Abstract
Type 2 diabetes is a risk factor for PAD, and insulin resistance is a key feature of diabetes and pre-diabetes. No longitudinal epidemiological study has examined the relation between insulin resistance and PAD. Our study analyzed the association of quartiles of the homeostatic model of insulin resistance (HOMA-IR) and the development of PAD defined by 2 methods. PAD was first defined as the development of an abnormal ankle brachial index (ABI) (dichotomous outcome) after six years of follow-up. PAD was alternatively defined as the development of clinical PAD (time to event analysis). The study samples included adults over the age of 65 who were enrolled in the Cardiovascular Health Study, had fasting measurements of insulin and glucose, had ABI measurements, and were not receiving treatment for diabetes. Multivariable models were adjusted for potential confounders, including age, sex, field center and cohort, BMI, smoking status, alcohol use, and exercise intensity. Additional models adjusted for potential mediators, including blood pressure, lipids, kidney function, and prevalent vascular disease. In the ABI analysis (n=2108), multivariable adjusted models demonstrated a positive relation between HOMA-IR and incident PAD (Odds Ratio=1.80 comparing the 4th versus 1st quartile of HOMA-IR, 95% confidence interval 1.20-2.71). In the clinical PAD analysis (n=4208), we found a similar relation (Hazard ratio=2.30 comparing the 4th versus 1st quartile of HOMA-IR, 95% confidence interval 1.15-4.58). As expected, further adjustment for potential mediators led to some attenuation of effect estimates. In conclusion, insulin resistance is associated with a higher risk of PAD in older adults.
Keywords: Peripheral artery disease, Diabetes, Insulin Resistance, Epidemiology
Introduction
Peripheral artery disease (PAD) of the lower extremities is a manifestation of systemic atherosclerosis with an increased prevalence in the elderly.1 Type 2 diabetes mellitus has been shown to be a strong risk factor for the development of PAD with the risk increasing with more advanced diabetes.2-4 The effect of insulin resistance on the vasculature is just one of multiple pathophysiological mechanisms implicated in the association between diabetes and vascular disease.5, 6 A cross-sectional study using the National Health and Nutrition Examination Survey has shown a positive association between the homeostatic model of insulin resistance (HOMA-IR) and PAD, as assessed by ankle brachial index (ABI).7 However, prospective studies on such an association are lacking.
To address the relation of insulin resistance and PAD, the current study prospectively assessed whether there is an independent association between HOMA-IR and incident PAD in the Cardiovascular Health Study (CHS), a population based study of older American adults.
Methods
Study Population
The CHS is a community-based longitudinal study of Medicare-eligible adults older than 65 years of age designed to evaluate the development and progression of cardiovascular disease (CVD). The initial cohort of 5201 CHS participants was recruited between 1989-1990, and a second cohort of 687 African American participants was enrolled between 1992-1993. Detailed descriptions of the CHS have been previously published.8, 9 Participants provided written informed consent, and the study protocol was approved by the investigational review board of each participating institution.
Participants were seen for yearly study visits until 1998-1999. Using yearly participant-reports and Medicare hospitalization records, discharge summaries have been requested for all hospitalizations and full medical records have been reviewed for all adjudicated outcomes. Prevalent PAD had been defined in CHS by the presence of exertional leg pain and either a physician diagnosis of PAD or a low ABI during the baseline CHS clinic examination. We additionally excluded individuals with abnormal ABI’s at baseline without leg symptoms to account for asymptomatic PAD. A normal ABI measurement at baseline was defined as ABI ≥ 0.9 and < 1.4. Individuals with ABI ≥ 1.4 were excluded due to the difficulty in diagnosing PAD in the presence of medial arterial calcification.10
Outcome Ascertainment
Incident PAD was defined in two ways. PAD was first defined as the development of an abnormal ABI (ABI <0.9 with a change of ≥ 0.15) between the 1992-93 and 1998-99 CHS examinations (dichotomous outcome) in participants with normal ABI’s and without prevalent PAD at baseline. The second definition of incident PAD in our study required the development of clinical PAD (time to event analysis) in participants with normal ABI’s and without prevalent PAD at baseline. During follow-up, potential clinical PAD outcomes were initially identified by any of the following methods: 1) report of a PAD diagnosis by the participant at a clinic visit or during a telephone call; 2) a PAD diagnosis found during review of medical records for another event; 3) active surveillance of CMS records for the ICD-9 codes 400.2 (atherosclerosis of the native arteries of the extremities) and 443.9 (peripheral vascular disease, unspecified). After PAD outcomes were identified by these methods, medical records were reviewed, and a final decision was adjudicated by the Morbidity Subgroups of the CHS Clinical Events Subcommittee. This analysis includes events that occurred through June 30 2007.
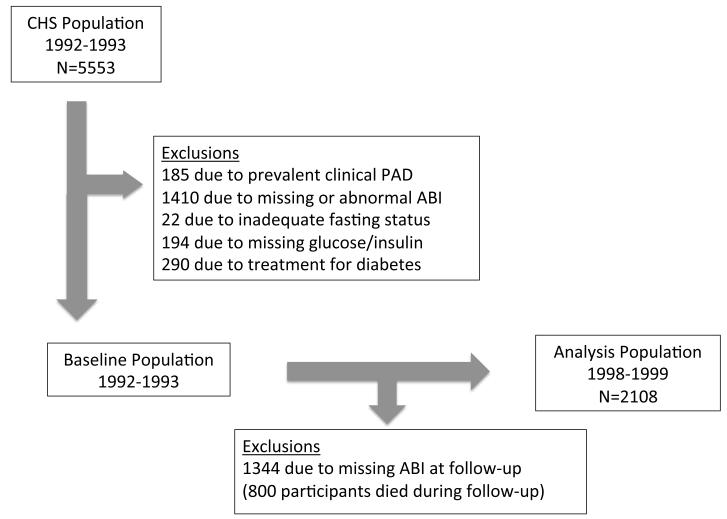
For the PAD outcome defined by ABI (dichotomous outcome), the initial population alive at the 1992-1993 examination included 5553 participants. Of these, 185 participants were excluded due to prevalent PAD, 653 due to abnormal ABI at baseline, 757 due to missing ABI measurements, 194 due to missing glucose or insulin data, 290 due to use of oral hypoglycemic agents or insulin therapy for treatment of diabetes, and 22 due to inadequate (<8 hours) or uncertain fasting status, each ascertained at the 1992-1993 exam. Participants receiving oral hypoglycemic or insulin therapy for diabetes were excluded due to the potential unreliability of HOMA-IR in this setting. We excluded subjects with an ABI <0.9 without leg symptoms to specifically exclude asymptomatic PAD. Participants with baseline diabetes not receiving drug therapy were included in the study population. Participants without ABI measurement at the 1998-99 clinic visit (n=1,344) were also excluded. A final sample size of 2108 participants was used for the ABI analysis (Figure 1).
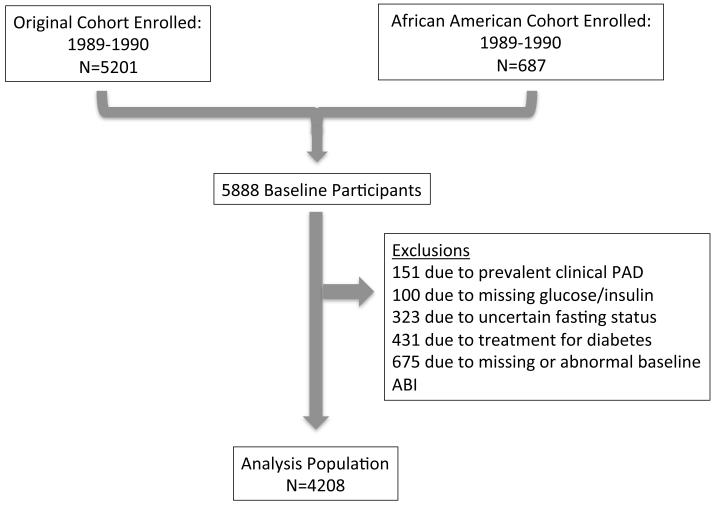
Figure 1.
Flowchart of participants: Flowchart describing selection of participants for the (A) ankle brachial index and (B) clinical peripheral artery disease analyses. CHS, Cardiovascular Health Study; PAD, peripheral artery disease; ABI, ankle brachial index.
For the clinical PAD outcome (time to event analysis) baseline measurements were obtained at participants’ initial examination (1989-1990 for members of the original cohort and 1992-1993 for members of the second cohort). For this analysis, 151 participants were excluded due to prevalent PAD at baseline, 100 due to missing glucose or insulin specimens, 323 participants due to uncertain fasting status, 431 participants due to use of oral-hypoglycemic agents or insulin as treatment for diabetes, and 675 due to missing or abnormal ABI measurements, each ascertained at the initial examination. The final sample included 4208 participants (Figure 1).
Insulin Resistance
Fasting (≥8 hour) blood specimens of glucose and insulin were collected at both the 1989-1990 and 1992-1993 study visits and were stored at -70°C. HOMA-IR was calculated using the following formula: [fasting glucose (mmol/L)]*[fasting insulin (U/mL)]/22.5.11
Ankle Brachial Index
The ABI protocol in CHS has been described previously.12 Briefly, manual blood pressure measurements were obtained after at least 5 min rest and with the subject in a supine position using a Doppler stethoscope (8 MHz, Huntleigh Technology, Inc., Luton, United Kingdom) over the right brachial artery and both posterior tibial arteries. When a blood pressure could not be measured in the right arm, the left arm was used. The ratio of the systolic blood pressure in the leg to the arm defined the leg-specific ABI. The lower of the leg-specific ABIs was used as the patient-specific ABI for this analysis. When arterial flow was not abolished with the leg blood pressure cuff inflated to >300 mm Hg, the artery was deemed incompressible.
Covariates
Covariates were ascertained at the 1992-1993 CHS exam for the primary dichotomous study outcome and at the initial CHS exam (1989-90 or 1992-1993 for the secondary time to event outcome). C-reactive protein (CRP), cystatin C, LDL, and HDL were measured from fasting blood specimens. Cystatin C concentrations were measured using a BN II nephelometer (Dade Behring Inc., Deerfield, Illinois) as described elsewhere.13 Cystatin C–based estimated glomerular filtration rate (eGFR)14 was calculated using the equation: eGFRcys=76.7*cystatin C [mg/l]−1.19 and expressed in ml/min/1.73m2. CRP was assessed with an enzyme-linked immunosorbent assay developed in the CHS laboratory. The Olympus Demand System (Olympus, Lake Success, New York) determined serum total and HDL cholesterol concentrations; LDL concentrations were calculated using the Friedewald equation.15
A prior history of coronary heart disease (CHD) (myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention), and prior cerebrovascular disease (stroke or transient ischemic attack) was determined by self report with validation by either a) information from the baseline CHS examination; b) medical record review or c) physician questionnaires. Information on age, race, exercise intensity and alcohol consumption were collected as previously described.8, 16 Participation in leisure time physical activity was collected using an instrument adapted from the Health Interview Survey, and this information was used to estimate kilocalories of energy expended and time spent per week in leisure-time physical activity. Alcohol consumption was assessed using a standardized questionnaire. A seated blood pressure was obtained and participants were classified as normotensive, prehypretensive or hypertensive depending on a combination of their systolic blood pressure, diastolic blood pressure, or treatment for hypertension. Diabetes was defined by treatment with medication for diabetes or a fasting glucose level ≥126 mg/dl (in participants not receiving treatment for diabetes). Smoking history was determined by questionnaire and categorized as current, past, or never smokers. Information on number of pack-years smoked was also collected by questionnaire.
Statistical Analysis
We compared the distribution of baseline characteristics across quartiles of HOMA-IR. We calculated odds ratios (ORs) and 95% confidence intervals (95% CIs) for incident PAD defined by ABI using logistic regression; HOMA-IR quartile-1 was used as the referent group. HOMA-IR was also analyzed as a continuous variable after log transformation. For the clinical PAD analysis, we used Cox proportional hazards models to assess the association of HOMA-IR with incident clinical PAD. Participants were censored if they died, were lost to follow-up, or at the end of the study. Assumptions for the proportional hazards models were tested by including main effects and product terms of covariates and time factor. These assumptions were met as all p values were >0.05.
Covariates were selected on the basis of their biological plausibility to either confound or mediate the relationship between insulin resistance and incident PAD. Initial models adjusted for age, gender, race, and CHS field center. A multivariable model also adjusted for potential confounders including smoking status, alcohol use, exercise intensity, and body mass index. Finally, we included potential mediating factors such as blood pressure (normotensive, prehypertensive or hypertensive), cholesterol ratio (LDL/HDL), use of lipid lowering medications, eGFR, and known CVD or cerebrovascular disease, and CRP. Tests of linear trend were computed using median values of HOMA-IR within each quartile. To further assess whether any association between insulin resistance and PAD existed after exclusion of participants with baseline diabetes, prevalent CHD, or current or prior smoking, sensitivity analyses excluded participants with these characteristics (each separately). We did not adjust for diabetes that might have developed during follow-up as the development of diabetes among individuals with insulin resistance likely reflects part of the causal pathway linking insulin resistance and subsequent PAD. In secondary analysis, we examined the association between fasting insulin and glucose individually and incident PAD as defined by the development of abnormal ABI. We also re-analyzed our time to event results using Fine and Gray competing risks proportional hazards regression to account for competing risk due to mortality in this older population.17 All analyses were completed using SAS version 9.2 (SAS Institute, Cary, NC). The significance level was set at 0.05.
Results
Baseline characteristics
Among the 2108 study participants included in the ABI analysis, the mean age was 73.4 years, 61% were female and 85% were Caucasian. Among the 4208 participants in the clinical PAD analysis, the mean age was 72.3 years, 60% were female and 86% were Caucasian. For the ABI analysis, follow-up consisted of the six years between the 1992-1993 and the 1998-1999 measurements of ABI. The median duration of follow-up for the clinical PAD analysis was 14.1 years. Compared to the HOMA-IR quartile-1, individuals in higher quartiles were younger and had higher BMI. Participants with higher HOMA-IR were less likely to be Caucasian, were less physically active, and were less likely to use alcohol. They were also more likely to have hypertension, diabetes, and prior coronary heart disease. In terms of laboratory values, a higher HOMA-IR was associated with higher CRP, higher LDL cholesterol, lower eGFRcystatin, and lower HDL cholesterol levels (all p <0.05) (Table 1). There was no significant difference in the baseline ABI by HOMA-IR quartile. For the ABI analysis, participants who attended the follow-up exam in 1998-1999 and had ABI measured appeared healthier than those who did not (Suppl. Table 1).
Table 1.
Baseline characteristics of elderly subjects according to quartiles of HOMA-IR
| Characteristics | HOMA-IR | |||
|---|---|---|---|---|
|
| ||||
| Quartile 1 <1.65 (N=530) |
Quartile 2 1.65-2.31 (N=528) |
Quartile 3 2.32-3.42 (N=526) |
Quartile 4 ≤ 3.43 (N=526) |
|
| Age (years) | 73.5±4.4 | 73.7±4.1 | 73.4±4.3 | 72.8±4.1 |
| Female gender (%) | 62.3 | 60.4 | 62.9 | 59.9 |
| White race (%) | 87.2 | 87.1 | 83.8 | 80.8 |
| Body mass index (kg/m2) | 24.3±3.4 | 25.8±3.5 | 27.6±4.1 | 29.6±4.6 |
| Current Smokers (%) | 11.9 | 11.2 | 10.7 | 6.7 |
| Moderate or high intensity exercise (%) |
58.5 | 48.9 | 48.9 | 46.0 |
| Current alcohol use (%) | 56.6 | 53.2 | 49.0 | 44.1 |
| Diabetes (%)c | 0.2 | 0.6 | 2.1 | 20.2 |
| Hypertension (%) | 24.7 | 30.5 | 36.6 | 50.4 |
| Known coronary heart diseasea (%) | 14.2 | 11.6 | 16.4 | 17.3 |
| Known cerebrovascular diseaseb (%) |
3.2 | 3.4 | 2.9 | 4.6 |
| Baseline fasting insulin (pmol/L)c | 40.3±6.3 | 59.0±6.9 | 80.6±10.4 | 142.4±79.9 |
| (μIU/ml) | 5.8±0.9 | 8.5±1.0 | 11.6±1.5 | 20.5±11.5 |
| Baseline fasting blood glucosec | 5.14±0.46 | 5.31±0.47 | 5.56±0.59 | 6.54±1.98 |
| (mmol/L) (mg/dl) |
92.6±8.2 | 95.6±8.5 | 100.1±10.7 | 117.9±35.6 |
| Low density lipoprotein cholesterol (mmol/L) |
3.22±0.81 | 3.33±0.82 | 3.44±0.80 | 3.30±0.84 |
| (mg/dl) | 124.4±31.1 | 128.5±31.8 | 132.7±30.9 | 127.5±32.4 |
| High density lipoprotein cholesterol (mmol/L) |
1.56±0.40 | 1.44±0.36 | 1.37±0.34 | 1.26±0.30 |
| (mg/dl) | 60.1±15.5 | 55.6±14.0 | 52.9±13.2 | 48.5±11.7 |
| Estimated GFR cystatin (ml/min/1.73m2) |
80.8±15.8 | 76.0±16.4 | 72.9±15.8 | 71.2±15.8 |
| C-reactive protein (nmol/L) | 27.6±37.1 | 41.9±82.9 | 47.6±88.6 | 61.9±112.4 |
| (mg/L) | 2.9±3.9 | 4.4±8.7 | 5.0±9.3 | 6.5±11.8 |
| Baseline ankle brachial indexd | 1.13±0.10 | 1.14±0.10 | 1.14±0.10 | 1.15±0.11 |
Continuous variables are presented as means ± standard deviations
Known coronary heart disease includes prior myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, or angina.
Known cerebrovascular disease includes prior cerebrovascular accident or transient ischemic attack
The study population only included participants with diabetes who were not receiving drug therapy
The study population was limited to participants with ankle brachial index ≥0.9 and ≤1.4.
Incident PAD by ABI
The odds of PAD increased across quartiles of HOMA-IR in a model adjusting for age, gender, race, and field center (Table 2a). The association was strengthened after further adjustment for potential confounders including smoking status, alcohol consumption, exercise intensity, and body mass index (Table 2a). Re-analysis of the relation using information on pack years of smoking led to overall similar findings (data not shown). Further adjustment for potential mediators of the relationship between insulin resistance and incident PAD, including blood pressure parameters, cholesterol ratio, use of lipid lowering therapy, eGFR, and prevalent CHD or cerebrovascular disease led to a modest attenuation as expected (odds ratios (95% confidence intervals) for increasing quartiles of HOMA-IR were 1.0 (referent), 0.73 (0.46-1.14), 1.4 (0.91-2.16), 1.66 (1.04-2.66) (p trend 0.003)). Addition of CRP to the other potential mediators of the association between HOMA-IR and PAD led to overall similar findings [OR’s (95% confidence intervals) were 1.0 (referent), 0.70 (0.44-1.10), 1.36 (0.88-2.09), and 1.59 (0.99-2.55)].
Table 2a.
Odds ratios (95% confidence intervals) of developing an abnormal ankle brachial index after 6 years of follow-up according to quartile of homeostatic model of insulin resistance
| HOMA-IR quartile |
Cases | Adjusted for age, race, gender, and field center |
Multivariable Model Aa |
Multivariable Model Bb |
|---|---|---|---|---|
| 1 | 63 | 1.0 | 1.0 | 1.0 |
| 2 | 55 | 0.85 (0.58-1.26) | 0.89 (0.60-1.32) | 0.73 (0.46-1.14) |
| 3 | 77 | 1.28 (0.89-1.84) | 1.43 (0.97-2.09) | 1.40 (0.91-2.16) |
| 4 | 83 | 1.46 (1.02-2.10) | 1.80 (1.20-2.71) | 1.66 (1.04-2.66) |
| p for linear trend |
0.006 | 0.001 | 0.003 |
Incident Clinical PAD
The risk of incident clinical PAD increased across increasing quartiles of HOMA-IR in simple and multivariable models (Table 2b). Further adjustment for potential mediators of the relationship between insulin resistance and incident PAD, including blood pressure parameters, cholesterol ratio, use of lipid lowering therapy, eGFR, and prevalent CHD or cerebrovascular disease led to a modest attenuation (Hazard ratios (95% confidence intervals) for increasing quartiles of HOMA-IR were 1.0 (referent), 0.84 (0.38-1.85), 0.96 (0.46-1.97), 1.62 (0.80-3.30) (p trend 0.007)). Addition of CRP to the other potential mediators of the association between HOMA-IR and PAD led to some attenuation of the hazard ratios in the higher quartiles (OR’s (95% confidence intervals) were 1.0 (referent), 0.85 (0.39-1.87), 0.86 (0.42-1.78) and 1.31 (0.64-2.68).
Table 2b.
Hazard Ratios (95% confidence intervals) of developing clinical PAD according to quartile of homeostatic model of insulin resistance
| HOMA-IR quartile |
Cases | Adjusted for age, race, gender, cohort and field center |
Multivariable Model Aa |
Multivariable Model Bb |
|---|---|---|---|---|
| 1 | 10 | 1.0 | 1.0 | 1.0 |
| 2 | 18 | 0.87 (0.40-1.88) | 0.91 (0.41-1.99) | 0.84 (0.38-1.85) |
| 3 | 41 | 1.23 (0.61-2.46) | 1.18 (0.58-2.41) | 0.96 (0.46-1.97), |
| 4 | 88 | 2.14 (1.11-4.14) | 2.30 (1.15-4.58) | 1.62 (0.80-3.30) |
| p for linear trend |
<0.001 | <0.001 | 0.007 |
additionally adjusted for potential confounders, including smoking status (never, former, current), alcohol consumption, exercise intensity, body mass index
adjusted for covariates in model-A plus potential causal intermediary factors, including blood pressure category (normotensive, prehypertensive or hypertensive), LDL/HDL ratio, use of lipid-lowering medications, estimated glomerular filtration rate using cystatin C, known cardiovascular disease, and known cerebrovascular disease
Secondary Analyses
When HOMA-IR was log-transformed and analyzed as a continuous variable, the findings were similar. For each 1 unit increase in log(HOMA-IR), the odds of PAD increased by 48% in a model that also controlled for smoking status, BMI, alcohol use, and exercise intensity (p=.002). In sensitivity analyses excluding participants with prevalent baseline diabetes, the findings from multivariable models that included confounders were largely unchanged for both the ABI and clinical PAD analyses (Table 3). As expected, inclusion of potential causal intermediary factors in the multivariable model had led to attenuation of the effect measures, and this attenuation was more pronounced in the analysis excluding participants with prevalent diabetes (Table 3). In analyses excluding participants with known coronary heart disease, the findings were also largely unchanged (data not shown).
Table 3.
The association of HOMA-IR and PAD after excluding subjects with baseline diabetes: A) Odds ratios (95% confidence intervals) of developing an abnormal ankle brachial index after 6 years of follow-up and B) Hazard ratios (95% confidence intervals) of developing clinical PAD according to quartile of homeostatic model of insulin resistance
| A) | ||||
|---|---|---|---|---|
| HOMA-IR quartile |
Cases | Adjusted for age, race, gender, and field center |
Multivariable Model Aa |
Multivariable Model Bb |
| 1 | 60 | 1.0 | 1.0 | 1.0 |
| 2 | 53 | 0.86 (0.58-1.27) | 0.89 (0.59-1.33) | 0.73 (0.46-1.16) |
| 3 | 72 | 1.28 (0.88-1.86) | 1.40 (0.94-2.08) | 1.39 (0.89-2.17) |
| 4 | 57 | 1.33 (0.89-1.98) | 1.57 (1.00-2.45) | 1.51 (0.90-2.52) |
| p for linear trend |
0.05 | 0.01 | 0.02 | |
| B) | ||||
|---|---|---|---|---|
| HOMA-IR quartile |
Cases | Adjusted for age, race, gender, cohort and field center |
Multivariable Model Aa |
Multivariable Model Bb |
| 1 | 10 | 1.0 | 1.0 | 1.0 |
| 2 | 16 | 0.79 (0.36-1.74) | 0.83 (0.37-1.87) | 0.76 (0.34-1.71) |
| 3 | 36 | 1.12 (0.55-2.26) | 1.11 (0.54-2.30) | 0.90 (0.43-1.88) |
| 4 | 62 | 1.79 (0.91-3.50) | 2.05 (1.01-4.16) | 1.50 (0.72-3.11) |
| p for linear trend |
0.001 | <0.001 | 0.01 | |
additionally adjusted for potential confounders, including smoking status (never,former, current), alcohol consumption, exercise intensity, body mass index
adjusted for covariates in model-A plus potential causal intermediary factors, including blood pressure category (normotensive, prehypertensive or hypertensive), LDL/HDL ratio, use of lipid-lowering medications, estimated glomerular filtration rate using cystatin C, known cardiovascular disease, and known cerebrovascular disease
Analyses excluding current and former smokers led to attenuation of the OR’s in multivariable models in the ABI analysis, but a similar positive relation between insulin resistance and PAD in the clinical PAD analysis. Specifically, the multivariable ORs (95% confidence intervals) across quartiles of HOMA-IR for the ABI analysis were 1.0 (referent), 0.73 (0.40-1.33), 1.01 (0.58-1.77), and 1.28 (0.74-2.21)) from the lowest to the highest quartile of HOMA-IR, respectively. The sample size for this analysis dropped from 2132 to 997 participants. In the clinical PAD analysis of never smokers, the multivariable hazard ratios (95% CI’s) were 1.0 (referent), 0.91 (0.42-2.00), 1.15 (0.56-2.35), and 2.26 (1.13-4.50) from the lowest to the highest quartile of HOMA-IR (p, trend <0.001). In secondary analyses examining fasting insulin and glucose as individual exposures, there was a significant linear trend across quartiles of glucose or insulin and incident PAD defined by ABI. For the highest quartile of glucose and insulin, the OR’s (95% CI) for incident PAD were 2.15 (1.43-3.22) and 1.65(1.11-2.46), respectively. Results with use of Fine and Gray competing risks proportional hazards regression were not materially different (data not shown).
Discussion
In this sample of elderly adults from the Cardiovascular Health Study, we found that higher levels of insulin resistance, as assessed by the homeostatic model, are associated with the development of PAD, defined by the development of an abnormal ABI. Survival analysis examining the risk of clinical PAD demonstrated similar findings. These associations persisted after exclusion of participants with baseline diabetes. Overall, these findings suggest a potential role for insulin resistance in the pathogenesis of PAD.
Diabetes is a well established risk factor for PAD2-4 and the severity of diabetes, as assessed by HbA1c, correlates with the degree of risk.2 Although basic and translational research has supported a role for insulin resistance in the pathogenesis of atherosclerosis perhaps through the development of endothelial dysfunction,18 epidemiologic studies of the association between insulin resistance and PAD are limited to a cross-sectional analysis.7 Other analyses have focused on the association of metabolic syndrome and incident PAD, but these analyses have considered all the components of metabolic syndrome together. Some of these components, such as hypertension, are already known to be associated with PAD. Our analysis expands upon this prior work by examining whether insulin resistance, which is often considered to be the underlying abnormality of metabolic syndrome, was associated with PAD after adjustment for other known risk factors, including other components of the metabolic syndrome. Our study is the first, to our knowledge, to demonstrate a longitudinal association between HOMA-IR and incident PAD. Longitudinal analysis is particularly important to assess the relationship of insulin resistance with PAD, because reverse causation, i.e. PAD causing insulin resistance through decreased ability to maintain physical fitness, is a plausible explanation for cross-sectional associations. We were able to demonstrate a temporal association between insulin resistance and PAD defined by both ABI and clinical PAD. In addition, our analysis showed that this association persisted after exclusion of prevalent baseline diabetes. Given the multifaceted associations between insulin resistance, CRP and PAD, we analyzed the effect of adding CRP to a model examining the relation between insulin resistance and PAD that already controlled for the potential confounders and mediators of this association. These results suggest that although biologically both insulin resistance and PAD are associated with inflammation, CRP did not appear to have a large effect beyond the other confounders and mediators.
Insulin resistance has been suggested as a precursor to endothelial dysfunction,18 which is itself postulated as a potential precursor to atherosclerosis.19-21 Therefore, endothelial dysfunction is an attractive biological intermediary between insulin resistance and PAD. Supporting this, prior research has suggested physiological mechanisms by which insulin resistance may lead to atherosclerosis by inducing endothelial dysfunction through the adverse effects of glucotoxicity, lipotoxicity and inflammation on the vascular endothelium.18 Insulin resistance is known to be strongly correlated with clinical risk factors, including obesity22 and dyslipidemia,23 which are themselves associated with endothelial dysfunction and atherosclerosis 24-26. However, our findings suggest a potential role of insulin resistance in the pathogenesis of PAD beyond its association with known cardiovascular risk factors. Although endothelial dysfunction provides at least one possible biological pathway between insulin resistance and atherosclerosis, including PAD, we did not have a measure of endothelial function to assess this. Furthermore, there may be other potential mechanisms that might explain a link between insulin resistance and atherosclerosis l,27 and the adverse effects of insulin resistance on the vasculature remains an area of active investigation.
Strengths of our study include the longitudinal nature and extended follow-up of the cohort. The availability of important covariates strengthened the multivariate analysis, and we were able to assess incident PAD by two methods. Some limitations warrant discussion. Participants in CHS are over the age of 65, which limits the generalizability of our findings to younger participants. However, a significant proportion of patients with PAD are elderly making this a relevant population to study. Because of the elderly population, a significant number of participants either died or did not return for follow-up. The baseline characteristics of those individuals who did and did not return for follow-up tended to be different (supplemental table 1). This limited the sample size for our logistic regression analysis and may have introduced survivor bias. However, the finding that insulin resistance is associated with incident PAD even among a healthier subset of participants is an important finding. Also, the findings with the clinical PAD outcome that used Cox proportional hazards analysis and did not require follow-up ABI’s were consistent with the ABI results. In addition, results from analyses which accounted for competing risks were not materially different. The ABI was defined by right arm blood pressures and without dorsalis pedis blood pressure. This may have led to outcome misclassification. However, since examiners were not aware of the HOMA-IR status of the study participants, such misclassification is more likely to be non-differential and might have led to attenuation of the true estimates.
Conclusion
In this cohort of older patients, insulin resistance was associated with the development of PAD assessed both by the development of a low ABI and clinical PAD. These results suggest a role for insulin resistance in the pathogenesis of atherosclerosis in the lower extremity. Additional research is needed to determine whether strategies aimed at ameliorating insulin resistance might be helpful in PAD prevention, and whether insulin resistance might be a therapeutic target for preventing progression of established clinical PAD.
Supplementary Material
Acknowledgements
This research was supported by NHLBI contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm.Evan L. Thacker was supported by NHLBI training grant T32 HL007902. Dr. Britton was supported by a Research Career Development Award (K12 HL083786) from the NHLBI.
Reference
- 1.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral Arterial Disease in the Elderly : The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–92. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 2.Beks P, Mackaay A, de Neeling J, de Vries H, Bouter L, Heine R. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn Study. Diabetologia. 1995;38:86–96. doi: 10.1007/BF02369357. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy M, Solomon C, Manolio TA, et al. Risk Factors for Declining Ankle-Brachial Index in Men and Women 65 Years or Older: The Cardiovascular Health Study. Arch Intern Med. 2005;165:1896–902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 5.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and Vascular Disease: Pathophysiology, Clinical Consequences, and Medical Therapy: Part I. Circulation. 2003;108:1527–32. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 6.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and Vascular Disease: Pathophysiology, Clinical Consequences, and Medical Therapy: Part II. Circulation. 2003;108:1655–61. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 7.Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of Insulin Resistance and Inflammation With Peripheral Arterial Disease: The National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118:33–41. doi: 10.1161/CIRCULATIONAHA.107.721878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 9.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 10.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. Journal of Vascular Surgery. 2008;48:1197–203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Newman AB, Shemanski L, Manolio TA, et al. Ankle-Arm Index as a Predictor of Cardiovascular Disease and Mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 13.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR Using Serum Cystatin C Alone and in Combination With Serum Creatinine: A Pooled Analysis of 3,418 Individuals With CKD. American Journal of Kidney Diseases. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:299–502. [PubMed] [Google Scholar]
- 16.Siscovick DS, Fried L, Mittelmark M, et al. Exercise Intensity and Subclinical Cardiovascular Disease in the Elderly: The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–86. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 18.Kim J-a, Montagnani M, Koh KK, Quon MJ. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction: Molecular and Pathophysiological Mechanisms. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 19.Yeboah J, Folsom AR, Burke GL, et al. Predictive Value of Brachial Flow-Mediated Dilation for Incident Cardiovascular Events in a Population-Based Study: The Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattace-Raso FUS, van der Cammen TJM, Hofman A, et al. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 21.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis. 2008;197:1–11. doi: 10.1016/j.atherosclerosis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) The Journal of Clinical Investigation. 1997;100:1166–73. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauss RM. Lipids and Lipoproteins in Patients With Type 2 Diabetes. Diabetes Care. 2004;27:1496–504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- 24.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical Correlates and Heritability of Flow-Mediated Dilation in the Community: The Framingham Heart Study. Circulation. 2004;109:613–9. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery,* Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–5. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 27.Wang CCL, Goalstone ML, Draznin B. Molecular Mechanisms of Insulin Resistance That Impact Cardiovascular Biology. Diabetes. 2004;53:2735–40. doi: 10.2337/diabetes.53.11.2735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.