Abstract
Background
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an autoimmune disorder in which the use of immunotherapy and the long-term outcome have not been defined.
Methods
In this multi-institutional observational study (2007-2012), all patients with GluN1 antibodies were assessed at symptom onset and 4, 8, 12, 18, and 24 months using the modified Rankin Scale (mRS). Treatment included first-line immunotherapy (steroids, intravenous immunoglobulin, plasmapheresis), second-line immunotherapy (rituximab, cyclophosphamide), and tumor removal. Predictors of outcome were determined at the Universities of Pennsylvania and Barcelona using generalized linear mixed models with binary distribution.
Results
577 patients (1-85 years, median 21) were studied, 212 were children (<18 years). Treatment effects and outcome were assessable in 501 (median follow-up 24 months): 472 (94%) underwent first-line immunotherapy or tumor removal, resulting in improvement within four weeks in 251 (53%). Of 221 patients who failed first-line therapy, 125 (57%) received second-line immunotherapy resulting in better outcome than those who did not (OR 2·69, CI 1·24-5·80, p=0·012). During the first 24 months, 394/501 reached good outcome (mRS 0-2; median 6 months), and 30 died. At 24 month follow-up 204/252 (81%) had good outcome. Outcomes continued to improve for up to 18 months after symptom onset. Predictors of good outcome were early treatment (OR 0·62, CI 0·50-0·76, p<0·0001) and lack of ICU admission (OR 0.12, CI 0·06-0·22,p<0·0001). 45 patients had one or multiple relapses (representing a 12% risk within 2 years); 46/69 (67%) relapses were milder than previous episodes (p<0·0001). In 177 children, predictors of good outcome and the magnitude of effect of second-line immunotherapy were comparable to those of the entire cohort.
Conclusions
Patients with anti-NMDAR encephalitis respond to immunotherapy. Second-line immunotherapy is usually effective when first-line therapies fail. Recovery can take more than 18 months.
Keywords: anti-NMDA-receptor antibodies, encephalitis, paraneoplastic, teratoma, behavior, seizures, treatment, outcome
Introduction
In 2005, a syndrome with prominent psychiatric symptoms, memory loss, decrease of level of consciousness and central hypoventilation was described in four young women with ovarian teratoma and antibodies against an antigen highly expressed in the hippocampus.1 Soon thereafter the target antigen was identified as the N-methyl-D-aspartate receptor (NMDAR).2 The disorder, named “anti-NMDAR encephalitis”, has since been recognized in patients of all ages, but more frequently in young adults and children with or without teratoma.3, 4 Epidemiological studies suggest that this disorder is the most common cause of autoimmune encephalitis after acute demyelinating encephalomyelitis (ADEM).5 In a center focused in the study of encephalitis of unclear etiology the frequency of anti-NMDAR encephalitis surpassed that of any specific viral etiologies in young individuals.6 Patients usually present with acute behavioral change, psychosis and catatonia that evolve to include seizures, memory deficit, dyskinesias, speech problems, and autonomic and breathing dysregulation.3 The syndrome resembles the phenotypes obtained with genetic and pharmacological decrease of levels and function of NMDAR.7, 8 Experiments where patients’ antibodies were added to cultures of hippocampal neurons or infused into the hippocampus of rodents showed specific antibody-mediated internalization of NMDAR and alteration of the mechanisms of synaptic plasticity.9, 10 Despite the severity of the disease, patients often improve after intensive care support, immunotherapy, and prolonged hospitalizations that require multidisciplinary care.3, 11 However, the efficacy of immunotherapy and its impact in the long-term outcome have not been established. A substantial number of patients fail to respond to first-line immunotherapy, such as steroids, plasmapheresis, and intravenous immunoglobulins (IVIg) and for these patients the treatment strategy and outcome are unknown. Moreover, studies suggest that the syndrome and response to treatment may differ between children and adults.4 To address these issues, we report 577 patients, focusing on the presentation of the disease, the spectrum of symptoms, immunotherapies used, timing of improvement, and long-term outcome.
Methods
Antibody studies
NMDAR antibodies were determined in serum or cerebrospinal fluid (CSF) of patients seen at the Hospitals of the Universities of Pennsylvania and Barcelona, or whose samples were sent to these Institutions for study from January 1, 2007 until January 1, 2012. One hundred and thirty-five patients were seen by the authors, the other patients’ serum and CSF were collected from 200 centers world-wide (32 countries). Samples were considered positive if they fulfilled previously reported criteria3 including (1) a characteristic pattern of immunostaining of the neuropil of rat brain, and (2) specific reactivity with HEK293 cells expressing GluN1 (also known as NR1) subunits of the NMDAR.
Patients, diagnostic studies, and assessment of treatment and outcome
All patients tested positive for NMDAR antibodies were included in the study without selection criteria other than availability of clinical information. Clinical information was obtained by the authors or referring physicians at the acute stage of the disease. Based on the reported manifestations of this disorder,3 symptoms were categorized in 8 groups: behavior and cognition, memory, speech, seizures, movement disorder, loss of consciousness, autonomic dysfunction, and central hypoventilation. Children were defined as patients younger than 18 years. Previous reports suggesting differences in symptom presentation and tumor association in children and adolescents4 led us to examine these two features in three groups of patients: pre-pubertal (< 12 years), post-pubertal (12-17 years), and adults. Follow-up information was obtained at regular intervals (4, 8, 12, 18, and 24 months) after symptom onset; neurological status was assessed with the modified Rankin scale (mRS).12 Only results from the first MRI, EEG and CSF studies were assessed in this study. All patients were screened at least once for systemic tumors. First-line immunotherapy was defined as the use of steroids, IVIg or plasma exchange alone or combined; second-line immunotherapy included rituximab or cyclophosphamide alone or combined. There was no predefined protocol establishing the order or combination of treatments within each line of therapies. Initial treatment was considered a failure if no sustained improvement occurred within four weeks after initiation of immunotherapy or tumor removal, and if the mRS score remained ≥4. Patients with a follow-up shorter than 4 months were excluded from analysis of effects of treatment and outcome. Relapse of encephalitis was defined as the new onset or worsening of symptoms occurring after at least two months of improvement or stabilization. Written consent for studies was obtained from families and patient’s representatives; studies were approved by the Institutional Review Boards of the Universities of Pennsylvania and Barcelona.
Overall, 577 patients were included for demographic analyses; of these, 501 had a follow-up of at least 4 months (median 24 months, range 4-186).
Statistical analysis
Demographic information and symptoms were analyzed with Fisher exact, Fisher-Freeman-Halton test (an extension of the Fisher exact test for r x c contingency tables), or Mann Whitney U test when appropriate. Because of a skewed distribution, log-transformation was used for age at symptom onset, duration of follow-up, and delay until initiation of treatment. For untreated patients, the last date of follow-up (maximum 24 months) was considered as the delay in initiation of treatment. Factors influencing outcome were assessed by univariable binary logistic regression (good outcome defined as mRS 0-2) independently of the treatments given. Factors associated with a good outcome (p<0·1) were included in generalized linear mixed models with binary distribution, declaring the subject as random and the other factors as fixed effects.13 SAS, procedure GLIMMIX, version 9.3 (SAS Institute Inc., Cary NC, USA) was used. All mRS scores at defined time points were used (4, 8, 12, 18, and 24 months; e.g. each patient’s follow-up could provide a maximum of 5 scores). The “time point from onset of disease” was included as an additional variable, allowing us to analyze patients with different duration of follow-up. After creation of this model, the effect of second-line immunotherapy was determined by adding second-line immunotherapy to the same model applied to only those patients who had failed first-line immunotherapy.
Funding source
The study sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit to publication.
Results
Demographics
Four hundred and sixty-eight patients were female (81%; Figure 1A and Table S1). Two hundred and eleven (37%) of all 577 patients were younger than 18 years, and 28 (5%) were 45 or older. The frequency of males was higher in age groups below 12 years (43: 39%) and above 45 (12: 43%, p<0·0001). Two hundred and twenty (38%) patients had an underlying neoplasm; of these, 213 were women (representing 46% of all women). The presence of a tumor predominated in patients between 12 and 45 years; only 4 females younger than 12 years (6%), and 7 males (6%, all adults) had a tumor (p<0·0005). Two hundred and seven tumors (94%) were ovarian teratomas, 4 (2%) extraovarian teratomas, and 9 (4%) other tumors (two of each, lung, breast, testicular; one ovarian carcinoma, one thymic carcinomas, and one pancreatic cancer). Six of the nine patients with tumors other than teratomas were older than 45 years. Asian and African-American patients were more likely to have a teratoma (81 and 38 patients: 45% and 48%) than Caucasian (58: 31%) or Hispanics (23: 27%, p=0·007, Figure S1).
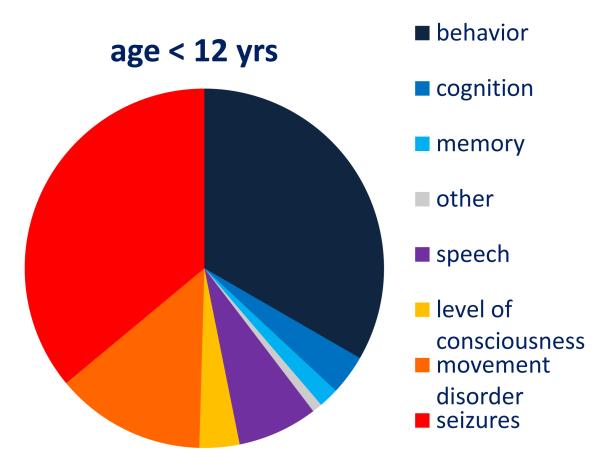
Figure 1. Demographic information, distribution by age of initial symptom, and cumulative symptoms during the first month of the disease.
Panel A: Patient’s age at disease onset
Panel B: Symptom presentation according to patients’ age (age <12 = 111 patients; 12-17 years = 99; ≥ 18 years = 364; 3 unknown). The frequency of abnormal behavior, movement disorder, and seizures is different among the three age groups (p<0·0001)
Panel C: Distribution by age of cumulative symptoms during the first month of the disease. For each color, the first dark column refers to patients < 12 years, the middle column to patients 12-17 years, and the right, light column to patients ≥ 18 years. *p = 0·002; **p < 0·0005; #p = 0·024.
Clinical and diagnostic features
Two hundred and thirty-eight adults (65%) presented with behavioral problems, while 55 children below 12 years (50%) presented with seizures or movement disorders (Figure 1B). In adolescents, the disease presented with a combination of symptoms from these groups. Within the first four weeks of the disease most patients developed a similar spectrum of symptoms regardless of age. While movement disorders were more frequent in children, memory deficits and central hypoventilation occurred more often in adults (Figure 1C). Atypical symptoms such as cerebellar ataxia or hemiparesis predominated in children. During the first month of the disease, 498 of 571 (87%) patients developed four or more of the 8 categories of symptoms; only 6 (1%) remained mono-symptomatic (Table 1). The assessment of disease severity showed that 495/571 patients (87%) had a maximum mRS of 5, and 435 (77%) were admitted to the ICU.
Table 1.
Number and severity of symptoms during the first month of the disease
| (n = 571)# | Non-tumor | Tumor | All | |||||
|---|---|---|---|---|---|---|---|---|
| number of symptoms * | 8 | 28 | 8% | 24 | 11% | 52 | 9% | 0·37 ** |
| 7 | 64 | 18% | 48 | 22% | 112 | 20% | ||
| 6 | 72 | 20% | 52 | 24% | 124 | 22% | ||
| 5 | 70 | 20% | 37 | 17% | 107 | 19% | ||
| 4 | 72 | 20% | 31 | 14% | 103 | 18% | ||
| 3 | 34 | 10% | 13 | 6% | 47 | 8% | ||
| 2 | 10 | 3% | 10 | 5% | 20 | 4% | ||
| 1 | 5 | 1% | 1 | 0% | 6 | 1% | ||
| maximum mRS | 5 | 296 | 84% | 199 | 91% | 495 | 87% | 0·086 † |
| 4 | 49 | 14% | 19 | 9% | 68 | 12% | ||
| 3 | 6 | 2% | 1 | 0% | 7 | 1% | ||
| Unknown | 1 | 0 | 1 | |||||
| ICU | Yes | 248 | 71% | 187 | 85% | 435 | 77% | < 0·0001 ‡ |
| No | 100 | 29% | 32 | 15% | 132 | 23% | ||
| Unknown | 4 | 4 | 4 | |||||
Clinical information was insufficiently detailed in six patients.
The eight symptom categories are: behavior/cognition, memory dysfunction, speech disorder/mutism, seizures, decrease in level of consciousness, movement disorder, autonomic dysfunction and central hypoventilation.
Fisher Exact test: 1-3 vs 4-8 symptoms, comparing non-tumor and tumor patients.
Fisher-Freeman-Halton test
Fisher Exact test
mRS = modified Rankin Scale; ICU = intensive care unit
MRI of the brain, EEG and CSF studies were abnormal in 180/540 (33%), 432/482 (90%) and 418/532 (79%) patients, respectively. Detection of NMDAR antibodies was compared in 250 paired serum and CSF samples randomly selected by use of an online random integer generator (http://www.random.org/integers/), showing more sensitivity in CSF than serum (100% vs 85%, p<0·0001, Table S2).
Treatments and outcome
Among the 501 patients with at least 4 months follow-up, 461 (92%) were treated with first-line immunotherapy, 134 (27%) with second-line immunotherapy (Figure 2 and Table 2), 10 (2%) with tumor removal without immunotherapy, and 29 (6%) were not treated (see below). The combination of first-line immunotherapy more frequently used was steroids and IVIG (202 patients, 44%). Of 197 patients with teratoma, 189 (96%) had tumor resection, 20 (11%) of them at neurological relapse or after recovery.
Figure 2. Flowchart of treatment groups.
Table 2.
Overview of treatments
| (n = 501) | Non-tumor | Tumor | All | Fisher Exact | ||||
|---|---|---|---|---|---|---|---|---|
| N | 304 | 197 | 501 | |||||
| Time from symptom onset | (median, IQ range) | 21 | (35) | 21 | (28) | 21 | (32) | 0·090 # |
| until treatment | (range; days) | (2-730) | (2-730) | (2-730) | ||||
| First-line immunotherapy | 283 | 93% | 179 | 91% | 462 | 92% | 0·40 | |
| Steroids | 265 | 87% | 156 | 79% | 421 | 84% | 0·024 | |
| IVIg | 221 | 73% | 125 | 63% | 346 | 69% | 0·030 | |
| Plasmapheresis | 80 | 26% | 83 | 42% | 163 | 33% | 0·0003 | |
| Second-line immunotherapy § | 93 | 31% | 41 | 21% | 134 | 27% | 0·017 | |
| Rituximab | 71 | 23% | 30 | 15% | 101 | 20% | 0·030 | |
| Cyclophosphamide | 50 | 16% | 31 | 16% | 81 | 16% | 0·90 | |
| Other immunotherapy † | 23 | 8% | 8 | 4% | 31 | 6% | 0·13 | |
| Time from symptom onset | (median, IQ range) | 1.4 | (1·9) | |||||
| until tumor removal | (range, months) | (−13 - 177) | ||||||
| Surgery ‡ | 14 | 5% | 189 | 96% | < 0·0001 | |||
| during initial episode | 14 | 169 | 86% | |||||
| at relapse | 0 | 7 | 4% | |||||
| after recovery | 0 | 13 | 7% | |||||
| Failure of first-line | yes | 145 | 48% | 76 | 39% | 221 | 44% | 0·069 |
| immunotherapy * | no | 138 | 45% | 103 | 52% | 241 | 48% | |
| surgery, no immunotherapy | 1 | 0% | 9 | 5% | 10 | 2% | ||
| no treatment | 20 | 7% | 9 | 5% | 29 | 6% | ||
Mann/Whitney U test
85% of the patients treated with rituximab received 4 weekly treatments of 375 mg/m2, 8% received 5-8 treatments; and 8% less than 4 treatments (most cases two treatments of 500 mg/m2 given two weeks apart). 73% of the patients treated with cyclophosphamide received 3-6 monthly cycles of 750 mg/m2, 15% received 7-12 cycles, and 12% 1-2 cycles. Four serious adverse events were reported including, one anaphylactic reaction and one infection due torituximab, both causing a delay in treatment, and one infection and one severe lymphopenia due to cyclophosphamide causing discontinuation of this drug. No treatment-related deaths or irreversible complications were identified.
Azathioprine, mycophenolate mofetil, tacrolimus or methotrexate
Eight patients with tumor did not have surgery: six had teratomas and five of them died of encephalitis; one was treated for an adenocarcinoma of the breast and the encephalitis improved; and 1 had a small cell lung cancer and died.
Twelve patients failed first-line therapies at relapse: seven without tumor had responded to first-line therapies at initial episode, the other five (one of them with teratoma) did not have immunotherapy at initial episode.
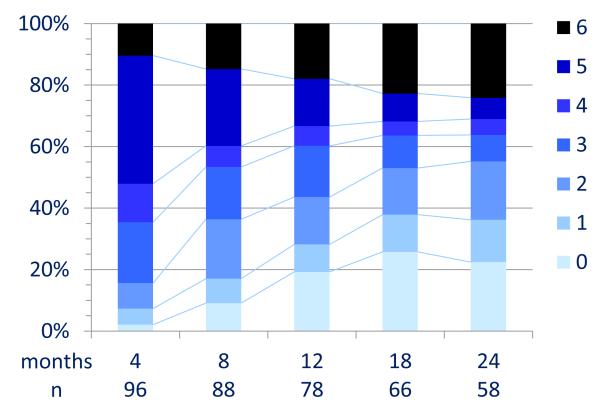
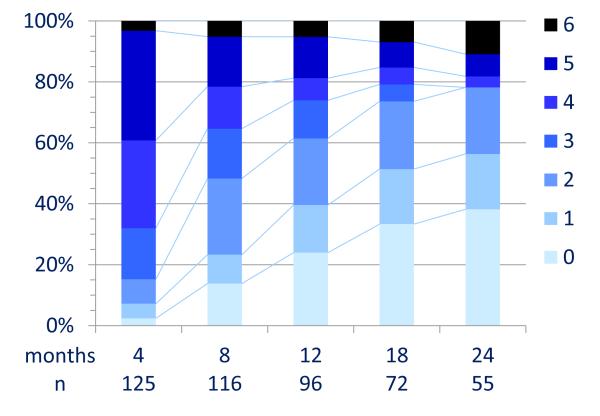
During the first 24 months, 394/501 reached an mRS of 0-2 (median 6 months). At 24 month follow-up 203/252 (81%) had a favorable outcome and 24 (9·5%) died (Figure 3A and Table S3). In univariable analysis, the factors associated with good outcome included, no need for ICU (p<0·0001), early treatment (p=0·009), and low severity of disease within four weeks of onset (maximum mRS, p=0·011). A longer follow-up was associated with a better outcome (p<0·0001, Table 3A). In multivariable analysis the factors associated with good outcome included, early treatment (OR 0·62, CI 0·50-0·76, p<0·0001) and no need for ICU (OR 0.12, CI 0·06-0·22, p<0·0001); the proportion of patients with good outcomes improved up until 18 months (Table 3B).
Figure 3. Clinical outcome after extended follow-up.
Panel A: All patients
Panel B: Patients who responded to first-line immunotherapy (steroids, IVIg, plasmapheresis)
Panel C: Patients who failed first-line immunotherapy and did not receive second-line therapy
Panel D: Patients who failed first-line immunotherapy and received second-line therapy (rituximab, cyclophosphamide, or both)
Outcome was measured by mRS. Twenty-nine patients included in panel A did not have immunotherapy or tumor removal.
Table 3.
Factors associated with good outcome (mRS 0-2).
| A. Univariable analysis | ||||||
|---|---|---|---|---|---|---|
| Factor | p | OR | 95% | CI | ||
|
|
||||||
| ICU-stay | < 0·0001 | 0·22 | 0·10 | 0·46 | ||
| time until treatment initiation (loge) | 0·009 | 0·79 | 0·66 | 0·94 | ||
| time of follow-up (loge) | < 0·0001 | 1·09 | 1·07 | 1·11 | ||
| maximum mRS | 0·011 | |||||
| 3 (n=7) | 0·569 | 1·86 | 0·22 | 15·59 | ||
| 4 (n=58) | 0·003 | 8·66 | 2·08 | 36·11 | ||
| 5 (n=436) | # | |||||
| age (loge) | 0·87 | 1·02 | 0·77 | 1·36 | ||
| age ≥18 | 0·92 | 0·98 | 0·62 | 1·53 | ||
| tumor | 0·55 | 1·15 | 0·74 | 1·78 | ||
| gender | 0·93 | 1·02 | 0·59 | 1·77 | ||
| B. Multivariable analysis | ||||||
| p | OR | 95% CI | events | |||
|
| ||||||
| ICU stay | < 0·0001 | 0·12 | 0·06 | 0·22 | 394 | |
| time until start treatment (loge) | < 0·0001 | 0·62 | 0·50 | 0·76 | 394 | |
| follow-up | < 0·0001 | |||||
| 4 months * | 0·04 | 0·02 | 0·06 | 224 | ||
| 8 months * | < 0·0001 | 0·20 | 0·12 | 0·35 | 110 | |
| 12 months * | 0·004 | 0·37 | 0·21 | 0·66 | 32 | |
| 18 months * | 0·007 | 0·76 | 0·42 | 1·37 | 22 | |
| 24 months * | 0·36 | 1·00 | 6 | |||
| maximum mRS | 0·51 | |||||
| C. Multivariable analysis first-line failure | ||||||
| p | OR | 95% CI | events | |||
|
| ||||||
| ICU stay | < 0·0001 | 0·07 | 0·02 | 0·25 | 133 | |
| time until start treatment (loget) | 0·005 | 0·55 | 0·36 | 0·83 | 133 | |
| follow-up | < 0·0001 | |||||
| 4 months * | 0·01 | 0·00 | 0·02 | 33 | ||
| 8 months * | < 0·0001 | 0·11 | 0·05 | 0·23 | 57 | |
| 12 months * | 0·004 | 0·26 | 0·12 | 0·54 | 22 | |
| 18 months * | 0·004 | 0·70 | 0·33 | 1·49 | 16 | |
| 24 months * | 0·35 | 1·00 | 5 | |||
| second-line treatment | 0·012 | 2·69 | 1·24 | 5·80 | 133 | |
A: Univariable analysis
B: Multivariable analysis for all patients (394/501 reached good outcome)
C: Multivariable analysis for patients who failed first-line immunotherapy (133/221 reached good outcome)
mRS 5 is the reference category
To assess continuous improvement over time, the outcome at each individual time point was compared with that of the previous time point, with the p values indicated as well as the amount of events in those specific months after the previous time point. After 8 months the number of patients achieving good outcome (or events”) becomes small (as reflected in the large confidence intervals). Patients improving from mRS 2 to 0 or 1 (or from 5 to 4 or 3) are not counted as events and therefore not visible in the table; these are all considered good outcome (or poor outcome) respectively.. The 24 month follow-up is the reference value for the odds ratios (and the associated lower and higher range of the confidence interval) of individual time points.
OR = odds ratio; CI = confidence interval; loge = natural logarithm; mRS = modified Rankin Scale; ICU = intensive care unit
Analysis of effects of immunotherapy
Of the 472 patients who were treated with first-line immunotherapy or tumor removal, 251 (53%) had symptom improvement within four weeks of treatment (Figure 2). During the first 24 months 241/251 reached an mRS 0-2 (median 3 months); at 24 month follow-up 111/115 (97%) of these patients had a good outcome (Figure 3B). Among the 221 (47%) patients who did not improve with these treatments, 125 (57%) received second-line immunotherapy and 96 (43%) had no further immunotherapy or continued receiving first-line immunotherapy (Figure 2).
Among patients who received second line immunotherapy, 84/125 reached an mRS 0-2 during the first 24 months, while among those who did not receive second line immunotherapy 49/96 reached an mRS 0-2 (median 10 and 15 months, respectively); at 24 month follow-up 43/55 (78%) and 32/58 (55%) had a good outcome (Figure 3C and 3D). Multivariable analysis identified the use of second-line immunotherapy as an additional factor for good outcome (OR 2·69, CI 1·24-5·80, p=0·012, Table 3C).
Twenty-nine patients were not treated with immunotherapy or surgery. The reasons for no treatment included, first diagnosis of the disease during relapses in 14 patients (initial episodes not treated), retrospective diagnosis in 9 patients (4 postmortem, and 5 using archived serum or CSF samples), and spontaneous improvement within a few weeks in 6 patients. Overall, in this group, 29% had a poor outcome (mRS 3-5 or dead).
Relapses
During the 24 month follow-up, 45 patients (representing a 12% risk) had clinical relapses, which in 15 patients (33%) were multiple (Table S4). Compared with the initial episode, 46/69 (67%) relapses were less severe (as reflected by a lower mRS score measured at the stage of maximum severity), more frequently mono-symptomatic (24, 35%), and resulted in fewer admissions (12, 17%) to the ICU (all p<0·0001). In 16 relapses (23%) the severity of symptoms was comparable to that of the initial episode, and in 7 (10%) was worse. Patients without a tumor had a higher frequency of relapses than those with a tumor (Figure 4A; p=0·0007). Only 12 patients with teratoma identified at symptom presentation or thereafter had neurological relapses (Table S4).
Figure 4. Frequency of relapses.
Panel A: Patients with tumor had fewer relapses than those without tumor (p = 0·0007).
Panel B: The use of immunotherapy associated with fewer relapses (p = 0·038). Second-line immunotherapy associated with fewer relapses in patients without tumor (see Figure S2).
Table S5 shows the treatment and outcome (best mRS) of the last relapse compared to those of the initial episode. The use of immunotherapy in the initial episode of encephalitis associated with a lower frequency of relapses (Figure 4B, p=0·038). A relapse-decreasing effect of second-line immunotherapy was observed in patients without tumor (Figure S2B, p=0·007). Similarly, the introduction of second-line immunotherapy during relapses decreased the frequency of subsequent relapses (Figure S3, p=0·024).
Treatment and outcome in patients younger than 18 years
Compared with adults, the time between symptom onset and initiation of treatment was shorter in children (21 versus 28 days, p=0·007, Table S6). Overall the outcome was similar to that of adults (p=0·92, Table 3A and Figure S4). Within the group of children, multivariable analysis identified similar risk factors as those of adults (Table S7). Eighty-two of 177 (46%) children failed first-line immunotherapy; in these patients, the magnitude of the effects of second-line immunotherapy (used in 53 cases, 65%; Table S5) was comparable to that of the entire cohort although due to the smaller sample size it did not reach significance (Table S6B, OR 3·35, CI 0·86-12·98, p=0·081).
Discussion
This large, observational study demonstrates that immunotherapy and tumor removal if applicable, result in substantial neurological improvement in 81% of patients with anti-NMDAR encephalitis after a median follow-up of 24 months. Moreover, second-line immunotherapy with rituximab, cyclophosphamide or both significantly improved the outcome of those who did not respond to first-line therapy, and decreased the frequency of relapses.
Symptom presentation varied between children and adults (“more neurological in children”, “more psychiatric in adults”), but in most cases the progression of symptoms evolved towards a similar syndrome. One month after disease onset, 87% of all patients had four or more of the indicated groups of symptoms. Recognition of this constellation of symptoms, which usually includes alteration of cognition and behavior (delusions, psychosis, catatonia) and abnormal movements (orofacial dyskinesias, choreoathetosis), should prompt testing for antibodies against GluN1 subunit of the NMDAR, especially in young individuals.
Identification of NMDAR antibodies confirms the diagnosis of the disorder and should lead to the search of a tumor which if present, is almost always an ovarian teratoma that contains nervous tissue and expresses NMDAR.14, 15 Teratomas located elsewhere and other tumors are uncommon. In the current study, the frequency of an underlying teratoma was significantly greater in females aged 12 years or older than in young children and males (52% versus 6%). Therefore, if a tumor is not found, the extent and frequency of tumor screening should take into consideration the patient’s age and gender. In females aged 12 years or older a screening approach similar to that of paraneoplastic syndromes is appropriate (e.g., MRI of the abdomen and pelvis every 6 months for 4 years)16 but in young children and males the need for repeat screening is unclear. If a tumor is found, removal is important because it expedites improvement and decreases relapses.3, 17
Two independent predictors of good outcome included the lower severity of symptoms assessed as no need for ICU support, and the prompt initiation of immunotherapy and tumor removal, if appropriate. A notable finding was the protracted phase of recovery that continued until the 18 month follow-up. By establishing the last follow-up assessment at 24 months, our study likely underestimates the frequency and level of recovery because some patients had a shorter follow-up, others continued to improve after 24 months, and those with substantial improvement tended to discontinue follow-ups (Figure S5A and B). For the same reasons, the frequency of mortality is likely overestimated; imputation analysis estimated 7% mortality at 24 months (Figure S5C).
Decisions about the type and duration of immunotherapy were made by the treating physicians and based on clinical symptoms, not antibody titers. Ninety-two percent of patients underwent first-line immunotherapy including steroids, IVIg, and plasmapheresis alone or combined. This treatment and tumor removal, if applicable, resulted in improvement within the first 4 weeks of therapy in 53% of the patients, and 97% of them showed a good outcome at 24 month follow-up (mRS 0-2). The main problem was posed by the 47% of the patients who did not improve with first-line therapy within the first four weeks of starting this treatment. These patients continued with poor mRS scores (median 5). In this group, patients who received rituximab, cyclophosphamide or both had better outcomes than those who continued with first-line immunotherapy or received no further immunotherapy. Similar results were obtained for pediatric patients; in this smaller group the effect of second-line immunotherapy did not reach statistical significance but the estimated benefit was similar to the entire cohort (odds ratios 3·35 vs 2·69). Second-line immunotherapy was well tolerated; only 4 patients had serious adverse effects, all reversible (Table 2).
In this study, the effect of individual treatments (e.g., plasma exchange vs IVIg or rituximab) could not be compared side-by-side statistically. This is because the effect or lack of effect of a certain treatment directly impacted physicians’ decisions about additional treatment leading to the apparent paradox “the more you treat, the worse the outcome”, while in fact “the worse the patient is doing, the more treatments are used”. However, we could statistically assess the impact of adding second-line immunotherapy to patients whose symptoms were refractory to first-line therapies. Indeed, after failing first-line immunotherapy, all these patients remained in a similar poor neurological status, enabling the comparison between those who subsequently received second-line immunotherapy with those who did not. The choice of adding second-line immunotherapy was based on physician’s preference, family acceptance of potential side effects, and availability of the drugs. Although selection bias cannot be ruled out in observational studies like this, we could not identify obvious bias between patients who after failing first-line therapies were treated with second-line therapy and those who did not: the severity of symptoms and duration of ICU stay was similar (92% vs 88%, and 88% vs 87%, respectively), and there were no differences in age, gender, ethnicity, and delay of initial immunotherapy.
The lower frequency of neurological relapses (12% versus 20-24% reported in previous studies)11, 18 is likely due to better recognition of the disorder, earlier treatment, and increasing use of second-line immunotherapy. Indeed, patients who received second-line immunotherapy during the initial episode of encephalitis had fewer relapses. While this finding should be considered with caution due to the small number of patients, a similar effect was observed in patients who had previous relapses; in these patients the introduction of second-line immunotherapy reduced the frequency of subsequent relapses. The presence of a teratoma not detected or untreated during the initial episode, or the possibility of tumor recurrence, should be considered in patients with a relapse.
The discovery of anti-NMDAR encephalitis has changed the diagnostic and treatment approach to several neurologic and psychiatric disorders, and has led to the identification of an expanding group of autoimmune encephalitis related to antibodies against synaptic proteins.19 Rapidly progressive alterations of memory, behavior, cognition, and psychosis previously thought to be idiopathic or viral-induced are now known to be autoimmune.20-23 To our knowledge, a systematic analysis of the effects of sequential (first-, second-line) immunotherapy and their impact on long-term outcome has not been previously done in any of these disorders or other antibody-mediated neurological diseases.
Our study has the limitations of not being randomized but sets the base for future trials to determine the efficacy of each individual treatment (e.g., steroids, IVIg, plasmapheresis, rituximab, and cyclophosphamide) and duration of immunotherapy. Moreover, the large number of events (patients achieving a good outcome) and prospective follow-up of many cases reported here, provide level II-2 evidence (US Preventive Services Task Force)24 of the usefulness of immunotherapy and tumor removal in anti-NMDAR encephalitis.
Research in context
Systematic review
We searched in Medline and Embase databases up until Oct 01, 2012 for articles published in any language with the search terms “Anti-N-Methyl-D-Aspartate Receptor Encephalitis”[MeSH Terms], ““Receptors, N-Methyl-D-Aspartate”[MeSH Terms]”, and “Encephalitis”[MeSH Terms]. We restricted searches to human studies. We also reviewed the reference lists of the papers identified by this search. Ten studies and one extended review describing at least three patients were identified; five of them included at least twenty-five patients.
Interpretation
Our study extends epidemiological and clinical knowledge on anti-NMDAR encephalitis. It confirms the female preponderance, the high frequency of the disease in children, and a higher risk for an underlying teratoma in African-Americans. The higher frequency of teratomas in Asian patients is also a novel finding. The manuscript establishes that the clinical presentation in children is often different from that of the adults; this has been previously suggested in small series but not confirmed until now. In the current study, the frequency of relapses was somewhat less frequent than in previous reports; in addition, the milder nature of the symptoms compared with the initial episodes was noted in most relapses. This is the first study to provide extensive and structured follow-up and prognostic factors determining outcome. The suggested better outcome of patients with teratoma compared with those without teratoma was not confirmed in this study; however, “ICU stay” and “time from symptom onset until initiation of treatment” were identified as significant prognostic factors. First-line immunotherapy was often insufficient to control the disease; in this group of patients the addition of second-line immunotherapy proved to associate significantly with a better outcome compared with those who did not receive second-line immunotherapy.
Supplementary Material
Acknowledgements
We would like to thank all physicians, patients and families that provided clinical information.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: MT is supported by a KWF fellowship 2009-4451 of the Dutch Cancer Society. Supported in part by NIH RO1NS077851 (JD), RO1CA89054 (JD), and RC1NS068204 (RB-G & JD); a McKnight Neuroscience of Brain Disorders award (RB-G & JD), Fondo de Investigaciones Sanitarias (FIS, Spain, 11/01780, JD; PS09/0193, FG; and 08/00285, EM-H), Fundació la Marató de TV3 (JD), and NIA (P50AG08702), the Alzheimer’s Association, the Alzheimer’s Disease Drug Discovery Foundation, and the Taub Institute (LSH). JD receives royalties from Athena Diagnostics for a patent for the use of Ma2 as autoantibody test, and licensing fees from Euroimmun for a patent for the use of NMDAR as autoantibody test. None of the funding sources had any influence on the data collection, analysis, interpretation or writing process.
Authors’ contributions: MJT: literature search, figures, study design, data collection, data analysis, data interpretation, writing, critical approval final manuscript, funding. LMcC: data collection, data analysis, critical approval final manuscript. IG: data collection, data analysis, critical approval final manuscript. TA: data collection, data analysis, critical approval final manuscript. CG: data collection, data analysis, critical approval final manuscript. TI: data collection, data analysis, critical approval final manuscript. LSH: data collection, data analysis, critical approval final manuscript. SB: data collection, data analysis, critical approval final manuscript. IK: data collection, data analysis, critical approval final manuscript. EMH: data collection, data analysis, critical approval final manuscript. EA: data collection, data analysis, critical approval final manuscript. NGA: data collection, data analysis, critical approval final manuscript. NRF: data collection, data analysis, critical approval final manuscript. AT: data analysis, data interpretation, critical approval final manuscript. AS: data collection, data analysis, critical approval final manuscript. MRR: data collection, data analysis, writing, critical approval final manuscript. RBG: data collection, data analysis, critical approval final manuscript. FG: data collection, data analysis, data interpretation, critical approval final manuscript, funding. JD: figures, study design, data collection, data analysis, data interpretation, writing, critical approval final manuscript, funding
Reference List
- 1.Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–8. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–44. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 6.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The Frequency of autoimmune N-Methyl-D-Aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 8.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 9.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–75. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikasova L, De RP, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–21. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 11.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–67. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 13.Verbeke G, Molenberghs G. Springer-Verlag. New York: 2005. Models for discrete longitudinal data. [Google Scholar]
- 14.Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–43. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabner M, McCluggage WG, Bundell C, et al. Ovarian Teratoma Associated With Anti-N-Methyl D-Aspartate Receptor Encephalitis: A Report of 5 Cases Documenting Prominent Intratumoral Lymphoid Infiltrates. Int J Gynecol Pathol. 2012;31:429–37. doi: 10.1097/PGP.0b013e31824a1de2. [DOI] [PubMed] [Google Scholar]
- 16.Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol. 2011;18:19–e3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabin TD, Jednacz JA, Staats PN. Case records of the Massachusetts General Hospital. Case 26-2008. A 26-year-old woman with headache and behavioral changes. N Engl J Med. 2008;359:842–53. doi: 10.1056/NEJMcpc0804644. [DOI] [PubMed] [Google Scholar]
- 18.Gabilondo I, Saiz A, Galan L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77:996–9. doi: 10.1212/WNL.0b013e31822cfc6b. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster E, Dalmau J. Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–90. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman MR, Vause HE. Anti-NMDA Receptor Encephalitis: Diagnosis, Psychiatric Presentation, and Treatment. Am J Psychiatry. 2011;168:245–51. doi: 10.1176/appi.ajp.2010.10020181. [DOI] [PubMed] [Google Scholar]
- 21.Kayser MS, Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J Neuropsychiatry Clin Neurosci. 2011;23:90–7. doi: 10.1176/appi.neuropsych.23.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruss H, Holtje M, Maier N, et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. 2012;78:1743–53. doi: 10.1212/WNL.0b013e318258300d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruss H, Kinke C, Holtje M, et al. NMDA receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012 Jul 16; doi: 10.1002/ana.23689. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S.Preventive ServicesTask Force . Guide to clinical preventive services: report of the U.S. Preventive Services Task Force. DIANE Publishing; Aug, 1989. p. 24. ISBN 978-1-56806-297-6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.