Abstract
BACKGROUND
Programmed death 1 (PD-1) protein, a T-cell coinhibitory receptor, and one of its ligands, PD-L1, play a pivotal role in the ability of tumor cells to evade the host’s immune system. Blockade of interactions between PD-1 and PD-L1 enhances immune function in vitro and mediates antitumor activity in preclinical models.
METHODS
In this multicenter phase 1 trial, we administered intravenous anti–PD-L1 antibody (at escalating doses ranging from 0.3 to 10 mg per kilogram of body weight) to patients with selected advanced cancers. Anti–PD-L1 antibody was administered every 14 days in 6-week cycles for up to 16 cycles or until the patient had a complete response or confirmed disease progression.
RESULTS
As of February 24, 2012, a total of 207 patients — 75 with non–small-cell lung cancer, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer — had received anti–PD-L1 antibody. The median duration of therapy was 12 weeks (range, 2 to 111). Grade 3 or 4 toxic effects that investigators considered to be related to treatment occurred in 9% of patients. Among patients with a response that could be evaluated, an objective response (a complete or partial response) was observed in 9 of 52 patients with melanoma, 2 of 17 with renal-cell cancer, 5 of 49 with non–small-cell lung cancer, and 1 of 17 with ovarian cancer. Responses lasted for 1 year or more in 8 of 16 patients with at least 1 year of follow-up.
CONCLUSIONS
Antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rates of 12 to 41% at 24 weeks) in patients with advanced cancers, including non–small-cell lung cancer, melanoma, and renal-cell cancer. (Funded by Bristol-Myers Squibb and others; ClinicalTrials.gov number, NCT00729664.)
Passive cancer immunotherapy that uses tumor-targeted monoclonal antibodies has achieved broad therapeutic efficacy.1 However, T-cell directed immunotherapy has been less successful.2 Despite the large number of tumor antigens induced by genetic and epigenetic changes found in all cancers, tumors resist immune attack by inducing tolerance among tumor-specific T cells and by expressing ligands that engage inhibitory receptors and dampen T-cell functions within the tumor microenvironment.3 Preclinical and clinical data show that antibody blockade of these immune checkpoints can significantly enhance antitumor immunity.4
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), an inhibitory receptor that down-modulates the initial stages of T-cell activation, was the first clinically validated checkpoint pathway target.5–9 Antagonist anti–CTLA-4 monoclonal antibodies mediate tumor regression, most notably in patients with melanoma, but are accompanied by frequent immune-related adverse events. Ipilimumab (Yervoy, Bristol-Myers Squibb), an anti–CTLA-4 antibody, was recently approved for the treatment of patients with stage IV melanoma on the basis of a randomized phase 3 trial that showed prolongation of overall survival.9
Programmed death 1 (PD-1) protein is another T-cell coinhibitory receptor with a structure similar to that of CTLA-4 but with a distinct biologic function and ligand specificity.10,11 PD-1 has two known ligands, PD-L1 (B7-H1)12,13 and PD-L2 (B7-DC).14,15 In contrast to CTLA-4 ligands, CD80 (B7-1) and CD86 (B7-2), PD-L1 is selectively expressed on many tumors16–18 and on cells within the tumor microenvironment in response to inflammatory stimuli.19 Blockade of the interaction between PD-1 and PD-L1 potentiates immune responses in vitro20 and mediates preclinical antitumor activity.16,17 PD-L1 is the primary PD-1 ligand that is up-regulated in solid tumors, where it can inhibit cytokine production and the cytolytic activity of PD-1+, tumor-infiltrating CD4+ and CD8+ T cells.16,21,22 These properties make PD-L1 a potentially promising target for cancer immunotherapy.
BMS-936559 is a high-affinity, fully human, PD-L1–specific, IgG4 (S228P) monoclonal antibody that inhibits the binding of PD-L1 to both PD-1 and CD80. Additional characterization of this anti–PD-L1 antibody is presented in the study protocol, available with the full text of this article at NEJM.org. In this report, we present clinical evidence regarding the safety, clinical activity, and pharmacokinetic and pharmacodynamic effects of anti–PD-L1 antibody in patients with selected advanced cancers.
METHODS
STUDY DESIGN
The primary objective of this phase 1 study was to assess the safety and adverse-event profiles of anti–PD-L1 antibody in patients with selected advanced cancers. Secondary objectives included assessment of the antitumor activity of the antibody and its pharmacokinetics. Pharmacodynamic measures were included as exploratory objectives. (Additional information is provided in the study protocol and in a detailed statistical analysis plan and the Methods section in the Supplementary Appendix, available at NEJM.org.)
PATIENTS
Patients were required to have documented advanced non–small-cell lung cancer, melanoma, renal-cell cancer, ovarian cancer, colorectal cancer, pancreatic cancer, gastric cancer, or breast cancer and have had tumor progression after at least one previous course of tumor-appropriate therapy for advanced or metastatic disease (except for those with pancreatic or gastric cancer, who were not required to have received previous treatment). Other inclusion criteria included an age of at least 18 years; a life expectancy of at least 12 weeks; an Eastern Cooperative Oncology Group performance status of 2 or less (in which 0 is asymptomatic, 1 is restricted in strenuous activity, and 2 is ambulatory but unable to work)23; measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (see Methods S3 in the Supplementary Appendix)24; and adequate hematologic, hepatic, and renal function. Patients with treated brain metastases were allowed to enroll if tumors were radiographically stable for at least 8 weeks.
Major exclusion criteria included a history of autoimmune disease or other diseases requiring systemic glucocorticoid or immunosuppressive therapy, previous therapy with T-cell modulating antibodies (including anti–PD-1, anti–PD-L1, and anti–CTLA-4), a history of human immunodeficiency virus infection, or active hepatitis B or C virus infection.
STUDY TREATMENT AND SAFETY EVALUATION
Patients were treated in 6-week cycles. Anti–PD-L1 antibody was administered as a 60-minute intravenous infusion on days 1, 15, and 29 of each cycle. Patients continued treatment for up to 16 cycles unless they had unacceptable toxic effects, disease progression, or withdrew consent. In clinically stable patients, treatment beyond initial disease progression was permitted until further progression was confirmed.
We conducted safety evaluations (clinical examination and laboratory assessments) in all treated patients at baseline and at regular intervals. We graded the severity of adverse events on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.25
DOSE ESCALATION
Patients with advanced non–small-cell lung cancer, melanoma, colorectal cancer, renal-cell cancer, and ovarian cancer were eligible to enroll in the dose-escalation phase of the trial. We used an accelerated titration design to assess safety at doses of 0.3, 1, 3, and 10 mg per kilogram of body weight. One patient was enrolled in each successive cohort until the occurrence of a treatment-related adverse event of grade 2 or more during cycle 1. Two additional patients were then enrolled at that dose level, and the study was transitioned to a standard 3+3 design, in which dose escalation proceeded when a minimum of three patients completed safety evaluation (at 42 days) at a given dose level with dose-limiting toxic effects in less than one third of patients (for details, see Methods S2 in the Supplementary Appendix). Intrapatient dose escalation or reduction was not permitted. The maximum tolerated dose was defined as the highest dose at which less than one third of patients had a dose-limiting toxic effect.
COHORT EXPANSION
To further assess the safety, side-effect profile, and clinical-activity profile of anti–PD-L1 antibody, disease-specific cohorts were enrolled. Initially, 5 expansion cohorts (with 16 patients per cohort) were enrolled in parallel and received 10 mg per kilogram for the treatment of non–small-cell lung cancer, melanoma, renal-cell cancer, ovarian cancer, and colorectal cancer. On the basis of initial signals of activity, additional expansion cohorts (with up to 16 patients per cohort) were enrolled for the treatment of melanoma (at 1 and 3 mg per kilogram), non–small-cell lung cancer (divided into cohorts with squamous or non-squamous subtypes and randomly assigned to receive 1, 3, or 10 mg per kilogram), and pancreatic, breast, and gastric cancer (all at 10 mg per kilogram).
PHARMACOKINETICS AND PHARMACODYNAMICS
For pharmacokinetic analyses, we collected serial blood samples to measure serum levels of anti–PD-L1 using an enzyme-linked immunosorbent assay. Peripheral-blood mononuclear cells were isolated from patients at baseline and after one treatment cycle to assay PD-L1 receptor occupancy by anti–PD-L1 on circulating CD3+ T cells by means of flow cytometry (see Methods S4 in the Supplementary Appendix).26
STUDY OVERSIGHT
The study was sponsored by Bristol-Myers Squibb, which provided the study drug, and was designed jointly by representatives of the sponsor and the senior academic authors, who collected, analyzed, and interpreted the study results. All the authors signed a confidentiality agreement with the sponsor. All drafts of the manuscript were prepared by the authors with editorial assistance from a professional medical writer paid by the sponsor. The authors vouch for the accuracy and completeness of the reported data and for the fidelity of the study to the trial protocol.
STATISTICAL ANALYSIS
We evaluated baseline characteristics and adverse events in all 207 patients who were receiving anti–PD-L1 antibody as of February 24, 2012. The efficacy population consisted of 160 patients in whom a response could be evaluated and who had initiated treatment by August 1, 2011. All adverse events were coded with the use of the Medical Dictionary for Regulatory Activities, version 14.1. We derived the best overall response for individual patients from radiographic measurements according to modified RECIST, version 1.0.24 Objective responses were confirmed by at least one sequential tumor assessment. (Additional details regarding statistical methods are provided in Methods S1 and the statistical analysis plan in the Supplementary Appendix.)
RESULTS
PATIENTS AND TREATMENT
From April 9, 2009, to February 24, 2012, we administered anti–PD-L1 antibody to 207 patients — 75 with non–small-cell lung cancer, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer; all the patients were included in the safety analysis. Efficacy was analyzed in 160 patients in whom a response could be evaluated and who initiated treatment by August 1, 2011.
The baseline demographic characteristics of patients in both the safety and efficacy populations were very similar (Table S1A in the Supplementary Appendix). Among treated patients, 86% had received previous chemotherapy, and 28% had received immunologic or biologic therapy. Previous therapies according to tumor type included immunotherapy (in 56%) and BRAF inhibitors (in 9%) in patients with melanoma; platinum-based chemotherapy (95%) and tyrosine kinase inhibitors (41%) in patients with non–small-cell lung cancer; and nephrectomy (94%), antiangiogenic therapy (82%), and immunotherapy (41%) in patients with renal-cell cancer (Table S1B in the Supplementary Appendix).
SAFETY
A maximum tolerated dose was not reached. The median duration of therapy was 12 weeks (range, 2 to 111 weeks) (Table S2A in the Supplementary Appendix). A relative dose intensity of at least 90% was achieved in 86% of patients. Of the 207 patients, 23 (11%) discontinued treatment because of an adverse event; of these events, 12 (6%) were considered by investigators to be related to treatment (Tables S2B and S3A in the Supplementary Appendix).
Adverse events of any grade were reported in 188 of 207 patients (91%) (Table S3A in the Supplementary Appendix). Investigator-assessed treatment-related adverse events were noted in 126 of 207 patients (61%) (Tables S3A and S3B in the Supplementary Appendix). The most common drug-related adverse events were fatigue, infusion reactions, diarrhea, arthralgia, rash, nausea, pruritus, and headache. Most events were low grade, with treatment-related grade 3 or 4 events noted in 19 of 207 (9%). The spectrum, frequency, and severity of treatment-related adverse events were similar among the dose levels, with the exception of infusion reactions.
Drug-related adverse events of special interest, with potential immune-related causes, were observed in 81 of 207 patients (39%) and included rash, hypothyroidism, hepatitis, and one case each of sarcoidosis, endophthalmitis, diabetes mellitus, and myasthenia gravis (Table 1, and Tables S3B and S4 in the Supplementary Appendix). These adverse events were predominantly grade 1 or 2 and were managed with treatment interruption or discontinuation. Nine patients were treated with glucocorticoids for the management of adverse events, with improvement or resolution of events in all patients. Furthermore, 4 of the 9 patients maintained disease control despite treatment with glucocorticoids. Endocrine adverse events (e.g., hypothyroidism and adrenal insufficiency) were managed with replacement therapy, and patients reinitiated treatment with anti–PD-L1 antibody at the discretion of the treating physician.
Table 1.
Adverse Events of Special Interest in 207 Patients Receiving Anti–PD-L1 Antibody.*
| Event | Anti–PD-L1, 0.3 mg/kg (N = 3) |
Anti–PD-L1, 1 mg/kg (N=37) |
Anti–PD-L1, 3 mg/kg (N = 42) |
Anti–PD-L1, 10 mg/kg (N = 125) |
Anti–PD-L1, Total (N = 207) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | |
| number of patients (percent) | ||||||||||
| Any adverse event of special interest† | 1 (33) | 0 | 18 (49) | 2 (5) | 14 (33) | 2 (5) | 48 (38) | 6 (5) | 81 (39) | 10 (5) |
| Skin or subcutaneous disorder | ||||||||||
| Any rash | 0 | 0 | 5 (14) | 0 | 1 (2) | 0 | 8 (6) | 0 | 14 (7) | 0 |
| Pruritus | 0 | 0 | 6 (16) | 0 | 3 (7) | 0 | 3 (2) | 0 | 12 (6) | 0 |
| Vitiligo | 0 | 0 | 3 (8) | 0 | 1 (2) | 0 | 1 (1) | 0 | 5 (2) | 0 |
| Pruritic rash | 0 | 0 | 1 (3) | 0 | 1 (2) | 0 | 2 (2) | 0 | 4 (2) | 0 |
| Macular rash | 0 | 0 | 2 (5) | 0 | 1 (2) | 0 | 0 | 0 | 3 (1) | 0 |
| Erythema | 0 | 0 | 2 (5) | 0 | 0 | 0 | 0 | 0 | 2 (1) | 0 |
| Erythematous rash | 0 | 0 | 0 | 0 | 1 (2) | 0 | 1 (1) | 0 | 2 (1) | 0 |
| Gastrointestinal disorder | ||||||||||
| Diarrhea | 1 (33) | 0 | 4 (11) | 0 | 6 (14) | 0 | 8 (6) | 0 | 19 (9) | 0 |
| Procedural complication | ||||||||||
| Infusion-related reaction | 0 | 0 | 0 | 0 | 2 (5) | 0 | 19 (15) | 1 (1) | 21 (10) | 1 (<1) |
| Endocrine disorder | ||||||||||
| Hypothyroidism | 0 | 0 | 0 | 0 | 1 (2) | 0 | 5 (4) | 0 | 6 (3) | 0 |
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 1 (2) | 1 (2) | 1 (1) | 0 | 2 (1) | 1 (<1) |
| Autoimmune thyroiditis | 0 | 0 | 2 (5) | 0 | 0 | 0 | 0 | 0 | 2 (1) | 0 |
| Eye disorder | ||||||||||
| Dry eye | 0 | 0 | 0 | 0 | 2 (5) | 0 | 0 | 0 | 2 (1) | 0 |
| Immune-system disorder | ||||||||||
| Hypersensitivity | 0 | 0 | 1 (3) | 0 | 0 | 0 | 2 (2) | 0 | 3 (1) | 0 |
| Laboratory investigation | ||||||||||
| Increased alanine aminotransferase | 0 | 0 | 1 (3) | 0 | 0 | 0 | 1 (1) | 0 | 2 (1) | 0 |
Listed events were reported in at least 1% of the patients. The following events that were categorized as adverse events of special interest occurred in one patient each: sarcoidosis, diabetes mellitus, and myasthenia gravis (in patients receiving the 10-mg dose) and endophthalmitis (in patients receiving the 3-mg dose).
The numbers reported within a column may not add up to the total number reported because patients who had more than one adverse event were counted for each event but were counted only once for “any adverse events of special interest.”
Infusion reactions were observed in 21 of 207 patients (10%), predominantly at the dose of 10 mg per kilogram. All such reactions were grade 1 or 2 with the exception of one grade 3 event in a patient receiving 10 mg per kilogram. Infusion reactions were generally rapidly reversible with antihistamines, antipyretics, and (in some cases) glucocorticoids. A prophylactic regimen of antihistamines and antipyretics was implemented during the study. Patients with grade 1 or 2 infusion reactions were able to continue treatment with the antibody with the use of prophylactic antihistamines and antipyretics and at a reduced infusion rate.
Serious adverse events that investigators considered to be related to treatment occurred in 11 of 207 patients (5%) (for a full list of events, see Table S4 in the Supplementary Appendix). At the time of data cutoff, 45 patients (22%) had died. The most common cause of death was disease progression (Table S5 in the Supplementary Appendix).
CLINICAL ACTIVITY
The efficacy population included 160 patients with non–small-cell lung cancer, melanoma, colorectal cancer, renal-cell cancer, ovarian cancer, or pancreatic cancer but not gastric cancer or breast cancer (Table S1A in the Supplementary Appendix). Clinical activity was observed at all doses of 1 mg per kilogram or higher. Objective responses (confirmed complete or partial responses) were observed in patients with melanoma, non–small-cell lung cancer, renal-cell cancer, and ovarian cancer (Table 2, Fig. 1 and 2, and Fig. S1 in the Supplementary Appendix), and many objective responses were durable. Four additional patients had a persistent reduction in target lesions in the presence of new lesions (consistent with an “immune-related” pattern of response27). However, for the purpose of calculating response rates, these patients were not categorized as having had a response. Antitumor responses or prolonged stable disease were observed in patients who had undergone a variety of previous therapies. Objective responses were observed even in patients with an extensive burden of metastatic disease. To date, there have been no objective responses in patients with colorectal or pancreatic cancer.
Table 2.
Clinical Activity of Anti–PD-L1 Antibody in the Efficacy Population.*
| Tumor Type and Dose | No. of Patients |
Objective Response† |
Duration of Response‡ |
Stable Disease ≥24 Weeks |
Rate of Progression- free Survival at 24 Weeks§ |
||
|---|---|---|---|---|---|---|---|
|
no. of patients |
% (95% CI) | mo |
no. of patients |
% (95% CI) | % (95% CI) | ||
| Melanoma | |||||||
| 0.3 mg/kg | 1 | 0 | 0 (0–98) | NA | 0 | 0 (0–98) | NA |
| 1 mg/kg | 18 | 1 | 6 (0–27) | 6.9 | 6 | 33 (13–59) | 39 (16–61) |
| 3 mg/kg | 17 | 5¶ | 29 (10–56) | 23.5+, 22.9+, 16.2+, 4.1+, 3.5 | 3 | 18 (4–43) | 47 (21–72) |
| 10 mg/kg | 16 | 3‖ | 19 (4–46) | 20.8+, 16.6, 2.8 | 5 | 31 (11–59) | 44 (19–68) |
| All doses | 52 | 9 | 17 (8–30) | 14 | 27 (16–41) | 42 (28–56) | |
| Non–small-cell lung cancer | |||||||
| All patients, 1 mg/kg | 11 | 0 | 0 (0–29) | NA | 0 | 0 (0–29) | NA |
| All patients, 3 mg/kg | 13 | 1 | 8 (0–36) | 2.3+ | 1 | 8 (0–36) | 34 (7–60) |
| Squamous subtype | 4 | 0 | 0 (0–60) | NA | 1 | 25 (0–81) | 50 (1–99) |
| Nonsquamous subtype | 9 | 1 | 11 (0–48) | ND | 0 | 0 (0–34) | 25 (0–55) |
| All patients, 10 mg/kg | 25 | 4 | 16 (5–36) | 16.6+, 12.6+, 9.8, 3.5 | 5 | 20 (7–41) | 46 (25–67) |
| Squamous subtype | 8 | 1 | 13 (0–53) | ND | 2 | 25 (3–65) | 47 (10–83) |
| Nonsquamous subtype | 17 | 3 | 18 (4–43) | ND | 3 | 18 (4–43) | 46 (20–72) |
| All patients, all doses | 49 | 5 | 10 (3–22) | 6 | 12 (5–25) | 31 (17–45) | |
| Squamous subtype | 13 | 1 | 8 (0–36) | ND | 3 | 23 (5–54) | 43 (15–71) |
| Nonsquamous subtype | 36 | 4 | 11 (3–26) | ND | 3 | 8 (2–23) | 26 (10–42) |
| Ovarian cancer | 1 | 0 | 0 (0–98) | NA | 0 | 0 (0–98) | NA |
| 3 mg/kg | 1 | 0 | 0 (0–98) | NA | 0 | 0 (0–98) | NA |
| 10 mg/kg | 16 | 1 | 6 (0–30) | 1.3+ | 3 | 19 (4–46) | 25 (4–46) |
| All doses | 17 | 1 | 6 (0–29) | 3 | 18 (4–43) | 22 (2–43) | |
| Renal-cell cancer, 10 mg/kg | 17 | 2 | 12 (2–36) | 17, 4 | 7 | 41 (18–67) | 53 (29–77) |
The efficacy population included 160 patients in whom a response could be evaluated and who initiated treatment by August 1, 2011. These patients had measurable disease at a baseline tumor assessment and at least one of the following: an assessment of tumor burden during the study, clinical progression, or death. NA denotes not applicable, and ND not determined.
Objective response rates (including both complete response and partial response) are based on confirmed responses only, with 95% confidence intervals calculated with the use of the Clopper–Pearson method.
The duration of response is the time from the first response to the time of documented disease progression, death, censoring of data (denoted by a plus sign), or last tumor assessment.
The rate of progression-free survival was the proportion of patients who did not have disease progression and were alive at 24 weeks, as calculated by the Kaplan–Meier method. The Greenwood method was used to calculate confidence intervals.
Two of these patients had a complete response.
One of these patients had a complete response.
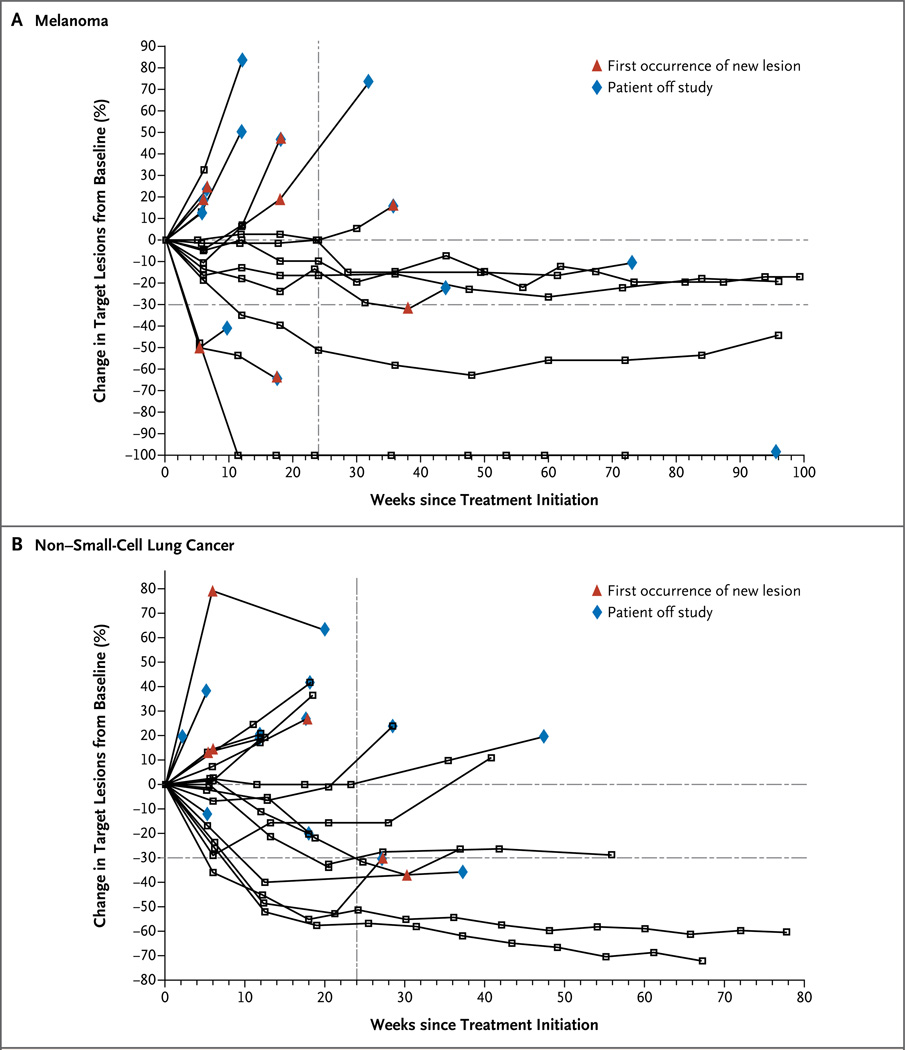
Figure 1. Activity of Anti–PD-L1 Antibody in Patients with Advanced Melanoma and Non–Small-Cell Lung Cancer.
Shown is the tumor burden (assessed as the longest linear dimension) over time in patients with melanoma (Panel A) and non–small-cell lung cancer (Panel B) who received 10 mg of anti–PD-L1 antibody per kilogram of body weight. In most patients who had an objective response, responses were durable and were evident by the end of cycle 2 (12 weeks) of treatment, regardless of the drug dose or tumor type. The vertical dashed line marks the 24-week time point at which the rate of progression-free survival was calculated. Tumor regression followed both conventional and immune-related patterns of response, such as a prolonged reduction in the tumor burden in the presence of new lesions.
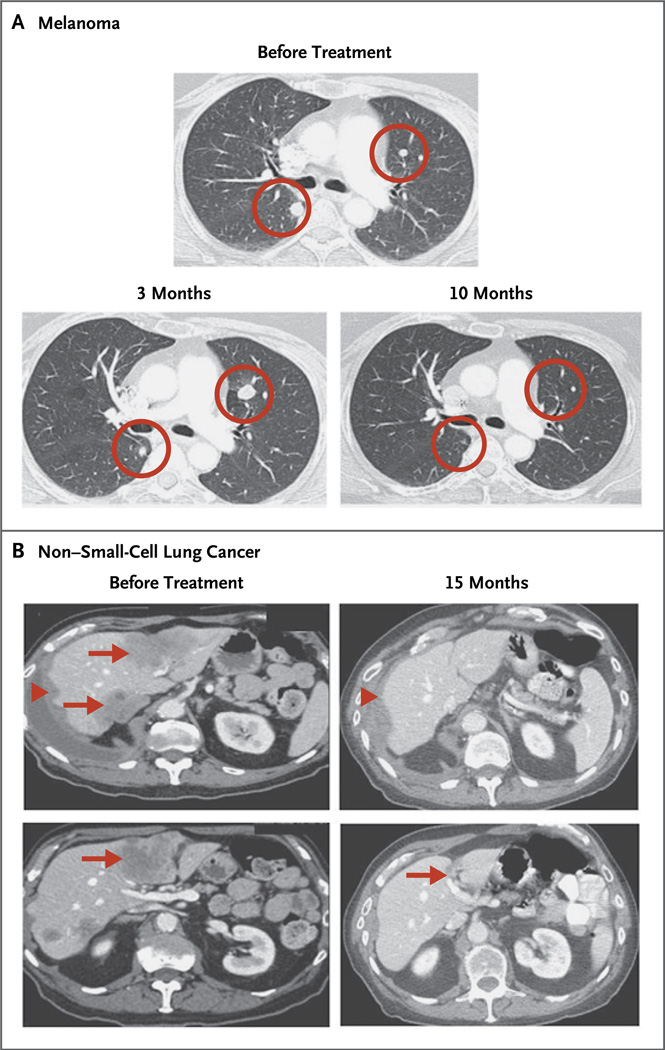
Figure 2. Computed Tomography after Receipt of Anti–PD-L1 Antibody.
Panel A shows a complete response in a patient with melanoma who received 3 mg of anti–PD-L1 antibody per kilogram. Circles indicate an initial increase in the size of pulmonary nodules at 6 weeks and 3 months, followed by complete regression at 10 months (i.e., an immune-related pattern of response). Panel B shows a partial response at 15 months in the liver (arrows) and right lung pleura (arrowheads) in a patient with non–small-cell lung cancer (nonsquamous subtype) who received 10 mg of anti–PD-L1 antibody per kilogram.
In patients with melanoma, there were nine objective responses among 52 patients receiving 1-mg, 3-mg, and 10-mg per kilogram doses, with response rates of 6%, 29%, and 19%, respectively (Table 2). Three patients with melanoma achieved a complete response. All 9 patients who had a response started treatment at least 1 year before data analysis, and of these patients, 5 had an objective response lasting at least 1 year. In addition, 14 of 52 patients with melanoma (27%) had stable disease lasting at least 24 weeks.
In patients with non–small-cell lung cancer, there were five objective responses (in four patients with the nonsquamous subtype and one with the squamous subtype) at doses of 3 mg and 10 mg per kilogram, with response rates of 8% and 16%, respectively. All five patients with an objective response started treatment at least 24 weeks before data analysis, and of these, three had responses lasting at least 24 weeks. Six additional patients with non–small-cell lung cancer had stable disease lasting at least 24 weeks.
In patients with ovarian cancer, 1 of 17 (6%) had a partial response, and 3 (18%) had stable disease lasting at least 24 weeks, all at the 10-mg dose.
In patients with renal-cell cancer, 2 of 17 (12%) had an objective response, all at the 10-mg per kilogram dose, with responses lasting 4 and 17 months. Seven additional patients with renal-cell cancer (41%) had stable disease lasting at least 24 weeks.
PHARMACOKINETICS AND PHARMACODYNAMICS
Serum levels of anti–PD-L1 antibody increased in a dose-dependent manner from 1 to 10 mg per kilogram in 131 patients who were evaluated. The geometric mean area under the curve (0 to 14 days) for doses of 1 mg, 3 mg, and 10 mg per kilogram were 2210, 7750, and 36,620 µg per milliliter per hour, respectively (coefficient of variation, 34 to 59%). After the first dose, geometric mean peak levels at these dose levels were 27, 83, and 272 µg per milliliter, respectively (coefficient of variation, 30 to 34%). The half-life of anti–PD-L1 antibody was estimated from population pharmacokinetics as approximately 15 days. PD-L1 receptor occupancy on CD3+ peripheral-blood mononuclear cells was assessed in 29 patients with melanoma at the end of one cycle of treatment, at doses of 1 to 10 mg per kilogram. Median receptor occupancy exceeded 65% for all groups (Fig. S2 in the Supplementary Appendix).
DISCUSSION
In this study, blockade of the immune inhibitory ligand PD-L1 by a monoclonal antibody produced both durable tumor regression (objective response rate, 6 to 17%) and prolonged (≥24 weeks) disease stabilization in patients with metastatic non–small-cell lung cancer, melanoma, renal-cell cancer, and ovarian cancer, including some who had been treated with extensive previous therapy. Grade 3 or 4 adverse events that were considered to be drug-related occurred in 9% of patients at doses up to and including 10 mg per kilogram. These findings are consistent with the mild autoimmune phenotype seen in mice that are homozygous for PD-L1 deletion.28
Although ipilimumab and anti–PD-L1 antibody have not been compared head to head, the toxic effects associated with ipilimumab are reported as more common and of higher grade, consistent with the more severe hyperproliferation seen in CTLA-4 knockout mice, as compared with PD-1 knockout mice.6,7,10 Most of the toxic effects that were associated with anti–PD-L1 were immune-related. The spectrum and frequency of adverse events are somewhat different between anti–PD-L1 and anti–CTLA-4, emphasizing the distinct biologic features of the two pathways.29 Infusion reactions were observed with anti–PD-L1 antibody, although they were mild in most patients. Severe colitis, an adverse event that is considered to be drug-related in some patients receiving ipilimumab, was infrequently noted in patients receiving anti–PD-L1.30
The objective response in 5 of 49 patients (10%) with advanced non–small-cell lung cancer who received anti–PD-L1 was quite unexpected. Although melanoma and renal-cell cancer are responsive to cancer immunotherapy (e.g., interleukin-2 and anti–CTLA-4), non–small-cell lung cancer has been considered to be nonimmunogenic and poorly responsive to immune-based therapies.31 Another important feature of anti–PD-L1 therapy was the durability of response across multiple tumor types. This was particularly notable given the advanced stage of disease and previous treatments of patients in our study. This durability appeared to be greater than that observed with most chemotherapies and kinase inhibitors used in these diseases, although no direct comparisons have been performed.
Because peripheral-blood T cells express PD-L1, it is possible to assess in vivo receptor occupancy by anti–PD-L1 antibody as a pharmacodynamic measure. Median receptor occupancy was more than 65% for the doses tested. Although these studies provide a direct assessment and evidence of target engagement in patients receiving anti–PD-L1 antibody, relationships between receptor occupancy in peripheral blood and the tumor microenvironment remain poorly understood.
A major implication of the clinical activity of immune checkpoint blockade is that significant endogenous immune responses to tumor antigens are generated, and these responses may be harnessed therapeutically to mediate clinical tumor regression on checkpoint inhibition. An emerging concept in cancer immunology is that inhibitory ligands such as PD-L1 are induced in response to immune attack, a mechanism termed adaptive resistance.22,32 This potential mechanism of immune resistance by tumors suggests that therapy directed at blocking interaction between PD-1 and PD-L1 might synergize with other treatments that enhance endogenous antitumor immunity.3,4,33 Longer follow-up will confirm whether patients continue to have tumor control after cessation of blockade of the pathway between PD-1 and PD-L1. Such tumor control could reflect a persistent antitumor immune response and the generation of effective immunologic memory to enable sustained control of tumor growth.
The concurrent clinical testing of antibodies that block an immune-regulatory receptor and one of its cognate ligands has not been reported. Our study and a companion study by Topalian et al.,34 now reported in the Journal, show remarkable similarities between the patterns of clinical activity observed with anti–PD-L1 and anti–PD-1 antibodies, which validate the role of this pathway in tumor immune resistance and support the notion that it may be a target for therapeutic intervention. However, the molecular interactions that are potentially blocked by these two antibodies are not identical. Anti–PD-1 antibody blocks interactions between PD-1 and its ligands, PD-L1 and PD-L2, whereas anti–PD-L1 antibody blocks interactions between PD-L1 and both PD-1 and CD80; the latter interaction has also been shown to down-modulate T-cell responses in vitro and in vivo.35–39 Although the agents were not compared directly in a randomized trial, the frequency of objective responses for anti–PD-L1 antibody appears to be somewhat lower than that observed for anti–PD-1 antibody in initial trials. The significance of these findings remains to be defined. The clinically appropriate dose of anti–PD-L1 will require further definition in future testing, including additional phase 2 dose-ranging trials.
Our findings show that antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rate of 12 to 41% at 24 weeks) in patients with select advanced cancers, including non–small-cell lung cancer, a tumor that has not been considered to be responsive to immunotherapy. These findings validate the pathway between PD-1 and PD-L1 as an important target for therapeutic intervention in some patients with cancer. Additional studies are needed to identify which patients are likely to have a response, to determine an appropriate clinical dose, and to define the spectrum of tumors in which targeting of this pathway will have antitumor effects.
Supplementary Material
Acknowledgments
Supported by Bristol-Myers Squibb and by grants (5R01 CA142779, to Drs. Topalian, Chen, and Pardoll) from the National Institutes of Health, and from the Melanoma Research Alliance (to Drs. Topalian and Pardoll and Ms. Salay).
We thank the patients who participated in this study; clinical faculty and personnel, including Cherylann Carr, Robert Gray, Marina Laiko, Dung Le, Evan Lipson, Beth Onners, Alice Pons, William Sharfman, and Lei Zheng of Johns Hopkins University School of Medicine and the Sidney Kimmel Comprehensive Cancer Center, and Alex Adjei and Grace Dy from the Roswell Park Cancer Institute; Mubing Li, the lead statistical programmer, and Michael Tagen (both of Bristol-Myers Squibb) for developing the population pharmacokinetic model of PD-L1; and Rebecca Turner of StemScientific for providing editorial support.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM. Blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 8.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [Erratum, N Engl J Med 2010;363:1290.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 13.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [Erratum, Nat Med 2002;8:1039.] [DOI] [PubMed] [Google Scholar]
- 17.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 19.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 20.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 22.Taube JM, Anders RA, Young JD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003689. 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Cancer Therapy Evaluation Program (CTEP) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. Bethesda, MD: National Cancer Institute; 2003. Apr, ( http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). [Google Scholar]
- 26.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of in-trahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 29.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 30.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt GE, Podack ER, Raez LE. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy. 2011;8:43–54. doi: 10.2217/thy.10.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 33.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–1105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Riella LV, Chock S, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187:1113–1119. doi: 10.4049/jimmunol.1100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butte MJ, Peña-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.