Abstract
Aim
As part of an on going investigation of novel anticancer agents from natural origin, the biological and cellular effects of (5Z)-7-oxozeaenol on cancer cells were investigated.
Materials and Methods
The expression of nuclear factor kappa B (NF-κB), IκB kinase (IKKα), IKKβ and caspase-3 were analyzed by western blot. Reactive oxygen species (ROS) fluorescence and caspase luminescent assays were used to assess the intracellular effects in HeLa cervical and HT-29 colon cancer cell lines. The mitochondrial transmembrane potential (MTP) was analyzed by fluorescence-activated cell sorting (FACS).
Results
Cells treated with (5Z)-7-oxozeaenol exhibited down-regulation of NF-κB in a dose-dependent manner. Treatment with (5Z)-7-oxozeaenol significantly enhanced the levels of ROS in HeLa and HT-29 cells. MTP was reduced in HT-29 cells. The expression of caspase-3 and -7 was induced in (5Z)-7-oxozeaenol treated HeLa cells, in comparison with those treated with paclitaxel.
Conclusion
Our findings suggest that (5Z)-7-oxozeaenol is a potent inhibitor of the NF-κB pathway and potentiates the production of ROS, as well as induces caspase-3 and -7 in HeLa and HT-29 cancer cells. Thus, (5Z)-7-oxozeaenol represents a new lead compound for drug development, particularly as a new cancer chemotherapeutic agent, since programmed cell death might be mediated through the activation of a caspase-arbitrated pathway.
Keywords: (5Z)-7-Oxozeaenol, NF-κB pathway, apoptosis, caspase, IKKα, IKKβ, ROS, HeLa, HT-29 cells
The transcription factor NF-κB is a heterodimer composed of subunits p50 and p65 (1). IκB kinase (IKKβ) and NF-κB are induced in response to extracellular inflammatory stimuli e.g. by proinflammatory cytokinins (IL-1), tumor necrosis factor-α (TNF-α), and epidermal growth factors (EGF) (2). When inactive, NF-κB is found sequestered in the cytoplasm, bound to subunit inhibitor of kappa-B (IκB). After phosphorylation of IκB by IKK, NF-κB is subsequently released and translocated to the nucleus, where it exerts its effects, activating a wide range of target genes that are involved in apoptosis inhibition, cell adhesion, and cell mediation. Induction of NF-κB prevents cancer cells from entering apoptosis (3). As a result, cancer cells are induced to survive instead of undergoing apoptosis. Thirty-five out of the 60 NF-κB-related genes are up-regulated in inflammatory breast cancer (4), which are related to apoptosis, immune response, proliferation, tumor promotion and angiogenesis. The activation of the NF-κB cell signaling pathway is responsible for survival, proliferative control, and, moreover, it promotes the resistance to certain tumorigenic agents and chemotherapy (4).
In this study, the effects of the resorcylic acid lactone, (5Z)-7-oxozeaenol (Figure 1), on the oxidative pathway of cervical (HeLa) and colonic (HT-29) cancer cells, were examined. (5Z)-7-Oxozeaenol was previously isolated from a filamentous fungus (5) and was reported by our group as an NF-κB inhibitor. It has previously been shown by the Matsumoto research group that (5Z)-7-oxozeaenol inhibits transforming growth factor β-activated kinase 1 (TAK1), a member of the mitogen-activated kinase (MAPK) family, likely by competitively binding at the ATP-binding site. Moreover, the inhibition of TAK1 resulted in decreased IKKβ activity (6). The data presented herein indicate that (5Z)-7-oxozeaenol inhibits the NF-κB survival pathway (7, 8), interfering with TNFα signaling, and possibly through targeting and inhibition of TAK1 (9). The expression of NF-κB p65, p50, IKKα, IKKβ and caspase-3 were studied by western blot analysis in HeLa cells. The production of intracellular ROS and the mitochondrial membrane potential were also evaluated using HT-29 cells.
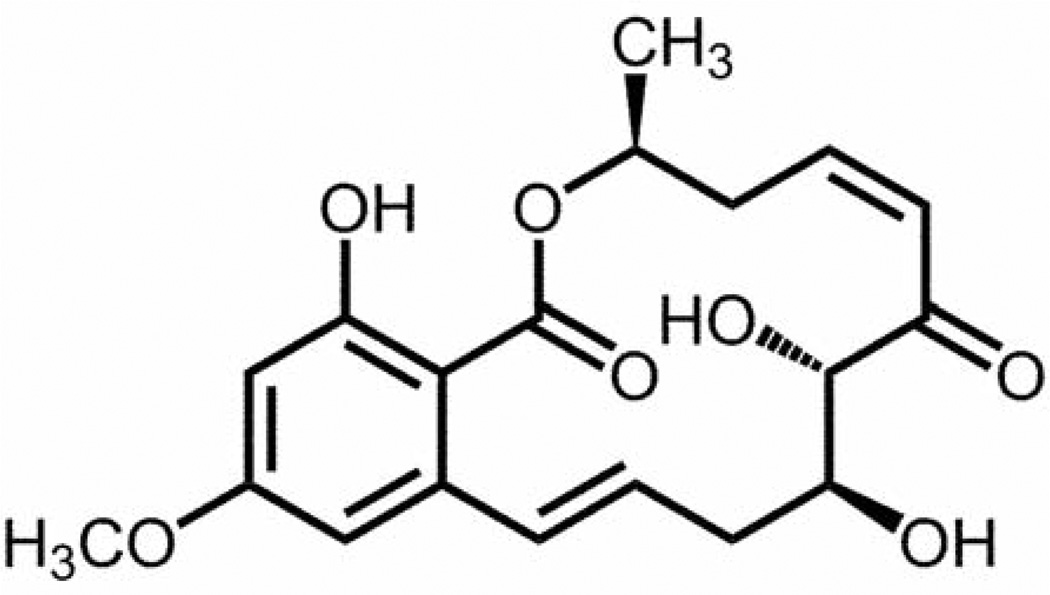
Figure 1.
Chemical structure of (5Z)-7-oxozeaenol (5).
Secondary metabolites of natural origin, such as (5Z)-7-oxozeaenol, represent new lead compounds in drug development, particularly as new cancer chemotherapeutic agents (10). For example, a closely related analog of (5Z)-7-oxozeaenol, termed E6201, is in phase II clinical trials by Eisai Inc. for the treatment of melanoma (11, 12), as well as being a possible topical agent for treatment of psoriasis (13).
Materials and Methods
Compound source
(5Z)-7-Oxozeaenol was isolated from a filamentous fungus by our research group, as part of the drug discovery program (5). Vitamin C, iron sulphate (FeSO4) and hydrogen peroxide (H2O2) were purchased from Sigma Aldrich (St. Louis, MO, USA). Rocaglamide was obtained from Enzo Life Sciences, Inc (Farmingdale, NY, USA).
Cell culture
Colonic cancer HT-29 and cervical cancer HeLa cell lines were obtained from the American Type Culture Collection, Manassas, VA, USA. HT-29 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine calf serum (FBS) (Gibco, Rockville, MD, USA) and 10% antibiotic-antimycotic. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine calf serum and 10% antibiotic-antimycotic from Gibco. Cells were kept at 37°C, in an atmosphere with 5% CO2.
Immunoblotting
To observe the effects of (5Z)-7-oxozeaenol on the NF-κB pathway, HeLa cells were treated over a range of concentrations (0.008, 0.016, 0.4, 2.0 and 10 µM) for 24 h. Briefly, cells were then lysed using PhosphoSafe Lysis Buffer (Novagen, Madison, WI, USA). Protein concentration in the lysates was determined by using the Bradford protein assay kit and the albumin standard (Thermo Scientific, Rockford, IL, USA). Optical density of protein solutions from the cell extracts and of the serial dilutions from the albumin standard were measured at 562 nm, using Fluostar Optima plate reader (BMG Labtech Inc, Durham, NC, USA). Lysates were analyzed by western blot. Equal amounts of protein (20 µg) were loaded together with lithium dodecyl sulphate (LDS) sample loading buffer (Invitrogen, Carlsbad, CA, USA) and resolved using Nu-PAGE 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) Bis-Tris gels, together with See Blue® Plus 2 Pre-Stained Standard Ladder (Invitrogen). Electrophoresis was performed using SDS-PAGE running buffer in a Nu-PAGE X Cell Sure Lock Module from Invitrogen. Proteins were transferred from the gel to a polyvinyldiene fluoride (PVDF) membrane using tris buffered saline with tween-20 buffer (TBS-T). The blots were then blocked at room temperature using non-fat milk and subsequently probed using primary antibodies (1:1000) against each target protein using 1% bovine serum albumine (BSA) in TBS-T overnight. Primary antibodies (caspase-3, NF-κB p65 (65kDa), p50 (50kDa), IKKα and IKKβ) were purchased from Cell Signaling Technologies (Beverly, MA, USA). The secondary, horseradish peroxidase (HRP) conjugated antibodies (1:2000) were obtained from Santa Cruz Biotechnology Inc, (Santa Cruz, CA, USA). Conjugated antibodies were detected using Chemiluminescent substrates Supersignal Femto LumiGLO kit from Thermo Scientific.
Mitochondrial transmembrane potential (ΨΔm) assay
The Mitochondria Transmembrane Potential (MTP) fluorescence-activated cell sorting (FACS) assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) was used to assess the MTP (ΨΔm) in colon cancer cells, after treatment using FACS analysis (14). The cells were seeded on 10-cm plates and treated with (5Z)-7-oxozeaenol for 24 h. After this time, the cells were harvested using Trypsin-EDTA (Gibco), washed in phosphate-buffered saline (PBS) and re-suspended in assay buffer. The potentiometric dye 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazoylcarbocyanine iodide (JC-1) was used to stain the HT-29 cells. A volume of 50 µl of JC-1 stain was added to the cells, followed by incubation for 15 min at 37°C in 5% CO2. At high ΨΔm, red fluorescent J-aggregates are formed in healthy cells; however, in apoptotic cells with low ΨΔm, JC-1 remains in the monomeric form, which only exhibits green fluorescence. Analysis was performed using FACS Canto II (BD Biosciences, San Jose, CA, USA). The mitochondrial function was assessed, and J-aggregates were detected using an excitation wavelength of 520–570 nm, and emission of 570–610 nm, respectively.
ROS assay
The assay was performed following a previously described procedure (15). The fluorescent probe 2’,7’-dichlorfluorescein-diacetate (DCFH-DA) was used to detect the presence of ROS in (5Z)-7-oxozeaenol treated cells, in the absence and in the presence of H2O2. Cells were seeded in 96-well plates and treated with (5Z)-7-oxozeaenol (0.28 µM-280 µM) or the controls, followed by a 5 h incubation before treated with H2O2 (1.25 mM) and FeSO4 (0.2 mM) for 30 min. Vitamin C (0.28 µM-280 µM) and H2O2 were used as controls. The fluorescent probe DCFH-DA was added to detect intracellular ROS. Fluorescence was detected and measured using FLUOstar Optima fluorescence plate reader (BMG Lab technologies GmbH, Inc., Durham, NC, USA), with an excitation wavelength of 485 nm and on emission wavelength of 530 nm. All treatments were performed in triplicate and the data shown are representative of at least two different experiments.
Caspase-3/7 assay
Caspase-3 and 7 activities were measured using the Caspase–Glo3/7® assay from Promega, Madison, WI, USA. HeLa cells were treated with different concentrations of (5Z)-7-oxozeaenol (0.08–10 µM) for 3 h. Paclitaxel (0.0001–1 µM) was used as a positive control. Caspase–Glo3/7® reagent was then added and luminescence was recorded using Fluostar Optima plate reader (BMG Lab technologies GmbH, Inc., Durham, NC, USA) at 37°C and the enzymatic activity was calculated. Each data point is an average of triplicate readings from two separate experiments.
Results
Immunoblotting
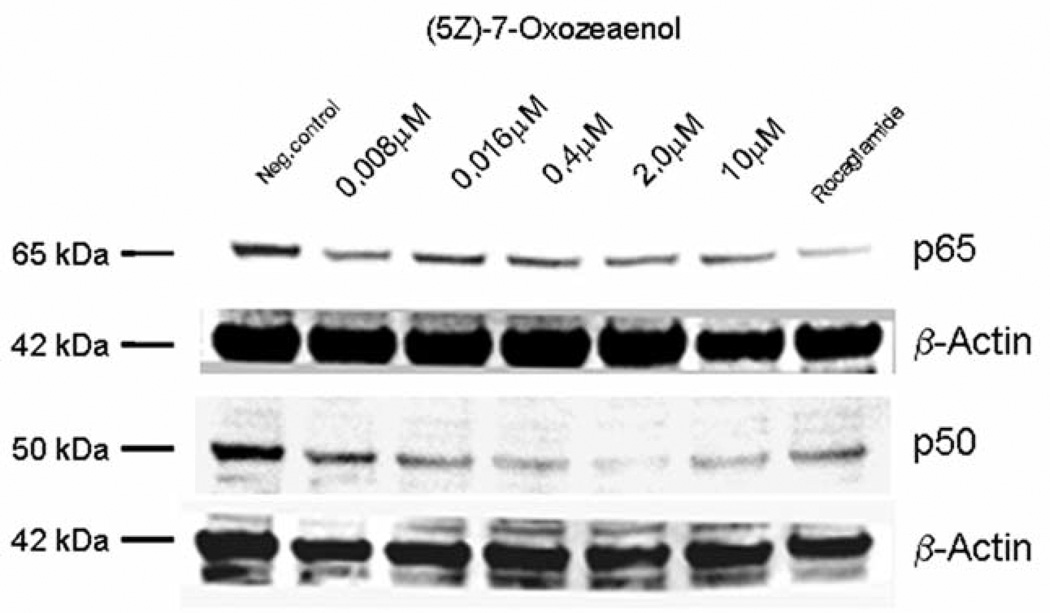
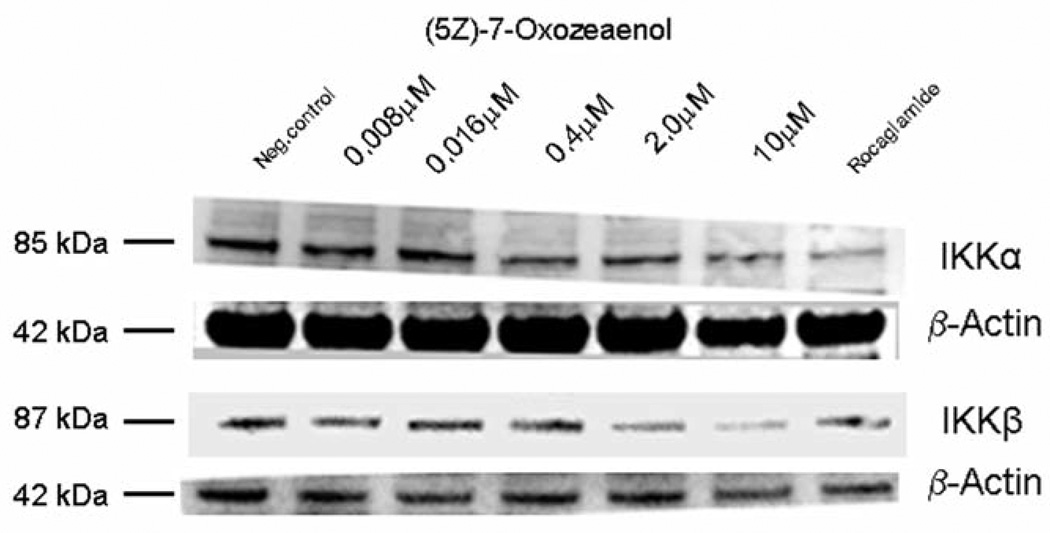
In a previous report, our natural product drug discovery program identified (5Z)-7-oxozeaenol (0.25 µM) (Figure 1) as being an NF-κB inhibitor in comparison with the positive control rocaglamide (0.075 µM) (5). In this study, NF-κB inhibition induced by (5Z)-7-oxozeaenol was further confirmed by western blot analysis. The inhibitory effects of (5Z)-7-oxozeaenol on mediators of the NF-κB pathway, and more specifically the attenuating effect on NF-κB p65 and p50, was found to be concentration-dependent (Figure 2). Similarly, the expression of IKKβ, an up-stream mediator in the pathway, was found to be down-regulated in a concentration-dependent manner in treated cells (Figure 3).
Figure 2.
Western blot analysis performed on HeLa cellular extracts showed that (5Z)-7-oxozeaenol suppresses NF-κB p65 and p50 activation. A decrease in activity was detected upon treatment with higher concentrations of (5Z)-7-oxozeaenol.
Figure 3.
Expression of IκB kinase alpha (IKKα) and IKKβ was down-regulated in treated HeLa cells. β-Actin was used as an internal control to establish equivalent loading.
MTP FACS assay
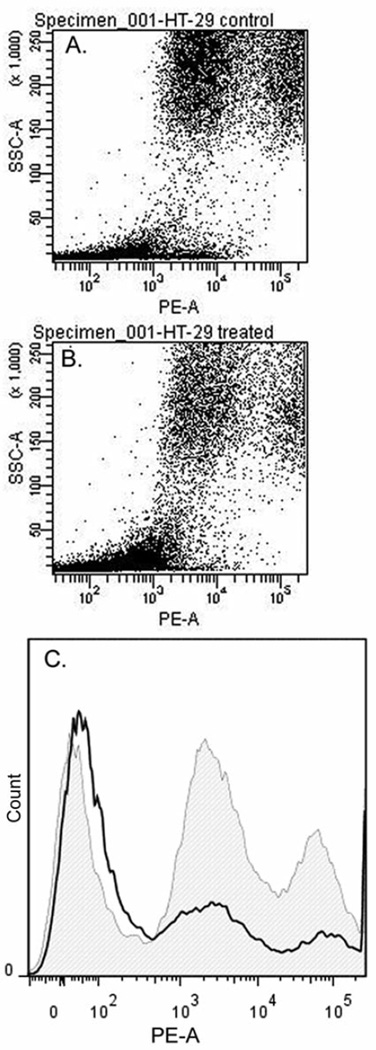
(5Z)-7-Oxozeaenol was also reported by our group previously, as having a moderate effect on the mitochondrial membrane potential after treatment for a period of 3 h. This effect was further examined here after treating HT-29 cells for 24 h, by using JC-1 staining to assess the ΨΔm. Treated HT-29 cells had fewer JC-1 aggregates than the untreated cells (Figure 4).
Figure 4.
JC-1 aggregates in untreated HT-29 cells detected at a wavelength of 564–606 nm (A). JC-1 monomers detected on treated cells (B). Mitochondrial transmembrane potential (ΔΨm) assay showed J-aggregates detected at 564–606 nm in untreated, non-apoptotic HT-29 cells (in gray), and apoptotic HT-29 cells after 24-h treatment with (5Z)-7-oxozeaenol (in black) (C).
ROS assay
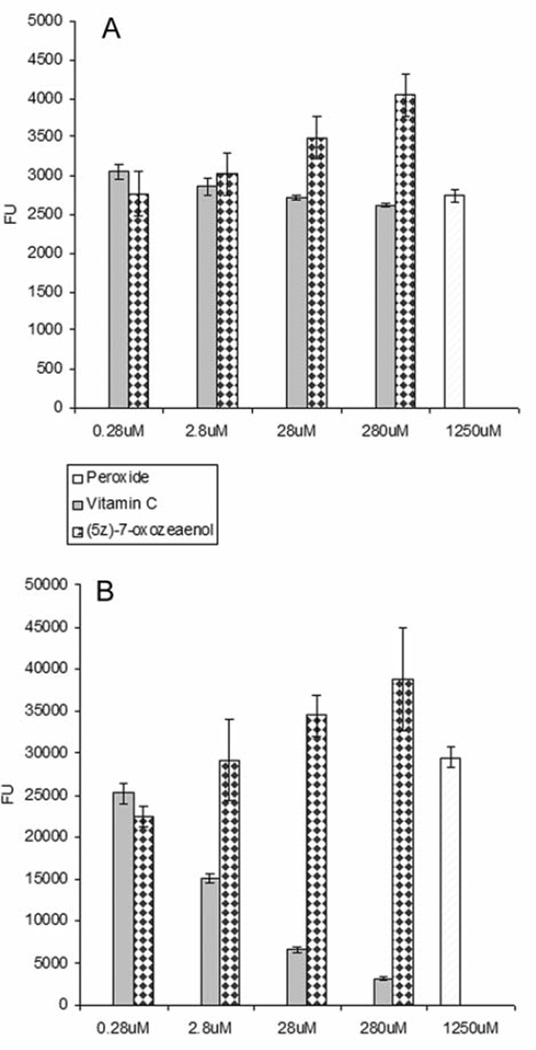
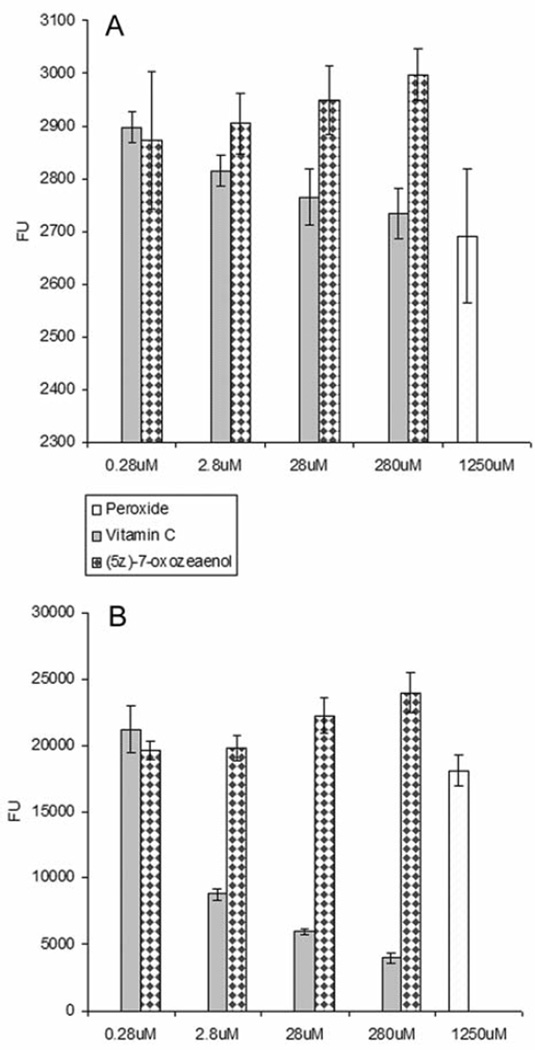
The ROS assay was performed in the absence and the presence of H2O2/FeSO4 in HeLa and HT-29 cells (Figures 5 and 6), respectively. H2O2 and Vitamin C were used as controls. The intracellular levels of ROS increased in a concentration-dependent manner when cells were treated with (5Z)-7-oxozeaenol in the absence of H2O2. (5Z)-7-Oxozeaenol was also tested in combination with H2O2 to evaluate its potentiating effect in increasing ROS levels. Noticeable, (5Z)-7-oxozeaenol induced significantly the elevation of intracellular ROS in the absence and in the presence of H2O2.
Figure 5.
Reactive oxygen species (ROS) evaluation of (5Z)-7-oxozeaenol using HeLa cells in the absence (A) and presence (B) of hydrogen peroxide (H2O2). In this assay, H2O2 and vitamin C were used as positive and negative controls, respectively. Cells were treated at four different concentration levels (0.28 µM–280 µM) for 5 h. ROS was detected with fluorescent probe 2’,7’-dichlorfluorescein-diacetate (DCFH-DA) at 485 nm emission wavelength and 530 nm excitation wavelength. Each experiment was carried out in triplicate and the graph represents the results of two separate experiments. Values in (A) and (B) represent the means of fluorescence units (FU) ± SEM of triplicate samples from two independent experiments.
Figure 6.
Reactive oxygen species (ROS) evaluation in HT-29 cells treated with (5Z)-7-oxozeaenol in the absence (A) and presence (B) of peroxide (H2O2). The cells were treated at four different concentrations (0.28 µM–280 µM) for 5 h. ROS were detected with fluorescent probe 2’,7’-dichlorfluorescein-diacetate (DCFH-DA) at 485 nm emission wavelength and 530 nm excitation wavelength. In this assay, H2O2 and vitamin C were used as positive and negative controls, respectively. Each experiment was carried out in triplicate and the graph represents the results of two separate experiments. Values in (A) and (B) represent the means of fluorescence units (FU) ± SEM of triplicate samples from two independent experiments.
Caspase-3/7 assays
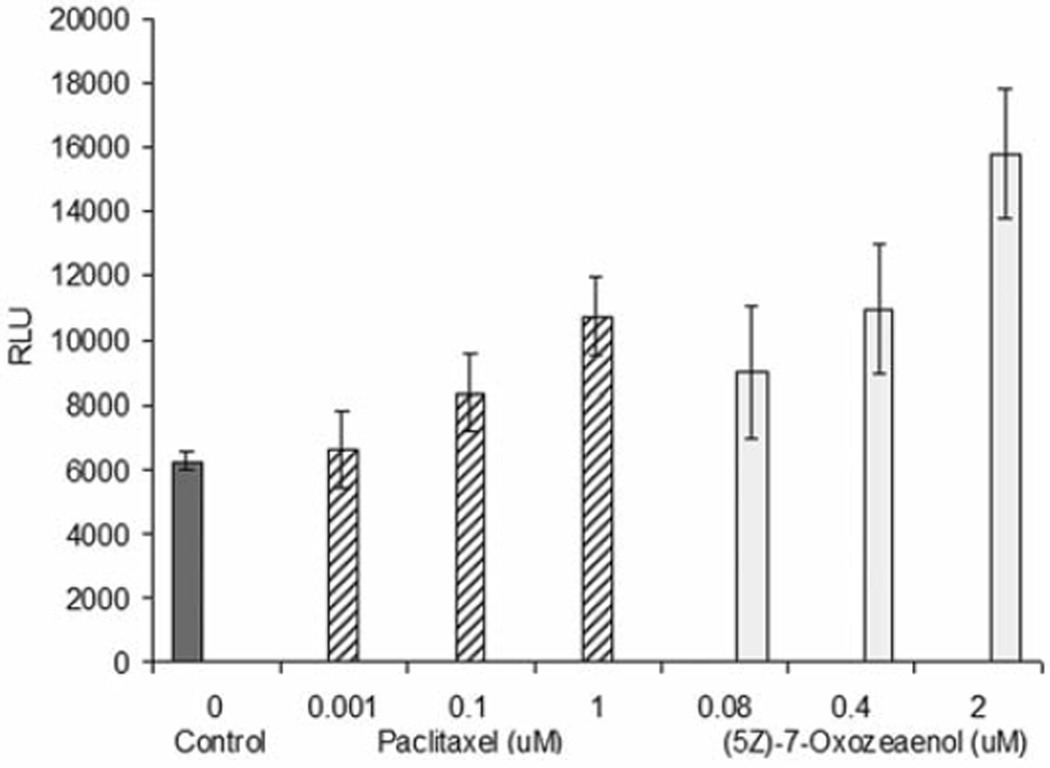
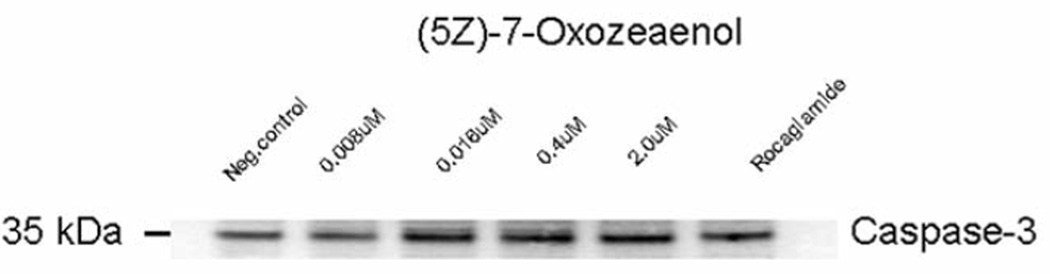
Caspase-3/7 enzyme activity was induced after 3 h of treatment with different concentrations of (5Z)-7-oxozeaenol in HeLa cells (Figure 7). Western blot analysis further confirmed that caspase-3 activity was induced in a dose dependent manner, when treating HeLa cells with (5Z)-7-oxozeaenol (Figure 8).
Figure 7.
Caspase-3/7 activity after 3 h of treatment with (5Z)-7-oxozeaenol. Positive control was paclitaxel. Bars on graph represent the means of relative luminescence units (RLU) ± SEM of triplicate samples from two independent experiments.
Figure 8.
HeLa cells treated with (5Z)-7-oxozeaenol expressed caspase-3 in a dose-dependent manner.
Discussion
Oxidative stress plays an important role in cellular status and oxygen-derived species such as superoxide radical, hydrogen peroxide, singlet oxygen and hydroxyl radical are well known for being cytotoxic and have been implicated in the etiology of a wide array of human diseases, including cancer. The dynamic state of cellular ROS depends on the equilibrium between the internal generation of ROS and the cell’s antioxidant system. The NF-κB pathway is generally thought to be a primary oxidative stress-response pathway, since nearly all pathways leading to NF-κB activation are blocked by a variety of antioxidative agents. Cancer cells in particular, in comparison to normal cells, have higher levels of ROS and are more susceptible to mitochondrial dysfunction due to their higher metabolic rate, making the oxidative pathway an attractive target for cancer drug discovery.
In this study, it was hypothesized that (5Z)-7-oxozeaenol is an NF-κB inhibitor that acts specifically on the TNFα-mediated pathway. The results of the present study show that even though (5Z)-7-oxozeaenol treatment did not inhibit K-Ras activity, induced by epidermal growth factor (EGF) (figure data not shown, but (5Z)-7-oxozeaenol exhibited less than 50% inhibition at 125 µM), it acts as an inhibitor of the TNFα-mediated NF-κB pathway. When HeLa cells were treated with (5Z)-7-oxozeaenol in the presence of TNFα, both NF-κB p65 and p50 were inhibited in a dose-dependent manner. The expression of IKKβ was also dose-dependently down regulated. Consequently, NF-κB was not activated, remaining in the cytoplasm in its inactive form, potentially inhibiting tumor survival signaling in treated cancer cells. (5Z)-7-Oxozeaenol also induced the production of ROS, both in the absence and in the presence of H2O2, in a dose-dependent manner, as shown in Figures 5 and 6. Inducing elevation of ROS levels in cancer cells might be a selective approach to preferentially kill cancer cells through pharmacological ROS insults, since cancer cells are already under high oxidative stress. This suggests that (5Z)-7-oxozeaenol might also affect mitochondrial function and that apoptosis may be induced through a caspase-dependent pathway. In the present study, increased intracellular levels of ROS were followed by loss of mitochondrial function, as well as cell death, as an effect of (5Z)-7-oxozeaenol treatment of HT-29 cells.
According to previous reports, there is a direct correlation between TNFα stimulation and mitochondrial ROS production that triggers TNFα-mediated apoptosis (16). The results of this investigation show that (5Z)-7-oxozeaenol inhibits TNFα-induced NF-κB activity in HeLa and HT-29 cells, thereby affecting the functions of mitochondria. There is increasing evidence (16, 17) suggesting that constitutively expressed NF-κB plays a fundamental role in the regulation of oxidative metabolism, and is moreover a vital physiological regulator of mitochondrial respiration in cancer cells. Previous work showed that NF-κB is involved in oncogenic transformation and cellular reprogramming for the metabolic adaptation required by cancer cells (17). Subsequently, (5Z)-7-oxozeaenol treatment might also lead to ROS-mediated cell death. Furthermore, IKKβ and NF-κB inhibition makes tumors highly susceptible to mitochondrial stress, both in vitro and in vivo (17). It has been shown that IKKβ inhibition sensitizes leukemia cells to starvation-induced cell death (16). In agreement with previous findings, this study shows that (5Z)-7-oxozeaenol inhibits IKKβ/NF-κB by targeting upstream signaling mediators. It is possible this inhibition would affect the ubiquitination of the NF-κB complex and subsequent proteasome degradation of the transcription element. Our results showed that the mitochondrial trans membrane potential and function were affected in treated HT-29 cells and increased production of ROS was also detected in a dose-dependent manner, likely promoting cell death. NF-κB inhibitory agents, such as (5Z)-7-oxozeaenol, which down-regulate and suppress the NF-κB survival pathway and induce cell death by ROS accumulation and activation of caspase-3 activity, are potential new anti-tumorigenic lead compounds (9). Analogs of (5Z)-7-oxozeaenol used in combination with other anticancer agents may increase the efficacy of existing treatments, possibly acting synergistically with chemotherapeutic agents.
Conclusion
In conclusion, these findings suggest that (5Z)-7-oxozeaenol prevents the activation of NF-κB, compromising the mitochondria membrane potential, and increasing the level of intracellular ROS in HeLa and HT-29 cells, leading to programmed cell death through the activation of a caspase-mediated pathway.
Acknowledgements
The Authors greatly acknowledge the financial support from the program project grant P01 CA125066 from the National Cancer Institute, NIH, Bethesda, MD and the Pelotonia fellowship from the Ohio State University for providing the support necessary for the presented work. The Authors would also like to express their gratitude to Mr. Jonathan Gladden for his assistance. Invaluable assistance was received from Dr. Mark E. Drew and his laboratory.
Footnotes
Conflict of Interest
The Authors confirm that there are no conflicts of interest in regards to this study.
References
- 1.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 2.Yin Y, Chen X, Shu Y. Gene expression of the invasive phenotype of TNF-alpha-treated MCF-7 cells. Biomed Pharmacother. 2009;63:421–428. doi: 10.1016/j.biopha.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Darnell JE. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 4.Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, Bieche I. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers S, Graf TN, Adcock AF, Kroll DJ, Matthew S, Carcache de Blanco EJ, Shen Q, Swanson SM, Wani MC, Pearce CJ, Oberlies NH. Resorcylic Acid Lactones with Cytotoxic and NF-kappaB Inhibitory Activities and Their Structure Activity Relationships. J Nat Prod. 2011;74:1126–1131. doi: 10.1021/np200062x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, (5Z)-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 7.Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 9.Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto M, Chow J, Muramoto K, Chiba K, Yamamoto S, Fujita M, Obaishi H, Tai K, Mizui Y, Tanaka I, Young D, Yang H, Wang YJ, Shirota H, Gusovsky F. E6201 [(3S,4R,5Z,8S,9S,11E)-8, 9,16-trihydroxy-3,4-dimethyl-3,4,9,19-tetrahydro-1H-2-benzoxacyclotetradec ine-1,7(8H)-dione], a novel kinase inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)-1 and MEK kinase-1: in vitro characterization of its anti-inflammatory and antihyperproliferative activities. J Pharmacol Exp Ther. 2009;331:485–495. doi: 10.1124/jpet.109.156554. [DOI] [PubMed] [Google Scholar]
- 11.Eisen A, Akerele C, Reyderman L, Verbel DA, Simon JS, Davis DW, Nomoto K, Wang J. CTC biomarker assessment to aid dosing selection of E6201, a potential MEK1 inhibitor, for treatment of BRAF-mutated melanoma. Ann Oncol. 2010;21:405–405. [Google Scholar]
- 12.Shen Y, Boivin R, Yoneda N, Du H, Schiller S, Matsushima T, Goto M, Shirota H, Gusovsky F, Lemelin C, Jiang Y, Zhang Z, Pelletier R, Ikemori-Kawada M, Kawakami Y, Inoue A, Schnaderbeck M, Wang Y. Discovery of anti-inflammatory clinical candidate E6201, inspired from resorcylic lactone LL-Z1640-2, III. Bioorg Med Chem Lett. 2010;20:3155–3157. doi: 10.1016/j.bmcl.2010.03.087. [DOI] [PubMed] [Google Scholar]
- 13.Muramoto K, Goto M, Inoue Y, Ishii N, Chiba K, Kuboi Y, Omae T, Wang YJ, Gusovsky F, Shirota H. E6201, a novel kinase inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-1 and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase-1: in vivo effects on cutaneous inflammatory responses by topical administration. J Pharmacol Exp Ther. 2010;335:23–31. doi: 10.1124/jpet.110.168583. [DOI] [PubMed] [Google Scholar]
- 14.Deng Y, Balunas MJ, Kim JA, Lantvit DD, Chin YW, Chai H, Sugiarso S, Kardono LB, Fong HH, Pezzuto JM, Swanson SM, Carcache de Blanco EJ, Kinghorn AD. Bioactive 5,6-dihydro-alpha-pyrone derivatives from Hyptis brevipes. J Nat Prod. 2009;72:1165–1169. doi: 10.1021/np9001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JA, Lau EK, Pan L, Carcache de Blanco EJ. NF-kappaB inhibitors from Brucea javanica exhibiting intracellular effects on reactive oxygen species. Anticancer Res. 2010;30:3295–3300. [PMC free article] [PubMed] [Google Scholar]
- 16.Fabre C, Carvalho G, Tasdemir E, Braun T, Ades L, Grosjean J, Boehrer S, Metivier D, Souquere S, Pierron G, Fenaux P, Kroemer G. NF-kappaB inhibition sensitizes to starvation-induced cell death in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007;26:4071–4083. doi: 10.1038/sj.onc.1210187. [DOI] [PubMed] [Google Scholar]
- 17.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, Moretti M, De Smaele E, Beg AA, Tergaonkar V, Chandel NS, Franzoso G. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–1279. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]