Abstract
BACKGROUND
Safe, accurate methods to reliably measure circulating red blood cell (RBC) kinetics are critical tools to investigate pathophysiology and therapy of anemia, including hemolytic anemias. This study documents the ability of a method using biotin-labeled RBCs (BioRBCs) to measure RBC survival (RCS) shortened by coating with a highly purified monomeric immunoglobulin G antibody to D antigen.
STUDY DESIGN AND METHODS
Autologous RBCs from 10 healthy D+ subjects were labeled with either biotin or 51Cr (reference method), coated (opsonized) either lightly (n = 4) or heavily (n = 6) with anti-D, and transfused. RCS was determined for BioRBCs and for 51Cr independently as assessed by three variables: 1) posttransfusion recovery at 24 hours (PTR24) for short-term RCS; 2) time to 50% decrease of the label (T50), and 3) mean potential life span (MPL) for long-term RCS.
RESULTS
BioRBCs tracked both normal and shortened RCS accurately relative to 51Cr. For lightly coated RBCs, mean PTR24, T50, and MPL results were not different between BioRBCs and 51Cr. For heavily coated RBCs, both short-term and long-term RCS were shortened by approximately 17 and 50%, respectively. Mean PTR24 by BioRBCs (84 ± 18%) was not different from 51Cr (81 ± 10%); mean T50 by BioRBCs (23 ± 17 days) was not different from 51Cr (22 ± 18 days).
CONCLUSION
RCS shortened by coating with anti-D can be accurately measured by BioRBCs. We speculate that BioRBCs will be useful for studying RCS in conditions involving accelerated removal of RBCs including allo- and autoimmune hemolytic anemias.
Red blood cells (RBCs) can be removed from the bloodstream at an accelerated rate by antibody-mediated mechanisms in a number of immunologic conditions including the following: 1) autoimmune hemolytic anemias, 2) transfusion of incompatible allogeneic RBCs into a recipient whose blood contains a corresponding alloantibody, 3) transfusion of passive antibodies into a recipient whose RBCs express the cognate antigen, 4) transplacental transfer of maternal allogeneic RBC antibodies into a fetus with RBCs expressing the cognate antigen resulting in alloimmune hemolytic disease of the fetus and newborn, and 5) posttransplantation transfer of viable donor lymphocytes capable of producing antibodies directed against recipient RBCs (e.g., group O donor into group A recipient). When caring for patients with these disorders, having an accurate and safe method for determining in vivo RBC kinetics would be of considerable value for establishing the diagnosis, defining the pathophysiology, and assessing the response to therapy. The availability of RBC survival (RCS) methods that address safety issues (e.g., avoidance of radioactivity) is particularly important for vulnerable patient populations including fetuses, neonates, children, and pregnant women.
Direct assessment of RCS requires the ability to distinguish cohort-labeled RBCs from the remaining RBCs in the circulation and to express their number as the relative proportion of labeled RBCs to total RBCs in that blood sample. The internationally recognized reference method for measuring RCS uses autologous or allogeneic RBCs labeled with chromium 51 (51Cr) and is based on concentration of 51Cr per unit volume of blood.1 Because transfusion of 51Cr-labeled RBCs exposes the recipient to radiation, this approach is viewed as unacceptable for research studies in the human fetus, neonate, and pregnant woman.
Our laboratory and others2-4 have developed methods for measuring RCS in humans based on labeling of RBCs with biotin at one5-10 or more11 densities. Here the term density refers to biotin labels per RBC and is determined by our selection of the concentration of biotinylating reagent per milliliter of RBCs in the biotinylation reaction mixture. Biotin-labeled RBCs (BioRBCs) are quickly and easily enumerated by flow cytometry.11 The biotin RBC method has the following safety and technical advantages over the standard 51Cr method: 1) study subjects including vulnerable populations are not exposed to radiation; 2) small blood volumes (e.g., 20 μL) are required for monitoring; and 3) multiple, independent RCS measurements can be made simultaneously in the same individual by labeling RBCs at more than one biotin density.
The aim of this study was to determine whether RBCs labeled with biotin at a single density can be used to accurately measure shortened RCS of RBCs experimentally coated (opsonized) with antibody simulating the situation present in immune-mediated hemolytic anemia. We hypothesized that short-term and long-term RCS measured using BioRBCs would agree with RCS determined using RBCs labeled with 51Cr.
MATERIALS AND METHODS
Definition of terms
RCS
RCS is a general term for the persistence of RBCs in circulation. In this study, three RCS variables were determined: posttransfusion recovery at 24 hours (PTR24), mean potential life span (MPL), and time to 50% decrease of the label (T50). These are defined as follows:
PTR24
PTR24 equals the percentage of RBC label (or of labeled RBCs) remaining in circulation 24 hours after infusion of the labeled RBCs relative to the first quantitation of the labeled RBCs, which was at 10 minutes in this study.
T50
The time from infusion to disappearance of 50% of the label (for 51Cr) or 50% of the labeled RBCs (for BioRBCs) is termed T50. Use of T50 values allows comparison of RCS in circumstances in which curvilinear disappearance curves prevent accurate estimation of MPL (see below). Use of T50 values does not require the tacit assumption that RBC disappearance is linear. In this study, T50 values for 51Cr-labeled RBCs are corrected for 51Cr decay and for 51Cr elution. T50 for BioRBCs is not corrected for biotin label loss from the RBC surface because no correction is necessary for enumeration methods.10,11
MPL
As discussed previously,10,11 survival curves for a uniformly labeled cohort population of RBCs in steady state erythropoiesis demonstrates a linear decline for most of the RBC life span.12 The value obtained by extrapolation of the linear proportion of the RBC disappearance curve to the time axis is termed the mean RBC life span.12,13 The value was originally referred to as “mean potential lifespan” and is commonly abbreviated as MPL.
Human studies
All studies were approved by the University of Iowa Committee on Research on Human Subjects (study performance site) and the institutional review board of the University of Arkansas for Medical Sciences (study analysis site). Written informed consent was obtained from each subject as part of the ongoing informed consent process.
Study population
Healthy adults of either sex were offered the opportunity to participate if they met the following inclusion criteria: 1) 18 to 65 years of age, 2) body weight greater than 50 kg, 3) blood hemoglobin concentration of at least 125 g/L, and 4) if their RBCs were D+. Exclusion criteria were the following: 1) presence of active chronic illness; 2) consumption of biotin supplements or raw eggs within 30 days; 3) blood donation in the previous 8 weeks; 4) blood loss in the previous 8 weeks due to epistaxis, gastrointestinal bleeding, trauma, diagnostic phlebotomy (>30 mL), or other bleeding; 5) premenopausal women, to avoid menstrual blood loss; and 6) treatment with antibiotics in the week before initiating study participation. The last criterion was adopted to avoid any suppression of erythropoiesis accompanying a putative infection.
Control population
A group of 10 normal subjects whose RBCs had been labeled with 51Cr or biotin at a density similar to that used in the study population served as historical controls. RCS has previously been reported for these subjects, and the results were used to assess the range for RCS variables in this small population of normal controls.10 For this group, healthy adults of either sex were screened according to the same inclusion and exclusion criteria—except for D antigen status, which was not determined because RBCs of these control subjects were not coated with anti-D.
Experimental design
Summary
Only healthy D+ adults were offered the opportunity to participate because we anticipated that the intravascular survival of their autologous RBCs would be shortened due to coating the RBC surface with purified immunoglobulin (Ig)G antibody to the D antigen. In clinical situations, transfusing D+ RBCs into recipients whose plasma contains anti-D can produce significant hemolysis. To minimize the risk of clinically significant hemolysis, highly purified monomeric IgG anti-D was used, and the study was conducted in two phases: first with “lightly coated” RBCs and 2) then with “heavily coated” RBCs (see “Safety considerations” under Results). All laboratory procedures were carried out with strict attention to maintaining sterility.
Blood collection
Two-hundred milliliters of venous blood was collected percutaneously from the antecubital vein in citrate phosphate dextrose blood collection bags. The RBCs were sedimented by centrifugation and washed with 11.1 mmol/L glucose, 20 mmol/L sodium bicarbonate, 2.3 mmol/L NaHPO4, 1.14 mmol/L Na2PO4, and 154 mmol/L NaCl (wash buffer); all components were “USP for injection” grade reagents.
Labeling of RBCs
Although there are several biotinylating reagents available commercially, there is no biotinylation reagent that is specifically approved for labeling products intended for human use. Therefore, we obtained and have annually renewed an investigational new drug approval from the US Food and Drug Administration for our human RBC biotinylation studies. Under the approved investigational new drug protocol, we filter sterilized the biotinylation reagent using a 0.22-μm filter. The method for biotin labeling used in this study has been reported in detail;10,14 this method was chosen to be the same single density method as used for the control group to have the most straightforward comparison. A separate publication provides an updated method for multidensity biotin labeling including recent improvements.15
In this study, biotin was attached to freshly washed RBCs by sterile incubation with sulfo-succinimido-biotin (Pierce Chemical Co., Rockford, IL) in wash buffer (pH 7.4) for 30 minutes. The biotinylation reaction was then stopped by washing the RBCs twice in fivefold greater volumes of wash buffer; this also served to remove most of the remaining biotinylating reagent and any hydrolysis reaction byproducts. Washed BioRBCs were then passed through an 18-μm filter to remove bacterial contaminants (Hemo-Nate syringe filter, Utah Medical Products, Inc., Midvale, UT). The BioRBCs for each subject were cultured for bacterial contamination; all cultures in this study were negative. The method used for 51Cr labeling was that recommended by the International Committee of Standardization in Haematology for the method using ascorbic acid.16
For each participant, separate aliquots of autologous RBCs were initially labeled with either 1) biotin at approximately 10 μg/mL RBCs (23 nmol/mL), a density well within a range shown to yield accurate estimates of in vivo RCS under normal conditions11 or 2) 31 μCi of 51Cr. The volume of RBCs to be infused was calculated to produce 1% to 5% enrichment of labeled RBCs in the total circulation. After mixing of the aliquots of BioRBCs and 51Cr-labeled RBCs, the mixtures were coated with highly purified monomeric IgG anti-D17 (WinRho SD, Univax Biologics, Inc., Rockville, MD); the RBC suspension was incubated with the antibody solution for 30 minutes at 37°C with gentle mixing every 5 minutes at one of two concentrations. Specifically, RBCs of the first four subjects (1-4; one woman) were treated with 0.1 μg anti-D/mL of resuspended RBCs (“light coating”). For the next six subjects (5-10; four women), RBCs were treated with 1.0 μg anti-D/mL of resuspended RBCs (10 times more antibody for “heavy coating”).
The pooled aliquot of labeled, opsonized RBCs, approximately 80 mL in volume, was passed through an 18-μm filter and infused as a bolus over approximately 15 minutes.The labeling, antibody coating, and infusion were all performed on a single day.
Posttransfusion testing for antibodies to BioRBCs
Before transfusion of BioRBCs and at intervals up to 6 months later, 1 mL of venous blood was collected from subjects for detection of antibodies to biotinylated antibodies. Plasma samples were tested for antibodies to BioRBCs using an antiglobulin technique against aliquots of group O RBCs from an adult human donor in which one aliquot was biotinylated and another was not (i.e., test and control RBCs). A positive reaction was defined as visible agglutination. RBCs with the K1 antigen (Kell) were reacted with anti-K1 to serve as a positive control.18
RCS measurements
RCS was determined using the standard 51Cr measurement procedure12 and the BioRBC enumeration method previously published. For RCS determinations, 7- to 10-mL venous blood samples in heparin at 15 U/mL were obtained at 10 minutes after infusion of the mixture of labeled RBCs; at 1, 2, 4, 7, 10, and 14 days; and at weekly intervals thereafter to the limit of quantitation. For 51Cr, the limit was approximately 5% of the original concentration of uncorrected radioactivity. For BioRBCs, the lower limit of quantitation is approximately 0.06% of total RBCs in the blood sample.10,11 Hence the duration over which RCS was tracked was not the same for the two methods, even for the same subject. For the graphic displays in Figs. 1 and 2 and for the regression analyses, survival points for which less than 20% of original radioactivity or 20% of BioRBCs remained were omitted as previously described.10,11
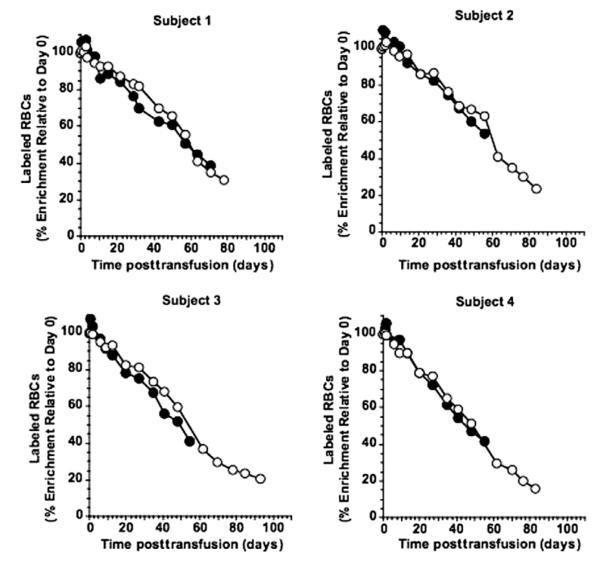
Fig. 1.
Survival curves of Subjects 1 to 4 for (●) 51Cr- and (엯) biotin-labeled RBCs lightly coated with anti-D before transfusion. 51Cr whole blood concentration was corrected for both elution and 51Cr decay. Values are expressed as percentage of Day 0 (10 min) value.
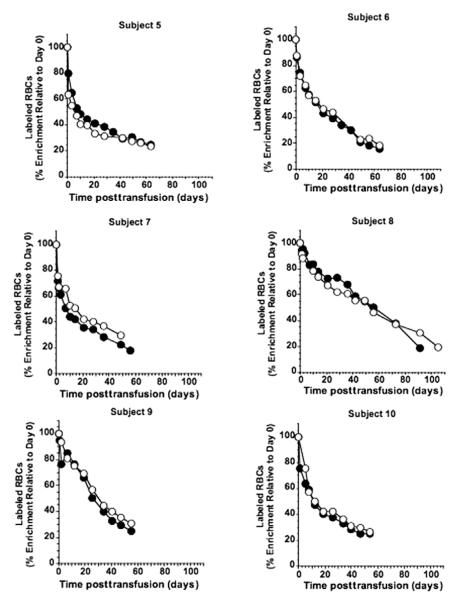
Fig. 2.
Survival curves for subjects 5-10 for (●) 51Cr- and (엯) biotin-labeled RBCs heavily coated with anti-D before transfusion. 51Cr: blood concentration of RBC-associated 51Cr as described in the legend to Fig. 1.
The blood concentrations of 51Cr-labeled RBCs were determined by gamma counting of 2 mL of whole blood using energy windows of 0 to 1000 keV with correction for radioactive decay by application of the formula dpm corrected = dpm measured × EXP((LN(2)/27.7 days) × [current time-zero time]) as described previously.10,19
51Cr data were also corrected for spontaneous elution of the label from RBCs using a mean value of 1.4% per day20 as described previously.10
The proportions of BioRBCs (expressed as percentage of total RBCs) were determined by flow cytometric enumeration. A 10-μL aliquot of blood was washed and the plasma-free BioRBCs were complexed to fluoresceinlabeled avidin for 30 minutes. The BioRBCs were washed to remove unbound dye and the fluoresceinavidin-BioRBC complex was enumerated by flow cytometry. The BioRBCs were expressed as percentages of the Day 0 value (10-min sample) and plotted versus time. Because both populations of labeled RBCs (e.g., corrected 51Cr and BioRBCs) exhibited approximately linear disappearance, MPL was calculated by extrapolation of the linear least squares regression to the time-axis intercept12 as previously described.10
Statistical analysis
For each of the three RCS variables for the two RBC labels (biotin or 51Cr), significance of the difference between the group means of light antibody coating versus controls was tested by Mann-Whitney U t test. Likewise, significance of the differences between the group means of heavy antibody coating versus controls were tested by Mann Whitney U t test for each RBC label. For the means testing of BioRBCs versus 51Cr-labeled RBCs in the same individuals, Wilcoxon rank test was used. Statistical analyses were performed with compture software (StatView 5.0, Abacus Concepts, Berkeley, CA); significance was set at α = 0.05 level.
RESULTS
Safety considerations
Largely to establish safety, we used a anti-D coating at a concentration (“light coating”) in the first phase of the study that we anticipated was unlikely to produce substantial hemolysis of the labeled RBCs and, importantly, would have only a modest chance of reducing RCS. Accordingly, we anticipated that RCS values might be normal. However, safety considerations mandated close assessment for hemolysis in this setting. All subjects were monitored by qualified personnel for evidence of transfusion reactions. Vital signs were take every 10 to 15 minutes, and subjects were queried about any symptoms such as pain, anxiety, or shortness of breath. A spot urine was collected 30 minutes after the infusion and, if any reddish color was noted, the urine sample was tested for blood; no reddish color was noted in any participant’s urine. Participants were instructed to drink 1.2 L water over the remaining day and to observe all voids for any reddish color; no reddish color was reported by any participant.
Short-term RCS (PTR24) by 51Cr-labeled RBCs and BioRBCs lightly coated with anti-D
Light anti-D coating did not significantly reduce short-term RCS as assessed by the 51Cr method. For these four subjects, the mean PTR24 by 51Cr was not significantly different from the mean PTR24 as assessed in 10 healthy subjects whose 51Cr-labeled RBCs were not coated with antibody, and the values for all four subjects fell within the range of the 10 controls (Table 1). Likewise, light anti-D coating did not significantly reduce the mean PTR24 by BioRBCs compared to the mean PTR24 in the 10 controls whose BioRBCs were not coated with antibody, and the four values were tightly grouped within the control range (Table 1).
TABLE 1.
PTR24 reduction by heavy but not light antibody coating and agreement of the biotin and 51Cr labeling methods for both coatings
| PTR24 (%)* |
|||
|---|---|---|---|
| Subjects | 51Cr | Biotin | p value† |
| Controls (n = 10)10 | 99 ± 5 (90-107) | 100 ± 6 (92-111) | |
| Light anti-D coating (n = 4) | 102 ± 3 (99-107) | 101 ± 1 (100-101) | 0.38 |
| p value‡ | 0.32 | 0.64 | |
| Heavy anti-D coating (n = 6) | 81 ± 10 (70-92) | 84 ± 18 (56-103) | 0.44 |
| p value§ | 0.0001 | 0.02 | |
Data are reported as mean ± SD (range).
p value of Wilcoxon test biotin versus 51Cr-labeled RBCs.
p value of Mann Whitney test light antibody coating versus controls.
p value of Mann Whitney test heavy antibody coating versus controls.
Long-term RCS by 51Cr-labeled RBCs and BioRBCs lightly coated with antibody
For these four subjects, survival curves for 51Cr-labeled RBCs that were not corrected for 51Cr elution exhibited the curvilinear survival previously described by others20 and by us.10 In contrast, the 51Cr disappearance curves that were corrected for elution (Fig. 1) exhibited survival that was linear; the mean correlation coefficient of the four linear regressions (mean ± SD) was 0.983 ± 0.006.The mean T50 for the corrected 51Cr survival curves of Subjects 1 to 4 was not different from that of the 10 healthy subjects whose 51Cr-labeled RBCs were not coated with antibody (Table 2), nor was the mean MPL different (Table 3). All of the individual values for T50 and MPL fell within the range of the 10 controls.
TABLE 2.
Shortened T50 by heavy antibody coating and agreement of the biotin and 51Cr methods
| T50 (days)* |
|||
|---|---|---|---|
| Subjects | 51Cr | Biotin | p value† |
| Controls (n = 10)10 | 58 ± 7 (45-69) | 45 ± 6 (35-52) | |
| Light antibody (n = 4) | 53 ± 7 (46-59) | 56 ± 6 (48-61) | 0.12 |
| p value‡ | 0.37 | 0.01 | |
| Heavy antibody (n = 6) | 22 ± 18 (8-56) | 23 ± 17 (5-53) | 0.56 |
| p value§ | 0.003 | 0.03 | |
Data are reported as mean ± SD (range).
p value of Wilcoxon test biotin versus 51Cr.
p value of Mann Whitney test light antibody coating versus controls.
p value of Mann Whitney test heavy antibody coating versus controls.
TABLE 3.
MPL* for the longest-lived RBCs was not shortened by heavy antibody coating and the biotin method agreed with the 51Cr method
| MPL (days)† |
|||
|---|---|---|---|
| Subjects | 51Cr | Biotin | p value‡ |
| Controls (n = 10)10 | 116 ± 17 (87-137) | 96 ± 10 (77-111) | |
| Light antibody (n = 4) | 101 ± 13 (88-113) | 108 ± 9 (95-115) | 0.12 |
| p value§ | 0.19 | 0.08 | |
| Heavy antibody (n = 6) | 103 ± 14 (89-122) | 110 ± 26 (87-147) | 0.31 |
| p value∥ | 0.12 | 0.64 | |
MPL for 51Cr and BioRBCs was derived from the longest-lived RBCs.
Data are reported as mean ± SD (range).
p value of Wilcoxon test biotin versus 51Cr.
p value of Mann Whitney test light antibody versus controls.
p value of Mann Whitney test heavy antibody versus controls.
Similar to the 51Cr findings, the disappearance curves for BioRBCs (Fig. 1) also exhibited linear survival; mean correlation coefficient of the four linear regressions (±SD) was 0.980 ± 0.011. Contrary to our expectation that long-term RCS might be shortened by light anti-D coating, mean T50 (Table 2) was significantly longer than the mean for the 10 healthy subjects whose BioRBCs were not coated with antibody (p = 0.01; Table 2); three of the four T50 values of subjects with light antibody-coated RBCs were greater than the upper limit of the range of BioRBC T50 values for the 10 controls. The mean MPL was not significantly longer (p = 0.08) than that of the 10 controls (Table 3). Importantly, when comparing the biotin method to the 51Cr method, neither the mean T50 nor the mean MPL were significantly different.
Short-term RCS by 51Cr and biotin in the subjects whose RBCs were heavily coated with anti-D
Heavy antibody coating significantly shortened RCS. For Subjects 5 to 10, the mean PTR24 by 51Cr was significantly shorter than the controls, with values from four of these six subjects being lower than the range of values from the ten controls (Table 1). BioRBCs accurately tracked the reduced short-term survival for these six subjects as judged by paired PTR24 values that were similar to those of 51Cr; the mean PTR24 by BioRBC was not significantly different from mean PTR24 by 51Cr (Table 1). The mean PTR24 by BioRBC was significantly shorter than controls, and PTR24 values from four of six were less than the lower limit of the controls (Table 1).
Long-term RCS by 51Cr and biotin in the subjects whose RBCs were heavily coated with anti-D
Corrected 51Cr survival curves exhibit rapid removal of 51Cr-labeled RBCs for approximately 2 weeks followed by decline that was approximately linear for the remainder of the RBC life span (Fig. 2). As a consequence of the rapid removal phase, mean T50 values derived from the 51Cr survival curves for Subjects 5 to 10 were significantly decreased compared to the controls (p = 0.003), and five of six were less than the lower limit of the controls (Table 2).
Similar to 51Cr, BioRBCs accurately tracked the reduced long-term RCS (Fig. 2). Mean T50 values derived from the BioRBC survival curves for Subjects 5 to 10 were significantly decreased compared to controls (p = 0.03) with five of six less than the lower limit of the control range (Table 2). The mean T50 by BioRBC was not significantly different from mean T50 by 51Cr (Table 2).
Because the disappearance curves for four of the six subjects (5, 6, 7, and 10) exhibited a distinctly slower terminal removal phase that was approximately linear over the last 4 to 6 weeks of the study, we extrapolated this terminal linear segment to the time axis to estimate of the MPL for this subset of RBCs. We used a similar time segment for the remaining two subjects to estimate MPL of their longest-lived RBCs. The mean MPL for the 51Cr-labeled RBCs in this phase was not significantly different from the 10 controls whose 51Cr-labeled RBCs were not coated with antibody (Table 3). Likewise, the MPL for the BioRBCs in this phase was not significantly different from the controls, and the MPLs were not different between biotin and 51Cr.
DISCUSSION
This study provides evidence that RCS in the bloodstream can be accurately tracked using BioRBCs under conditions in which the survival of the BioRBCs is experimentally shortened by antibody coating (opsonization); in this instance we report results using highly purified monomeric IgG anti-D to RBCs expressing D antigen. Importantly, these studies provide evidence that tracking the survival of BioRBCs by enumerating their persistence in circulation using flow cytometry produced survival curves quite similar to the reference 51Cr-labeled RBCs. BioRBCs accurately tracked the 51Cr-labeled RBCs despite the acceleration and the curvilinear nature of the disappearance curves.
Because appreciable RCS shortening was not induced by light antibody coating, the studies conducted on Subjects 1 through 4 confirmed our original observations10 that the biotin method can accurately measure RCS under normal survival conditions—even when the RBC surface has been altered by a light coating of IgG antibodies. We have no definitive explanation regarding why the T50 values for three of these four subjects whose BioRBCs were lightly coated with anti-D were longer than any of the 10 controls whose BioRBCs were not coated with antibody. The most plausible explanation is that the numbers of participants was too few to avoid the chance for observing a random difference in normal survival times.
The findings of a similar study by Mollison and coworkers21 in which RBCs were labeled in vitro with anti-D and followed in vivo for survival are relevant to this study in at least two aspects with the caveats that the antibodies used by Mollison and coworkers were isolated from patients’ blood, were stored frozen and subsequently thawed, and were studied by methods nearly 50 years old. Although the studies by Mollison and coworkers were quite elegant and sophisticated for the time, accurate comparison to our current studies using a highly purified monomeric IgG antibody is difficult. Nonetheless, Mollison and coworkers used much greater doses of anti-D (e.g., 13.4 μg/mL RBCs) and observed much faster RBC removal rates (e.g., T50 of 36 min). The largest dose used in this study was less than the smallest dose used in the study by Mollison and coworkers (1.6 μg anti-D/mL RBC). At that lowest dose, Mollison and coworkers observed no loss of the RBCs at 65 minutes and observed T50 of “>24 hours” at 1 day. This observation is roughly consistent with the mean PTR24 (i.e., the “Day 1 sampling”) of 81 and 84% observed in our study for 51Cr and BioRBCs. Lack of a detectable effect of 0.01 μg of anti-D/mL RBCs was anticipated by us but was studied first to minimize the risk of a significant hemolytic reaction for safety reasons.
To speculate further about this finding, it is useful to recall that assessment of RCS depends on the disappearance of label from the circulation; in turn, the disappearance of label is the consequence of two processes: 1) loss of the RBCs from bloodstream and (at steady state) with dilution by release of unlabeled RBCs from the bone marrow and 2) shedding of the label from the RBCs that remain in the bloodstream. For 51Cr, the rapid 4% loss during the first 24 hours is believed to be explained by loss of loosely bound 51Cr per se.20 Thereafter, a mean of 1.4% of the 51Cr elutes per day, but this number varies substantially among individuals. A key advantage of the biotin method relative to the 51Cr method is that loss of biotin label cannot be misinterpreted as loss of the BioRBCs because the biotin method is based on enumeration of BioRBCs rather than quantitation of the blood concentration of biotin label.10,11 Loss of biotin label from the RBC surface does occur and is manifested as a small shift in peak intensity per RBC in the histogram; this shift does not introduce an artifact into quantitation of the proportion of BioRBCs.10,11,22
Despite this advantage of the biotin method, antibodies binding to the D antigens expressed on the RBC membrane could theoretically interfere with the binding of fluorescenated avidin to the biotin moieties covalently linked to the lysine residues of proteins on the membrane surface; such a mechanism could render the BioRBCs undercounted or even undetectable. We saw no evidence of such interference either when examining the flow cytometric histograms or when comparing survival of BioRBCs to the 51Cr-labeled RBCs. We conclude that flow cytometric enumeration of BioRBCs accurately reflects in vivo RCS over the 7- to 10-week study period.
Shortening of survival by heavy antibody coating was evident primarily in the first few weeks after reinfusion of the labeled autologous RBCs; thereafter, RBCs exhibited a linear survival. The MPL of the labeled RBCs still in circulation during the later phase was not significantly different from normal. These biphasic survival curves offer potential insight into the process of antibody-mediated removal of RBCs from the circulation. The observation of roughly normal MPL for long-lived RBCs in the terminal phase is consistent with the inference that, if antibody-coated RBCs have not been removed within a certain time, the remaining cells will exhibit normal life spans. We further speculate that two alternative mechanisms might be at work. First, all RBCs may have been coated uniformly ex vivo and were initially removed at an accelerated rate. Sufficient anti-D may then have been removed from the RBCs remaining in circulation by an undefined process (e.g., elution/shedding or slight degrees of piecemeal phagocytosis). Thereafter, these RBCs may have no longer attracted sufficient attention from the reticuloendothelial system for rapid removal and consequently had a normal life span. Mollison and coworkers observed a bimodal removal of antibody-coated RBCs that was particularly pronounced for antibodies that eluted rapidly.21 Second, RBCs may not have been coated uniformly ex vivo. The more heavily coated cells were removed at an accelerated rate until this subpopulation was completely depleted. The RBCs with little or no initial coating would have remained in circulation and lived out their normal life span. Because the late survival curves are so linear, we favor the first mechanism.
This study, in which exogenous highly purified monomeric anti-D was added ex vivo to RBCs expressing the cognate antigen, differs from in vivo conditions in patients, such as autoimmune hemolytic anemia, in which production of antibodies (often a mixture of polyclonal gamma globulins) to an antigen on the RBC surface is ongoing and, possibly, changing over time. Hence the kinetics of RBC removal in vivo are likely to vary among different clinical scenarios. Notwithstanding, we contend that biotinylated autologous or allogeneic RBCs will be detectable by the method described here and consequently will reflect the true in vivo survival (normal or shortened) of the autologous or allogeneic RBCs being studied. This assertion is based on the fact that the affinity-binding constant for biotin and avidin is 103 to 106 greater that the affinity-binding constants for most antigen and antibody pairs, on the observation that the BioRBCs heavily coated with antibody to D were equally well detected as uncoated RBCs before and after infusion, and the observation that in vivo RCS of 51Cr-labeled RBCs that have no surface modification was accurately tracked by BioRBCs.
In addition to its application to immune hemolytic anemias, the BioRBC method offers a tool for the nonradioactive measurement of RCS in the human fetus, neonate, and pregnant woman. This method can be employed to study the physiology of normal and impaired erythropoiesis, the pathophysiology of anemia, and the response to various therapies such as transfusing fresh or stored RBCs,23 transfusing autologous placental blood,24 and therapy with human recombinant erythropoietin.25
ACKNOWLEDGMENT
The authors acknowledge the technical assistance of Marie Tippett for graphics.
This work was supported by the National Heart, Lung, and Blood Institute Grant P01 HL046925.
ABBREVIATIONS
- BioRBC(s)
biotinylated red blood cells
- MPL
mean potential red blood cell life span in circulation
- PTR24
posttransfusion recovery of red blood cells after 24 hours in the circulation
- RCS
red blood cell survival
- T50
time to disappearance of 50% of the labeled red blood cells from the circulation.
Footnotes
CONFLICT OF INTEREST The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
REFERENCES
- 1.International Committee for Standardization in Haematology Recommended methods for radioisotope red-cell survival studies. Br J Haematol. 1980;45:659–66. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 2.Franco RS, Lohmann J, Silverstein EB, Mayfield-Pratt G, Rucknagel DL, Joiner CH, Weiner M. The use of biotinylated RBC to determine the in vivo survival characteristics of normal and sickle cells. Blood. 1996;88:11A. [Google Scholar]
- 3.Russo V, Barker-Gear R, Gates R, Franco R. Studies with biotinylated RBC: (1) use of flow cytometry to determine posttransfusion survival and (2) isolation using streptavidin conjugated magnetic beads. In: Magnani M, DeLoach JR, editors. The use of resealed erythrocytes as carriers and bioreactors. Plenum Press; New York: 1992. pp. 101–7. [DOI] [PubMed] [Google Scholar]
- 4.Franco RS, Lohmann J, Silbertein EB, Mayfiled-Pratt G, Palascak M, Nemeth TA, Joiner CH, Weiner M, Rucknagel DL. Time-dependent changes in the density and hemoglobin F content of biotin-labeled sickle cells. J Clin Invest. 1998;101:2730–40. doi: 10.1172/JCI2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson I, Cooke A, Holland B, Houston A, Jones JG, Turner T, Wardrop CA. Red cell volume and cardiac output in anaemic preterm infants. Arch Dis Child. 1990;65:672–5. doi: 10.1136/adc.65.7_spec_no.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson IR, Cavill IA, Cooke A, Holland BM, Hoy TG, Trevett D, Turner TL, Wardrop CA. Biotin labeling of red cells in the measurement of red cell volume in preterm infants. Pediatr Res. 1990;28:199–202. doi: 10.1203/00006450-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Holland BM, Wardrop CA. Anaemias of the preterm infant. In: Turner TL, editor. Perinatal haematological problems. John Wiley & Sons, Ltd.; London: 1991. pp. 121–35. [Google Scholar]
- 8.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117:93–8. doi: 10.1542/peds.2004-1773. [DOI] [PubMed] [Google Scholar]
- 9.Aladangady N, Aitchison TC, Beckett C, Holland BM, Kyle BM, Wardrop CA. Is it possible to predict the blood volume of a sick preterm infant? Arch Dis Child Fetal Neonatal Ed. 2004;89:F344–7. doi: 10.1136/adc.2003.039008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin labeled red cells: validation against 51Cr labeled red cells. Transfusion. 1999;39:156–62. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]
- 11.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Committee for Standardization in Haematology Recommended methods for radioisotope red-cell survival studies. Br J Nutr. 1971;21:241–50. doi: 10.1111/j.1365-2141.1971.tb03435.x. [DOI] [PubMed] [Google Scholar]
- 13.Garby L, Mollison PL. Deduction of mean red-cell life-span from 51Cr survival curves. Br J Haematol. 1971;20:527–36. doi: 10.1111/j.1365-2141.1971.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 14.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of circulating red blood cell volume using biotin labeled red cells: validation against 51Cr labeled red cells. Transfusion. 1999;39:149–55. doi: 10.1046/j.1537-2995.1999.39299154728.x. [DOI] [PubMed] [Google Scholar]
- 15.Mock DM, Matthews NI, Strauss RG, Burmeister LF, Schmidt R, Widness JA. Red blood cell volume can be independently determined in vitro using sheep and human red cells labeled at different densities of biotin. Transfusion. 2009;49:1178–85. doi: 10.1111/j.1537-2995.2009.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Committee for Standardization in Haematology Recommended methods for measurement of red-cell and plasma volume. J Nucl Med. 1980;21:793–800. [PubMed] [Google Scholar]
- 17.Fresco R, Busch S, Hanson ML, Huestis DW. A simple method of preparing “coated” red blood cells for use as a control in antiglobulin testing. Transfusion. 1964;4:262–4. doi: 10.1111/j.1537-2995.1964.tb02869.x. [DOI] [PubMed] [Google Scholar]
- 18.Cordle DG, Strauss RG, Lankford GL, Mock DM. Antibodies provoked by the transfusion of biotin-labeled red cells. Transfusion. 1999;39:1065–9. doi: 10.1046/j.1537-2995.1999.39101065.x. [DOI] [PubMed] [Google Scholar]
- 19.Mock DM, Lankford GL, Burmeister LF, Strauss RG. Circulating red cell volume and red cell survival can be accurately determined in sheep using the [14C]cyanate label. Pediatr Res. 1997;41:916–21. doi: 10.1203/00006450-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Mollison PL, Engelfriet CP, Conteras M. Blood transfusion in clinical medicine. Blackwell Scientific Publications; London: 1993. [Google Scholar]
- 21.Mollison P, Crome P, Hughes-Jones N, Rochna E. Rate of removal from the circulation of red cells sensitized with different amounts of antibody. Transfusion. 1965;11:461–70. doi: 10.1111/j.1365-2141.1965.tb06609.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann-Fezer G, Mysliwietz J, Mortlbauer W, Zeitler JJ, Eberle E, Honle U, Thierfelder S. Biotin labeling as an alternative nonradioactive approach to determination of red cell survival. Ann Hematol. 1993;67:81–7. doi: 10.1007/BF01788131. [DOI] [PubMed] [Google Scholar]
- 23.Strauss RG, Burmeister LF, Johnson K, James T, Miller J, Cordle DG, Bell EF, Ludwig GA. AS-1 red blood cells for neonatal transfusions: a randomized trial assessing donor exposure and safety. Transfusion. 1996;36:873–8. doi: 10.1046/j.1537-2995.1996.361097017172.x. [DOI] [PubMed] [Google Scholar]
- 24.Bifano EM, Dracker RA, Lorah K, Palit A. Collection and 28-day storage of human placental blood. Pediatr Res. 1994;36:90–4. doi: 10.1203/00006450-199407001-00016. [DOI] [PubMed] [Google Scholar]
- 25.Widness JA, Strauss RG. Recombinant erythropoietin in treatment of the premature newborn. Semin Neonatol. 1998;3:163–71. [Google Scholar]