equine encephalitis virus RNA. Proc Soc Exp Biol Med. 1971 May;137(1):13–18. doi: 10.3181/00379727-137-35502. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Robinson W. S. Replication of Sendai virus. I. Comparison of the viral RNA and virus-specific RNA synthesis with Newcastle disease virus. Virology. 1968 Aug;35(4):537–549. doi: 10.1016/0042-6822(68)90284-5. [DOI] [PubMed] [Google Scholar]
- Bratt M. A. RNA synthesis in chick embryo cells infected with different strains of NDV. Virology. 1969 Jul;38(3):485–488. doi: 10.1016/0042-6822(69)90163-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Isolation of the nucleic acid of Newcastle disease virus (NDV). Proc Natl Acad Sci U S A. 1965 Sep;54(3):794–800. doi: 10.1073/pnas.54.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Chenault S. S., Stevenson D., Acton J. D. Effect of interferon on polymerization of single-stranded and double-stranded mengovirus ribonucleic acid. J Bacteriol. 1966 Mar;91(3):1230–1238. doi: 10.1128/jb.91.3.1230-1238.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallum J. V., Thacore H. R., Youngner J. S. Factors affecting the sensitivity of different viruses to interferon. J Virol. 1970 Aug;6(2):156–162. doi: 10.1128/jvi.6.2.156-162.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallum J. V., Younger J. S. Quantitative aspects of inhibition of virus replication by interferon in chick embryo cell cultures. J Bacteriol. 1966 Oct;92(4):1047–1050. doi: 10.1128/jb.92.4.1047-1050.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mécs E., Sonnabend J. A., Martin E. M., Fantes K. H. The effect of interferon on the synthesis of RNA in chick cells infected with Semliki forest virus. J Gen Virol. 1967 Jan;1(1):25–40. doi: 10.1099/0022-1317-1-1-25. [DOI] [PubMed] [Google Scholar]
- RAKE A. V., GRAHAM A. F. KINETICS OF INCORPORATION OF URIDINE-C14 INTO L CELL RNA. Biophys J. 1964 Jul;4:267–284. doi: 10.1016/s0006-3495(64)86782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Wong K. T., Robinson W. S., Merigan T. C. Effect of interferon on the replication of Sendai virus. J Gen Virol. 1970 Nov;9(2):141–150. doi: 10.1099/0022-1317-9-2-141. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Cells persistently infected with Newcastle disease virus. 3. Thermal stability of hemagglutinin and neuraminidase of a mutant isolated from persistently infected L cells. J Virol. 1971 Jan;7(1):53–58. doi: 10.1128/jvi.7.1.53-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with Newcastle disease virus. II. Ribonucleic acid and protein synthesis in cells infected with mutants isolated from persistently infected L cells. J Virol. 1970 Jul;6(1):42–48. doi: 10.1128/jvi.6.1.42-48.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. R. Biological studies of interferon. I. Suppression of cellular infection with eastern equine encephalomyelitis virus. Virology. 1961 Mar;13:323–337. doi: 10.1016/0042-6822(61)90152-0. [DOI] [PubMed] [Google Scholar]
- Wong K. T., Robinson W. S., Merigan T. C. Inhibition of rubella virus-specific RNA synthesis by interferon. Proc Soc Exp Biol Med. 1971 Feb;136(2):615–618. doi: 10.3181/00379727-136-35324. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


