Abstract
The possibility of using stem cells to regenerate damaged myocardium has been actively investigated since the late 1990s. Consistent with the traditional view that the heart is a “post-mitotic” organ that possesses minimal capacity for self-repair, much of the preclinical and clinical work has focused exclusively on introducing stem cells into the heart, with the hope of differentiation of these cells into functioning cardiomyocytes. This approach is ongoing and retains promise but to date has yielded inconsistent successes. More recently, it has become widely appreciated that the heart possesses endogenous repair mechanisms that, if adequately stimulated, might regenerate damaged cardiac tissue from in situ cardiac stem cells. Accordingly, much recent work has focused on engaging and enhancing endogenous cardiac repair mechanisms. This article reviews the literature on stem-cell based myocardial regeneration, placing emphasis on the mutually enriching interaction between basic and clinical research.
Keywords: regeneration, stem cell, infarction, myocardium
Introduction
Congestive heart failure (CHF) is the consequence of myocardial cell death and subsequent cardiac remodeling. Despite noteworthy therapeutic advances during the past half-century, CHF continues to be a major cause of morbidity and mortality and is the leading cause of hospitalization among people aged 65 years and older (Roger et al., 2012). Systematic exploration of regenerating lost myocardium in CHF began in the late 1990s. This article reviews progress in this area, emphasizing mutually beneficial interactions between basic and clinical research.
The preclinical and clinical studies of different exogenous stem cells
Skeletal myoblasts
The era of active research on cardiac regeneration may be dated to initial attempts to employ skeletal myoblasts for cardiac repair reasoning that sufficient plasticity might exist between precursors of related cell lineages, in this case muscle (Koh, Klug, Soonpaa, & Field, 1993). A noteworthy attempt was reported in the 1998 publication by Taylor et al., describing the transplantation of autologous skeletal myoblasts into myocardial scar in a rodent model of acute MI. The transplanted skeletal myoblasts created islands of skeletal and cardiac cells and improved myocardial contractility in the infarcted area (Taylor et al., 1998). In 2001, Menasche et al. translated these observations to patients by injecting skeletal myoblasts into scarred regions of the heart during coronary artery bypass surgery (CABG) and subsequently showed improved contractility and viability in the grafted scar on echocardiography and positron emission tomography 5 months after cell delivery (Menasche et al., 2001).
Safety of skeletal myoblast injections
Several key safety concerns emerged early in the translational development of skeletal myoblast therapy. Early work on skeletal myoblasts resulted in high incidence of sustained ventricular tachycardia, suggesting that cell injections could be pro-arrhythmic (Menasche, et al., 2001). In a subsequent large randomized, placebo-controlled trial, injection of skeletal myoblasts in patients with ischemic cardiomyopathy during CABG resulted in reverse left ventricular (LV) remodeling and no improvement in left ventricular ejection fraction with increased risk of arrhythmia in the early post-operative period (Menasche et al., 2008). There are ongoing preclinical investigations on a subpopulation of skeletal myoblasts called myoendothelial cells, which have a great propensity to differentiate into cardiomyocytes and endothelial cells compared to the undifferentiated pool of myoblasts. Although there is great promise for higher efficacy in cardiac repair using myoendothelial cells, the risk of arrhythmia will not be eliminated due to their inability to express connexin-43 and form gap junctions with existing cardiac myocytes (Menasche, 2008). Cardiac transplantation of myoblasts overexpressing connexin43 has been shown to improve the electrical coupling of skeletal myoblasts and cardiomyocytes, and in some studies eliminated pro-arrhythmogenic effect of skeletal myoblasts in small animal models of myocardial infarction (Fernandes et al., 2009).
Bone-marrow derived stem cells
Testing a new hypothesis that greater plasticity could be brought to bear for cardiac regeneration, Orlic et al. reported myocardial and vascular regeneration after myocardial injury resulting from injection of bone marrow derived c-kit+, lin- stem cells (Orlic et al., 2001). The subsequent preclinical and translational studies using bone-marrow derived stem cells ignited research on myocardial regeneration and cardiac cell therapy (Menasche, 2011; Orlic, et al., 2001; Rota et al., 2005), leading to multiple clinical trials of whole bone marrow or its constituents. Meta-analyses examining the clinical trials of autologous bone marrow cells demonstrated safety and temporary improvement in left ventricular ejection fraction (Abdel-Latif et al., 2007; Menasche, 2011). These clinical trials by and large are small, heterogeneous in design with respect to cell type, dosage, timing, route of administration and have varied outcomes.
Lessons learned from clinical trials of bone marrow stem cells
The lessons learned from this heterogeneous group of clinical trials, as well as from subsequent mechanistic studies in basic science laboratories, influenced the design of subsequent clinical trials. In an earlier study of intracoronary autologous bone-marrow cell transfer after myocardial infarction, there was modest, but temporary improvement in left ventricular ejection fraction (LVEF) at 6 months (Wollert et al., 2004), which did not persist at 18-month follow up (Meyer et al., 2006). In the landmark REPAIR-AMI (Reinfusion of Enriched Progenitor Cell and Infarct Remodeling) trial, intracoronary infusion of bone marrow cells within 3-7 days of myocardial infarction (MI) resulted in a modest improvement in left ventricular ejection fraction (LVEF) (Schachinger et al., 2006), which persisted in a 2-year follow up (Assmus et al., 2010a). A more recent study examining the effect of intracoronary infusion of bone marrow cells 2-3 weeks post-MI failed to show a similar improvement in LVEF (Traverse et al., 2011).
Taken together, the findings of these studies suggest that the timing of stem-cell transplantation following an acute myocardial injury may be a crucial determinant of the regenerative efficacy. Specifically, it appears that early transplantation, within a few days following acute myocardial infarction (AMI), is necessary for benefit. An important concept has emerged from these early trials, that clinical benefits might derive out of proportion to improvements in cardiac performance, and in this regard it is noteworthy that a two-year follow up in the REPAIR-AMI trial showed improved mortality regardless of whether and to what degree the ejection fraction improved (Assmus et al., 2010b). Accordingly, it is now important to start considering the inclusion of clinical endpoints in trial design of cell-based therapies for heart disease.
In the above studies, the working hypothesis was that benefits attained from the transplantation of exogenous stem cells were due to engraftment and differentiation of implanted cells into cardiomyocytes and possibly blood vessels. More recent data have shown that the benefits of cell therapy can arise from more elaborate, multi-factorial mechanisms. Animal studies have demonstrated that transplanted exogenous stem cells release paracrine factors that reduce apoptosis of native cardiomyocytes and improve LV remodeling (Burdon, Paul, Noiseux, Prakash, & Shum-Tim, 2011). These studies also raise the possibility that a time-dependent paracrine influence on the remodeling process may explain why early but not late transplantation is effective, post myocardial infarction.
The results of the earlier clinical trials and the recent mechanistic discoveries influenced the design of two large randomized, placebo-controlled trials. The TIME trial is an ongoing double-blind trial of 120 patients that is evaluating the safety and efficacy of administering bone marrow mononuclear cells at 3 vs. 7 days following AMI (Traverse et al., 2009). The European Society of Cardiology for Stem Cells and Cardiac Repair is planning to conduct a large 3000-patient trial to evaluate all-cause mortality in patients who receive bone-marrow derived mononuclear cells following AMI. This will be the first study to evaluate, as a primary outcome measure, the effect of stem cells on an outcome other than LVEF.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are an emerging and highly promising therapeutic option. MSCs, which are widely distributed in tissues, can be easily isolated and expanded from bone marrow aspirates. As they lack MHC cell-surface markers, MSCs do not induce an immune response, and may therefore facilitate allogeneic transplantation of these cells. As a practical matter, it is convenient to use allogeneic cells because they can be harvested from a pool of individuals for transplant into multiple patients. As a result, MSCs have emerged as a viable candidate for stem cell transplantation in cardiac diseases (Williams & Hare, 2011). As demonstrated in preclinical and clinical studies, MSCs can differentiate into cardiomyocytes, endothelial cells, and vascular muscle cells (Amado et al., 2005; Quevedo et al., 2009; Toma, Pittenger, Cahill, Byrne, & Kessler, 2002). MSCs also have cytoprotective effects via paracrine factors, although the efficacy of injected cells exceeds those of the MSC conditioned medium containing the paracrine factors (Hatzistergos et al., 2010b). The history of MSCs in cardiac regeneration provides a robust example of translation from preclinical mechanistic studies into clinical trials. The translational influence is particularly evident regarding routes of administration of cells including intravenous, intracoronary, and transendocardial cell delivery methods.
Lessons learned from different routes of administration of MSCs
Intravenous injection of MSCs following AMI in animal models demonstrated that only 0.4% of the MSCs were subsequently identified within the myocardium (Toma, et al., 2002). Furthermore, the majority of MSCs were found sequestered in the lungs (Halkos et al., 2008). This is not surprising, given that the lung is the first capillary bed encountered following intravenous (IV) administration. Beyond merely diverting cells from the cardiac targets, the pulmonary accumulation of pluripotent cells raised concerns that cell growths of unpredictable lineages might occur in the lung. Although a subsequent clinical trial of IV MSCs following AMI showed an excellent safety profile associated with 6 EF unit improvement in LVEF at 3 months (Hare et al., 2009) (Figure 1), persistent concerns about pulmonary sequestration led to a greater emphasis on intracoronary and transendocardial routes of cell administration.
Figure 1. Impact of human MSC treatment on prevention of LV remodeling following myocardial infarction, with permission from Hare et al., JACC 2009; 54(24): 2277-2286.
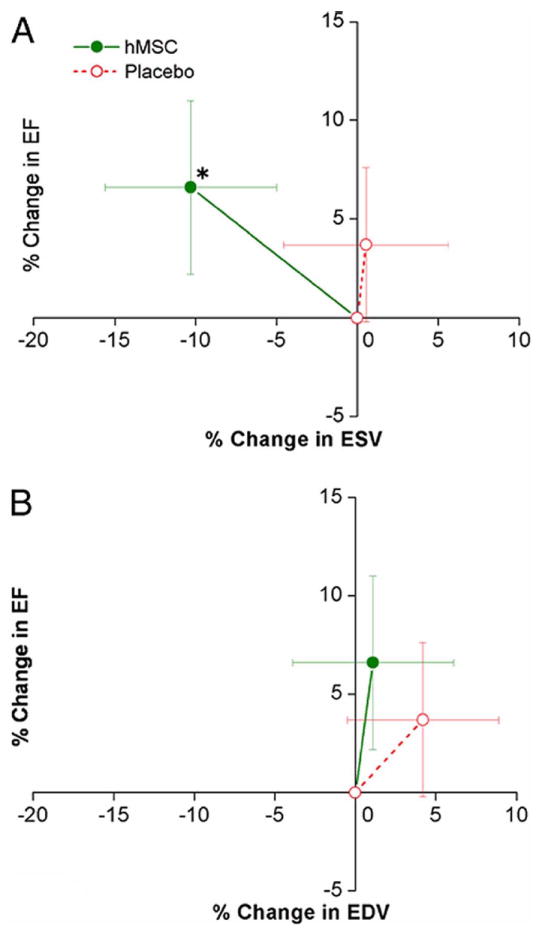
Changes in left ventricular (LV) ejection fraction (EF) are plotted against the changes in LV end-systolic volume (ESV) (A) and end-diastolic volume (EDV) (B) during follow-up. Human mesenchymal stem cell (hMSC) patients (n = 21 at 3 months, n = 18 at 6 and 12 months) exhibit evidence of attenuated remodeling with no increase in LV EDV and a decline in LV ESV, whereas placebo patients (n = 13 at 3 months, n = 11 at 6 and 12 months) demonstrate evidence of LV chamber enlargement consistent with progressive ventricular remodeling. *p = 0.005 versus baseline.
A potential advantage of intracoronary administration of MSCs is that it delivers a higher concentration of stem cells to the heart than the intravenous route. Also, the conventional over-the-wire balloon angioplasty catheters can be used for injection of the cells (Wang et al., 2009). In a swine model of AMI, intracoronary delivery of MSCs resulted in decreased scar size and improved EF (Qi et al., 2008). Similarly, an earlier clinical trial of 69 patients receiving intracoronary MSCs following AMI resulted in an improvement in LVEF (Chen et al., 2004).
In the transendocardial approach, cells are injected across the endocardial wall directly into ventricular muscle tissue via a needle-tipped catheter (Sherman, Martens, Viles-Gonzalez, & Siminiak, 2006). Using a canine model of AMI, Perin et al. compared the retention rates of the delivered stem cells via the transendocardial vs. intracoronary routes. They found that transendocardial delivery resulted in greater cardiac retention of delivered cells and a more robust functional recovery (Perin et al., 2008). Transendocardial delivery of MSCs has also been studied in swine models of both AMI and chronic ischemic cardiomyopathy (Amado, et al., 2005; Quevedo, et al., 2009). In these studies, the approach was found to be safe and effective, with evidence of engraftment and differentiation of the MSCs into cardiomyocytes, vascular smooth muscle cells and endothelial cells. Also, scar size was reduced, while there was an improvement in blood vessel density, tissue perfusion and EF (Amado, et al., 2005; Hatzistergos et al., 2010a; Quevedo, et al., 2009).
Based on these positive findings, transendocardial MSC delivery was studied in clinical trials. Transendocardial injection of MSCs in 8 patients with ischemic cardiomyopathy resulted in improved contractility in the region of the injections. At 12 months, there was a reduction of end-diastolic and end-systolic volumes. Importantly, there was no increase in the risk of arrhythmias during 12 months of follow up (Williams et al., 2011). The promising findings of this study have led to several larger trials of endomyocardial delivery of MSCs and bone marrow cells in patients with ischemic (Trachtenberg et al., 2011), and non-ischemic cardiomyopathy (clinicaltrials.gov NCT01392625).
The issue of immunogenicity of MSCs is being examined in the PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis (POSEIDON-DCM) trials, (clinicaltrials.gov NCT01087996, and NCT01392625). In these randomized clinical trial, the safety and efficacy of transendocardial injection of allogeneic and autologous MSCs on LV function recovery will be compared head to head. Two parallel trials will test this hypothesis first in patients with ischemic cardiomyopathy and then in patients with non-ischemic dilated cardiomyopathy.
Endogenous cardiac repair and the paradigm shift
Cardiac Stem Cells
In early 2000, the concept of endogenous cardiac repair via resident cardiac stem cells (CSCs) was introduced, and challenged the old belief that the heart is a post-mitotic organ (Anversa & Nadal-Ginard, 2002; Hierlihy, Seale, Lobe, Rudnicki, & Megeney, 2002). Resident CSCs are self-renewing, clonogenic and multipotent. They can differentiate into cardiomyocytes, smooth muscle and vascular endothelial cells (Linke et al., 2005; Oh et al., 2003). Several laboratories have identified CSCs expressing different stem cell antigens including c-kit, Sca-1, MDR1, and CD31 (Linke, et al., 2005; Messina et al., 2004b; Oh, et al., 2003; Pfister et al., 2005). The different phenotypes of CSCs are believed to represent different stages of maturation of the resident cardiac stem cells (Laflamme & Murry, 2011). Accumulating evidence supports the concept that the dominant cell in the hierarchy of cardiac precursors expresses the c-kit receptor (Ferreira-Martins et al., 2012). Clusters of CSCs form structural and functional units called “stem cell niches” that are connected to fibroblasts and cardiomyocytes via junctional and adhesion proteins (Urbanek et al., 2006).
The paradigm shift in cardiac biology and the recent advances in our understanding of cardiomyocyte homeostasis have generated exciting opportunities for cardiac regeneration. There is growing interest in either using isolated cardiac stem cells as a line of therapy or developing strategies to potentiate the existing endogenous myocardial repair mechanisms (Linke, et al., 2005). Pre-clinical studies have shown that CSCs have the potential to reduce the infarct size and improve LV remodeling in animal models of myocardial injury (Johnston et al., 2009; Rota et al., 2008).
The robust preclinical data with CSCs has already been translated into clinical trials. The phase I SCIPIO trial (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) demonstrated the safety and efficacy of intracoronary delivery of autologous c-kit+, lin- cardiac stem cells in patients with ischemic cardiomyopathy undergoing CABG (Bolli et al., 2011) (Figure 2). In this trial the c-kit+ cells were amplified from right atrial appendage tissue, were shown to have telomere lengths that were 7.5 kbp, and had minimal evidence of lineage commitment as shown by expression of c-kit in 75-98%, and expression of NKX2.5, GATA4, and Mef2c in only 0.1% of the cells. These findings suggested that the amplified cells retained the features of primitive stem cells, yet to undergo lineage commitment towards adult cellular constituents of the heart, and as such represent a population of true cardiac stem cells.
Figure 2. Echocardiographic analysis of CSC-treated patients and controls in SCIPIO trial, with permission from Bolli et al., Lancet 2011; 378(9806): 1847-1857.
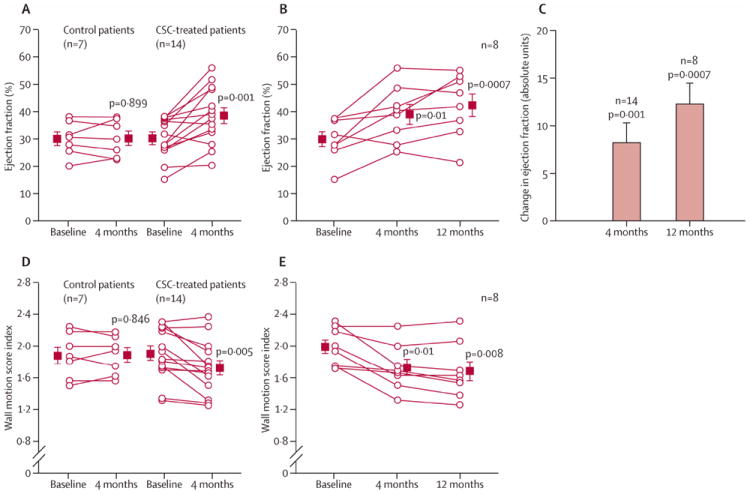
(A) Left ventricular ejection fraction (measured by use of three-dimensional echocardiography) at 4 months after baseline in control and CSC-treated patients. (B) Ejection fraction at 4 months and 12 months after baseline in the CSC-treated patients who had 1 year of follow-up. (C) Change in ejection fraction from baseline at 4 months and 12 months in CSC-treated patients. (D) Wall motion score index at 4 months after baseline in control and CSC-treated patients. (E) Wall motion score index at 4 months and 12 months after baseline in the CSC-treated patients who had 1 year of follow-up. Boxes represent the mean values and (error bars represent SE). P values are reported for difference between baseline and 4 months and between baseline and 12 months. CSC=cardiac stem cell. As shown, CSC-treated patients had improvement in the regional wall motion score index, and overall ejection fraction at 4 and 12 months following treatment.
Another approach to cell-based therapy employing a cell product amplified from myocardial tissue entails the culturing of cardiospheres generated from endomyocardial biopsies (Messina et al., 2004a). Cardiospheres are clusters of undifferentiated, clonogenic stem cells, and were studied in a porcine model of acute MI, and were shown to reduce infarct size, and improve LV hemodynamics (Johnston, et al., 2009). In a recent phase I trial, CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction (CADUCEUS), the safety of intracoronary infusion of cardiosphere derived cells, grown from endomyocardial biopsies, was examined in patients following myocardial infarction (Makkar et al., 2012). This approach led to a reduction in scar tissue, and augmentation of viable tissue. In a somewhat paradoxical observation, this trial failed to detect an increase in ejection fraction. Future larger studies will be required to compare the effects of cardiospheres vs other cell approaches in populations of patients with well-defined cardiac disorders.
Lessons learned from the paradigm shift
The recent paradigm shift and discovery of CSCs has expanded our understanding of the mechanism of action of other types of stem cells. For example, we have deomstrated that MSCs can mobilize clusters of c-kit+ CSCs into the infarct zone (Figure 3), and form structures resembling stem cell niches (Hatzistergos, et al., 2010a; Urbanek, et al., 2006). The mechanism of activation of endogenous cardiac stem cells are not well known, but paracrine factors (Mohsin, Siddiqi, Collins, & Sussman, 2011a), and cell-cell interaction between exogenous stem cells and stem cell niches (Williams & Hare, 2011) are two suggested mechanisms. Identification of the paracrine factors involved in stimulation of CSCs can help optimize the effect of cell therapy by preconditioning stem cells or using genetic engineering to enhance their efficacy in activating endogenous regeneration (Mohsin, Siddiqi, Collins, & Sussman, 2011b).
Figure 3. MSCs stimulate endogenous CSCs, with permission from Hatzistergos et al. Circulation Research 2010;107:913-922.
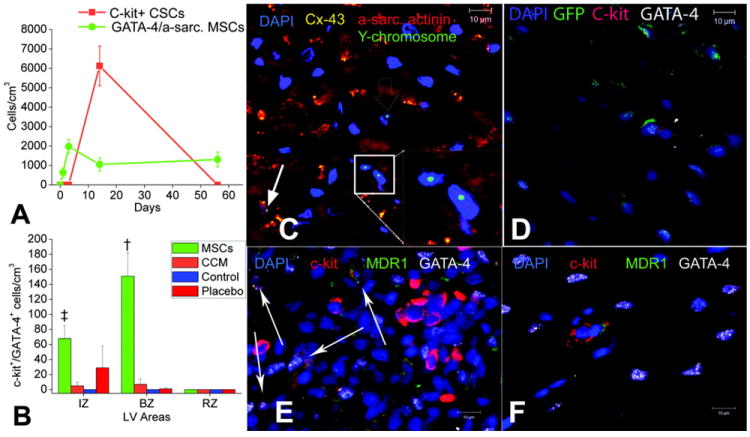
(A) Graph depicting the contribution of cardiomyocyte precursors following exogenous administration of MSCs (green line) and endogenous CSCs (orange line) during cardiac repair after MI. At 2 weeks, MSCs activate endogenous expansion of c-kit+ CSCs (orange line) (B) Two weeks following TEI, the number of C-kit+ cells co-expressing GATA-4 is greater in MSCs versus non-MSCs treated hearts. (C and D) The 2-week-old chimeric myocardium contains mature cardiomyocytes (open arrow), immature MSCs (inset), and cardiac precursors of MSCs origin (arrow), coupled to host myocardium by connexin-43 gap junctions. Interestingly, endogenous c-kit+ CSCs are found in close proximity to MSCs (D). (E) Cluster of c-kit+ CSCs in an MSC-treated heart; numerous CSCs are committed to cardiac lineage documented by GATA-4 and MDR-1 co-expression (arrows). (F) Few isolated c-kit+ cells were found in non-MSC-treated animals.
Pluripotent stem cells
Embryonic stem cells (ESCs) are derived from the inner cell mass of mammalian blastocysts, while induced pluripotent stem (iPS) cells are generated from somatic cells such as fibroblasts using retrovirus-mediated transduction of four transcription factors. iPS cells were first described by Takahashi and Yamanaka in 2006 in a mouse animal model (Takahashi & Yamanaka, 2006). The following year, human iPS cells were isolated from human fibroblasts (Takahashi et al., 2007). The morphology and gene expression of human iPS cells are similar to human ESCs. The two main challenges with iPS cells are: 1) retrovirus-mediated transduction imposes an increased risk of tumor formation due to reactivation of the integrated transgenes, and 2) there is poor efficacy of iPS differentiation into cardiomyocytes (Yoshida & Yamanaka, 2011). There are ongoing efforts to improve the method of iPS cell generation using adenoviruses and plasmid vectors for transduction of transcription factors while reducing the risk of tumor formation (Yoshida & Yamanaka, 2011). The focus is also shifted to directed differentiation of the iPS cells using signaling molecules to improve the efficacy of cardiomyocyte differentiation (Narazaki et al., 2008).
In a rodent model of MI, intramyocardial delivery of human iPS cells demonstrated in-situ generation of cardiomyocytes, smooth muscle cells and endothelial cells, and improvement of LV contractility (Nelson et al., 2009). However, the translation of this field into clinical studies will depend on improvements in the safety profile of the iPS cells and higher efficacy of generating these cells.
Future translational findings
In the last decade, the efforts in the field of stem cell therapy in cardiovascular disease have resulted in generating a wealth of data on efficacy and safety of different types of stem cells. The key element in moving the field forward has been the constant mutually reinforcing interactions between basic and clinical/translational research approaches. Although some of the more rigorous clinical trials have failed to reproduce earlier positive results from smaller trials, the findings in larger clinical trials highlighted possible mechanistic questions that should be answered by more focused experiments in the basic science laboratories.
Ongoing efforts are needed to optimize the cell delivery techniques, cell preparations and cell survival. The discovery of CSCs and their role in endogenous repair of the myocardium has opened a new chapter in studying the cellular constituents and pathways involved in myocardial repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Latif A, Bolli R, Tleyjeh et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of internal medicine. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. Research Support, N.I.H., ExtramuralReview. [DOI] [PubMed] [Google Scholar]
- Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. Comparative StudyResearch Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415(6868):240–243. doi: 10.1038/415240a. Research Support, U.S. Gov’t, P.H.S.Review. [DOI] [PubMed] [Google Scholar]
- Assmus B, Rolf A, Erbs S, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circulation Heart failure. 2010a;3(1):89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. Multicenter StudyRandomized Controlled Trial Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Assmus B, Rolf A, Erbs S, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010b;3(1):89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243.CIRCHEARTFAILURE.108.843243 [DOI] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. Clinical Trial, Phase I Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone marrow research. 2011;2011:207326. doi: 10.1155/2011/207326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. The American journal of cardiology. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. Clinical Trial Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- Fernandes S, van Rijen HV, Forest V, et al. Cardiac cell therapy: overexpression of connexin43 in skeletal myoblasts and prevention of ventricular arrhythmias. J Cell Mol Med. 2009;13(9B):3703–3712. doi: 10.1111/j.1582-4934.2009.00740.x.JCMM740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Martins J, Ogorek B, Cappetta D, et al. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res. 2012;110(5):701–715. doi: 10.1161/CIRCRESAHA.111.259507.CIRCRESAHA.111.259507 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Halkos ME, Zhao ZQ, Kerendi F, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic research in cardiology. 2008;103(6):525–536. doi: 10.1007/s00395-008-0741-0. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. Clinical Trial, Phase I Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation research. 2010a;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS letters. 2002;530(1-3):239–243. doi: 10.1016/s0014-5793(02)03477-4. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120(12):1075–1083. 1077–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh GY, Klug MG, Soonpaa MH, Field LJ. Differentiation and long-term survival of C2C12 myoblast grafts in heart. The Journal of clinical investigation. 1993;92(3):1548–1554. doi: 10.1172/JCI116734. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–335. doi: 10.1038/nature10147. Research Support, N.I.H., Extramural Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A, Muller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(25):8966–8971. doi: 10.1073/pnas.0502678102. Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. Clinical Trial, Phase I Multicenter Study Randomized Controlled TrialResearch Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P. Skeletal myoblasts for cardiac repair: Act II? Journal of the American College of Cardiology. 2008;52(23):1881–1883. doi: 10.1016/j.jacc.2008.07.066. Comment Editorial. [DOI] [PubMed] [Google Scholar]
- Menasche P. Cardiac cell therapy: lessons from clinical trials. Journal of molecular and cellular cardiology. 2011;50(2):258–265. doi: 10.1016/j.yjmcc.2010.06.010. Review. [DOI] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. Clinical Trial, Phase II Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279–280. doi: 10.1016/S0140-6736(00)03617-5. Case Reports Letter Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation research. 2004b;95(9):911–921. doi: 10.1161/01.RES.0000147315.71699.51. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011a;109(12):1415–1428. doi: 10.1161/CIRCRESAHA.111.243071.109/12/1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circulation research. 2011b;109(12):1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. Research Support, N.I.H., Extramural Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118(5):498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120(5):408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. In Vitro Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10344–10349. doi: 10.1073/pnas.181177898. Research Support, U.S. Gov’t, P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Silva GV, Assad JA, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. Journal of molecular and cellular cardiology. 2008;44(3):486–495. doi: 10.1016/j.yjmcc.2007.09.012. Comparative Study. [DOI] [PubMed] [Google Scholar]
- Pfister O, Mouquet F, Jain M, et al. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circulation research. 2005;97(1):52–61. doi: 10.1161/01.RES.0000173297.53793.fa. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- Qi CM, Ma GS, Liu NF, et al. Transplantation of magnetically labeled mesenchymal stem cells improves cardiac function in a swine myocardial infarction model. Chinese medical journal. 2008;121(6):544–550. Research Support, Non-U.S. Gov’t. [PubMed] [Google Scholar]
- Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. Comparative Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Anversa P, et al. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circulation research. 2005;97(12):1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circulation research. 2008;103(1):107–116. doi: 10.1161/CIRCRESAHA.108.178525. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. The New England journal of medicine. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S57–64. doi: 10.1038/ncpcardio0446.ncpcardio0446 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nature medicine. 1998;4(8):929–933. doi: 10.1038/nm0898-929. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. [DOI] [PubMed] [Google Scholar]
- Trachtenberg B, Velazquez DL, Williams AR, et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. American heart journal. 2011;161(3):487–493. doi: 10.1016/j.ahj.2010.11.024. Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA : the journal of the American Medical Association. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. American heart journal. 2009;158(3):356–363. doi: 10.1016/j.ahj.2009.06.009. Clinical Trial, Phase II Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9226–9231. doi: 10.1073/pnas.0600635103. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jameel MN, Li Q, Mansoor A, et al. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120(11 Suppl):S238–246. doi: 10.1161/CIRCULATIONAHA.109.885236. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation research. 2011;109(8):923–940. doi: 10.1161/CIRCRESAHA.111.243147. Research Support, N.I.H., Extramural Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circulation research. 2011;108(7):792–796. doi: 10.1161/CIRCRESAHA.111.242610. Clinical Trial, Phase I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yamanaka S. iPS cells: a source of cardiac regeneration. Journal of molecular and cellular cardiology. 2011;50(2):327–332. doi: 10.1016/j.yjmcc.2010.10.026. Research Support, Non-U.S. Gov’t Review. [DOI] [PubMed] [Google Scholar]


