Abstract
We behaviorally determined the audiograms of three Common vampire bats (Phyllostomidae, Desmodus rotundus), a species specialized to exist exclusively on blood. The bats were trained to respond to pure tones in a conditioned suppression/avoidance procedure for a blood reward and a mild punisher for failures to detect the tones. Common vampire bats have a hearing range from 716 Hz to 113 kHz at a level of 60 dB. Their best hearing is at 20 kHz where they are slightly more sensitive than other bats, and they have a second peak of good sensitivity at 71 kHz. They have unusually good sensitivity to low frequencies compared to other bats, but are less sensitive to low frequencies than most mammals. Selective pressures affecting high-frequency hearing in bats and mammals in general are discussed.
Keywords: Audiogram, chiroptera, mammals, evolution of hearing, low-frequency hearing
1. Introduction
Despite a common inner ear design and organization of the central auditory system, mammals vary widely in their hearing abilities. Even within a single specialized order, such as bats, there are differences in hearing range and absolute sensitivity, passive sound localization acuity, and echolocation (e.g., Heffner et al., 2001b; Jones and Teeling, 2006; Neuweiler, 2003). The presence of variation among evolutionarily closely related species allows us to explore differences in hearing that may be affected by lifestyle and enabled by differences in anatomy and physiology. The family, Phyllostomidae, offers such an opportunity. It is a large family of New-World leaf-nosed bats with more than 150 species that range from the southwestern United States through Central and South America to Argentina (Greenhall et al., 1983). We have previously reported on the hearing abilities of three species in this family (Short-tailed fruit bat, Jamaican fruit bat, and Greater spear-nosed bat), ranging in size from 18–110 g, and having different specializations for foraging —nectar, fruit, or insects and other mammals (Heffner et al., 2003; Koay et al., 2002, 2003). These different diets entail different demands on echolocation, from hovering and echolocating in leaf clutter for fruit or nectar, to finding prey in open flight, to gleaning prey from a substrate (Kalko and Condon, 1998); these in turn may exert different pressures on hearing that should become apparent in a more extensive sample of bats.
Perhaps the most unusual lifestyle in Phyllostomidae is that of vampire bats, of which the Common vampire, Desmodus rotundus, is the most studied. There is little that is not remarkable about vampire bats (e.g., Greenhall and Schmidt, 1988). Their prey are more than four orders of magnitude larger than themselves. Their digestive system is highly derived enabling them to feed off the blood of large mammals such as capybara, peccaries, tapir, and cattle; hence they are an important pest of livestock and, occasionally, of humans. Unlike other bats, vampire bats spend considerable time on the ground and often approach prey from below, climbing up a leg to bite near the foot, or onto the back to bite other highly vascular areas. They are extraordinarily agile on the ground and can take flight from a standing leap to escape the defensive reactions of their large prey—they are the chiropteran equivalent of harrier jets (Greenhall and Schmidt, 1988). Vampire bats are also unusual in that they have infrared heat detectors in three pits surrounding their noseleaf that help them detect blood vessels near the surface (Kurten and Schmidt, 1982). Finally, it has been reported that they use passive hearing to locate prey and have the capacity to identify specific individuals by their breathing sounds (Gröger and Wiegrebe, 2006; Schmidt 1988; Schmidt et al., 1991).
Common vampire bats may also have atypical hearing in comparison to other bats. Physiological responses from the cochlea and midbrain of vampire bats and the appearance of their brainstem auditory nuclei suggest that they may have significantly better low-frequency hearing than other bats (Kuwabara and Bhatnagar, 1999; Schmidt et al., 1991; Vernon and Peterson, 1966). However, the only behavioral test of hearing in this species used narrow band noises and, while demonstrating that vampire bats have good sensitivity in the midrange of their audiogram, did not determine the entire frequency range of their hearing (Gröger and Wiegrebe, 2006). Thus far no echolocating bat has been found to hear significantly below 1.7 kHz, even though the ability to hear frequencies lower than that is nearly universal among non-chiropteran mammals, among which low frequency hearing limits range from 17 Hz to 3.6 kHz (e.g., Heffner et al., 2006; Heffner et al., 2001a). Therefore it seemed especially worthwhile to determine whether a small echolocating bat could indeed hear below 1 kHz. Accordingly, we here report the audiogram of the Common vampire bat Desmodus rotundus, and compare its hearing to that of other mammals.
2. Methods
The bats were tested with a conditioned suppression/avoidance procedure in which a bat continuously licked a spout to receive a steady trickle of blood. It was then trained to break contact with the spout whenever it detected a tone to avoid impending shock (Heffner and Heffner, 1995). Thresholds were determined by successively reducing the intensity of the tone until the bats could no longer detect it above chance.
2.1. Subjects
Three D. rotundus (A, B, and C; all males; 27–31 g), approximately 2.5–3.5 years old, were used in this study. While being tested, the bats were individually housed in wood and plastic mesh cages (48 × 39 × 95 cm) and allowed to fly daily in the test chamber. They had free access to water and received their food, which consists entirely of defribinated blood (see Barnard, 2011) during the test sessions. Supplements of defribinated blood were given as needed to maintain a healthy body weight. Blood was collected from local cattle at slaughter and each gallon was mixed with 11g sodium citrate, 4 g citric acid, and 12.5 g dextrose. The blood was strained and frozen in approximately 100 ml quantities for up to 3 months; once thawed, it was refrigerated for up to 2 days before being used or discarded. The use of animals in this study was approved by the University of Toledo Animal Care and Use Committee.
2.2. Behavioral Apparatus
Testing was conducted in a carpeted, double-walled acoustic chamber (IAC model 1204; 2.55 × 2.75 × 2.05 m), with the walls and ceiling lined with egg-crate foam. The equipment for stimulus generation and behavioral contingencies was located outside the chamber and the bats were observed via closed-circuit television.
The bats were tested in a cage (28 × 21 × 24 cm) constructed of 1.5-cm wire mesh, and raised 92 cm above the floor on a tripod (for a drawing of the test cage, see Koay et al., 2002). A reward spout (3 mm-diameter brass tube, topped with a brass bowl, 8-mm diameter × 5-mm deep, attached at a 45° angle) projected vertically through the floor at the front of the cage 7 cm above the floor. The spout was attached with silicone tubing to a 10-cc plastic syringe that contained the blood reward. The blood was dispensed using a syringe pump (Yale Apparatus YA-12). To eliminate the noise generated when the pump was activated, it was housed in a high-density particleboard box (40 × 30 × 30 cm) lined with egg-crate foam and placed on the floor behind the cage.
During testing, a bat would climb onto a small platform (15 × 8 × 6 cm) and approach the reward spout to feed. The tip of the reward spout was positioned 1 cm higher and 1 cm in front of the platform, thus minimizing obstructions between the animal’s ears and the loudspeaker while it was eating from the spout. The platform was covered with a piece of dampened carpet to provide good traction and facilitate electrical contact with the spout. A contact circuit, connected between the spout and platform, was used to detect when an animal contacted the spout and to activate the syringe pump. Requiring the bat to maintain mouth contact with the spout served to keep its head in a fixed position within the sound field.
A shock generator (Coulbourn AC-Resistive Small Animal Shocker) was also connected between the reward spout and platform. The shock was adjusted for each individual to the lowest level that produced a consistent avoidance response of backing away slightly or lifting its head from the spout. Shock levels ranged from .05 mA to .15 mA. A 25-W light, mounted .5 m below the cage, was turned on and off with the shock to provide feedback for a successful avoidance and to indicate when it was safe to return to the spout.
2.3. Acoustical apparatus
Pure tones were generated using a signal generator (Zonic A & D 3525 for frequencies of 100 kHz and below; Agilent 33220A Waveform Generator for frequencies above 100 kHz). The tones were pulsed (Coulbourn S53-21, 400 ms on and 100 ms off for five pulses) and routed through a rise-fall gate (Coulbourn S84-04, set to 20 ms rise-decay for frequencies 4 kHz and above; 40 ms rise-decay for frequencies below 4 kHz). The signal was then bandpass filtered (Krohn-Hite 3202, 24 dB/oct rolloff starting 1/3-octave above and below the test frequency) and the intensity attenuated (Hewlett Packard 350D) as needed for threshold determination. Finally, the electrical signal was amplified (Crown D75), monitored with an oscilloscope (Tektronix TDS210), and routed to a loudspeaker in the test chamber. The loudspeaker was placed approximately 1–1.5 m in front of the cage, directly facing the bat (at a height of 1 m) when it was eating from the spout.
Various loudspeakers were used to present the tones—for frequencies of .25–2.8 kHz either a 15-in (38 cm), 12-in (30.4 cm) or a 6-in (15.2 cm) woofer (Infinity RS2000) was used, whereas for frequencies above 2.8 kHz, a ribbon tweeter (Panasonic EAS-10TH400C) or a piezoelectric tweeter (Motorola KSN1005) was used. Loudspeakers were regularly interchanged to check for the possibility that a threshold might be influenced by a specific loudspeaker. Although pure tone thresholds are usually determined at octave intervals, intermediate frequencies were also tested when the bat audiogram showed unexpected discontinuities. As a result, thresholds were obtained for all bats at the following frequencies: .25, .5, .625, .8, 1, 1.25, 1.6, 2, 2.36, 2.8, 3.35, 4, 5, 6.3, 8, 10, 12.5, 16, 20, 25, 32, 40, 50, 63, 71, 80, 90, 100, 112, and 116 kHz.
2.4. Sound level measurement
Sound level measurements were taken by placing the microphone in the position normally occupied by a bat’s head and ears while it ate from the spout, and pointing it directly at the loudspeaker. The sound pressure level (SPL re 20 μNewton/m2) for frequencies of 100 kHz and below was measured daily with a 1/4-in (.64-cm) microphone (Brüel and Kjaer 4939), preamplifier (Brüel and Kjaer 2669), and measuring amplifier (Brüel and Kjaer 2608). For measuring frequencies above 100 kHz, a 1/8-in (.32-cm) microphone (Brüel and Kjaer 4138, corrected for free-field with the protection grid on) was used in place of the 1/4-in microphone. The output of the measuring amplifier was then routed to a spectrum analyzer (Zonic A & D 3525) to monitor the speaker output for harmonics or distortion. Any measurable harmonics were at least 50 dB below the fundamental frequency and at least 20 dB below the animals’ thresholds and thus did not contribute unwanted cues. Care was also taken to produce a homogeneous sound field (within ±1 dB) in the area occupied by the animal’s head and ears when it was eating from the spout.
2.5. Behavioral procedure
A hungry bat was initially trained to climb onto the platform and drink from the reward spout. Requiring the bat to maintain contact with the spout served to orient it towards the loudspeaker while also activating the syringe pump to dispense a steady trickle of blood. A train of five 400-ms tone pulses was then presented at random intervals, followed at its offset by a mild electric shock delivered between the spout and platform. Although the shock was programmed to last 300 ms, its effective duration was much less as the bats broke contact with the spout as soon as they felt the shock. Thus, the bat learned to avoid the shock by breaking contact with the spout whenever it heard the tones and returned to the spout after the shock had been delivered (as indicated by the offset of the shock-indicator light).
The bats were tested daily during the early evening hours when they were normally active. Test sessions were divided into 2.4-s trials, separated by 1.0-s intertrial intervals. Approximately 22% of the trial periods contained a pulsing tone (warning signal), whereas no sound was presented in the remaining trial periods (safe signal). The contact circuit was used to detect whether the bat was in contact with the spout during the last 150 ms of each trial. If the bat broke contact for more than half of the 150-ms response period, a detection response was recorded. This response was classified as a hit if the trial had contained a tone (i.e., a warning signal) or as a false alarm if the trial had been silent (i.e., a safe signal). The hit and false alarm rates were then determined for each stimulus intensity, with a single intensity presented in a consecutive block of 5–10 warning trials (with approximately 20–40 associated safe trials). Finally, the hit rate was corrected for false alarms to produce a performance measure (Heffner and Heffner, 1995) according to the formula: Performance = Hit rate − (False alarm rate × Hit rate). This measure proportionately reduces the hit rate by the false alarm rate associated with each intensity (i.e., each block of trials) and varies from 0 (no hits) to 1 (100% hit rate with no false alarms).
Auditory thresholds were determined by successively reducing the intensity of the tones first in 10-dB steps then in 5-dB steps as threshold was approached (in blocks of 5–10 warning trials with the larger trial blocks near threshold) until the bat no longer responded to the warning signal above chance (i.e., the hit and false alarm rates did not differ reliably; p > .05, binomial distribution). Threshold was defined as the intensity at which the performance measure equaled 0.50, which was usually obtained by linear interpolation. Testing was considered complete for a particular frequency when the thresholds obtained in at least 3 different sessions were within 3 dB of each other and were no longer showing consistent improvement. Once an audiogram had been completed, selected frequencies were rechecked to ensure reliability.
3. Results
The initial adaptation of the bats to the apparatus, learning to drink from the spout, learning to respond to sound, and becoming reliable observers, altogether required approximately 60–70 sessions. In a typical session lasting approximately 30 min, a bat would consume up to 15 ml of blood (approximately half their body weight). However, we observed that the bats quickly become lethargic after about 12 ml and were less vigilant to warning sounds than they were earlier in the session. Thus data were recorded while a bat consumed the first 12 ml or less, which allowed enough time to present up to 60 warning trials (together with approximately 240 safe trials) and determine a threshold for a single frequency. The bats were allowed to continue feeding to satiation to maintain health, but responses at the end of the sessions were not included in determinations of threshold.
The thresholds of the three D. rotundus (Figure 1) show good agreement between individuals. Beginning with an average threshold of 72 dB SPL at 500 Hz, sensitivity improved rapidly at higher frequencies up to 20 kHz, the frequency of the animals’ best hearing, where their mean threshold was −5 dB SPL. Hearing sensitivity then steadily decreased to 20 dB SPL at 63 kHz, followed by a pronounced improvement in sensitivity to 1 dB SPL at 71 kHz, thus forming a secondary peak of sensitivity. Above this secondary peak at 71 kHz, sensitivity declined steeply to 116 kHz, the highest frequency tested, where the mean threshold was 74.5 dB SPL. At an intensity of 60 dB SPL, the hearing of D. rotundus ranges from 716 Hz to 113 kHz, a span of 7.3 octaves. Even at the much lower intensity of 30 dB SPL, the Common vampire can still hear frequencies from approximately 1.9 kHz to 102.5 kHz, a range of 5.8 octaves, indicating good sensitivity over a broad range of frequencies.
Figure 1.
Audiogram of three Common vampire bats (Desmodus rotundus). Note the close agreement between the individual bats. The arrow originating at 80 dB at 250 Hz indicates that no response could be elicited at that frequency at 80 dB from any of the bats. The gray bars indicate the dominant harmonics in their echolocation call (Greenhall, Joermann, and Schmidt, 1983).
Somewhat unusual are the two discontinuities in the low-frequency part of the audiogram. These appeared in all three individuals. Thresholds at 2.36 and 5 kHz were slightly worse than thresholds at nearby octave points of 2 and 4 kHz, leading to additional testing at 1.6, 2.8, 3.35, and 6.3 kHz to explore the extent and reliability of the discontinuities in the audiogram. Thresholds at 2.36 and 5 kHz were thoroughly rechecked in as many as ten sessions and adjacent frequencies in as many as seven sessions. These thresholds remained stable throughout the extended testing and the discontinuities appear to be valid. Finally, it is noteworthy that at 250 Hz (the lowest frequency tested) none of the bats responded above chance to the loudest intensity, 80 dB SPL.
4. Discussion
4.1 Auditory sensitivity of vampire bats
4.1.1. Previous behavioral studies
A previous behavioral study of auditory thresholds for D. rotundus was conducted by Gröger and Wiegrebe (2006) using narrow bands of noise centered from 3 to 80 kHz (bandpass filtered with a −3 dB attenuation at ±10% of the center frequency). The behavioral task consisted of walking to the source of a sound in a 3-choice apparatus, and a statistical definition of threshold was applied. (A statistical definition of threshold applied to the psychophysical functions in this report would have resulted in slightly lower thresholds but would not have changed the pattern of the audiogram.) As shown in Figure 2, although it is limited in extent, the noise band audiogram is consistent with the pure-tone audiogram, the main differences being for noise bands below 5 kHz and above 35 kHz. The noise audiogram did not show the discontinuities in the audiometric curve at 4 kHz and 71 kHz, indicating that although a narrowband noise audiogram may give reasonable estimates of detectability, a pure-tone audiogram is necessary to reveal fine variations in sensitivity common among bats.
Figure 2.
Mean pure-tone audiogram for three D. rotundus with narrow-band noise thresholds (Gröger and Wiegrebe, 2006) for comparison. Note good agreement in the midrange of frequencies between about 5 kHz–35 kHz.
4.1.2. Physiological studies
It is of some interest to examine how physiological recordings reflect the hearing ability of the whole organism (Figure 3). The comparisons are necessarily tentative for several reasons. The stimuli used are not identical since, unlike behavioral responses, neural responses do not occur to tones of gradual onset. The neural thresholds were determined “audiovisually” as the lowest intensity at which a neural response could be detected with the available equipment (Schmidt et al., 1991), whereas behavioral thresholds are traditionally defined as the intensity that is detected half the time, a necessarily more conservative definition. Finally, the behavioral audiogram depicts the mean 50% detection of three individuals, whereas the Inferior Collicular line in Figure 3 depicts the most sensitive multi-unit recorded from any of nine bats. Despite these standard differences between approaches to judging sensitivity, the multi-unit thresholds obtained from the Inferior Colliculus are consistent with behavioral pure-tone thresholds in the frequency range of best hearing and higher (Schmidt et al., 1991). The most sensitive multi-unit response occurred between about 10 kHz–25 kHz, with best sensitivity near −10 dB at 15 kHz, which agrees closely with the average best hearing of three Common vampire bats of −5 dB at 20 kHz. The lowest frequency that elicited any response in the Inferior Colliculus was 700 Hz, again consistent with the behavioral hearing limit of 710 Hz at 60 dB. Despite this overall agreement, the best neural responses, as might be expected, were somewhat more sensitive than the average behavioral responses. Perhaps more important, the neural responses revealed only a hint of the discontinuity at 71 kHz. This may be attributable to the adjustment during neural recordings of the position of the speakers in azimuth and elevation to elicit the maximum neuronal response, which would have reduced the effect of the pinna’s filtering characteristics. These peaks and dips of sensitivity at high frequencies around 63–70 kHz are known to be important contributors to sound localization (Heffner et al., 2003; Wotton et al., 1995; Wotton and Jenison, 1997; Wotton and Simmons, 2000). The discontinuities in the lower frequencies were not revealed, perhaps because very few neurons responded to frequencies below 8 kHz, even though those frequencies constitute more than 3 octaves of the audiogram.
Figure 3.
Comparison of the average 50% detection thresholds for three Common vampire bats to the most sensitive multi-unit recordings in the Inferior Colliculus (Schmidt et al., 1991) and the most sensitive cochlear microphonic responses (Vernon and Petersen, 1966).
Cochlear microphonic responses (Vernon and Petersen, 1966) varied over a 15–30 dB range among the nine recordings making comparisons especially difficult; nevertheless they were not consistent with the behavioral thresholds, being far less sensitive overall and showing best sensitivity at 60 kHz. These cochlear microphonic recordings are often cited as evidence that Common vampire bats can hear sounds as low as 100 Hz (e.g., Greenhall et al., 1983), however none of the bats tested behaviorally could respond to tones as low as 250 Hz, even at a level of 80 dB SPL. As with the neural responses in the Inferior Colliculus, the cochlear microphonic responses did not reveal the peaks of sensitivity found in the behavioral audiogram. Nevertheless they did show that Common vampire bats are more sensitive to frequencies below 10 kHz compared to Little brown bats (Myotis lucifugus), suggesting that bats may vary in their low-frequency hearing and that Common vampires may be especially sensitive.
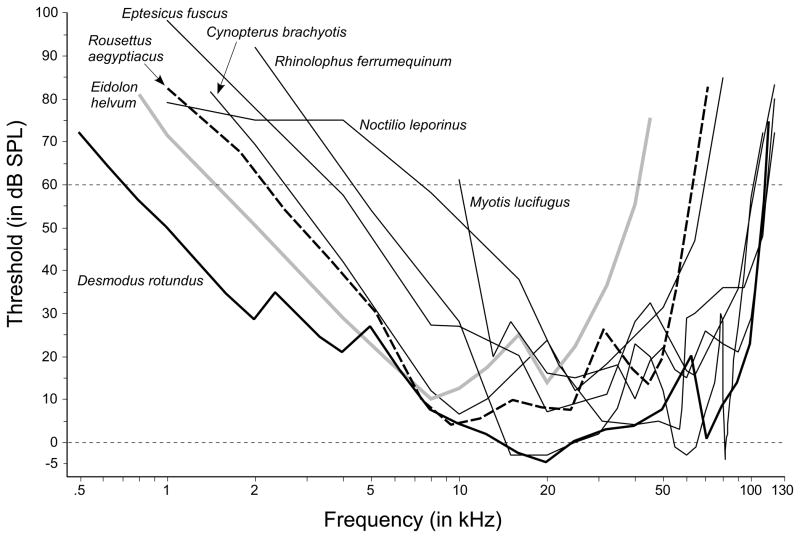
4.2. Vampire bats compared to other bats
To place vampire bats in perspective, we can compare their hearing first within their family, Phyllostomidae (Figure 4), then with that of other bats, including non-echolocators (Figure 5). Figure 4 illustrates the behavioral audiogram of D. rotundus along with behavioral audiograms of three other Phyllostomidae. There are several observations worth noting in this comparison. Vampire bats are clearly the most sensitive to low frequencies—hearing more than an octave lower than any other phyllostomid bat—with the next most sensitive species being the far larger Phyllostomus hastatus that hears only down to about 1.77 kHz at 60 dB SPL. Figure 5 allows us to compare Common vampire bats with species outside its family. It shows that Common vampire bats even have better low-frequency hearing any other bat so far tested, including the large megachiropteran bats (Yingpterochiroptera): Eidolon helvum (a non-echolocator) and Rousettus aegyptiacus (that echolocates using tongue clicks; Yovel et al., 2011). Thus the Common vampire bat is unusual among bats in its low-frequency sensitivity and it is possible that some of that sensitivity is used to detect sounds generated by its prey, such as rustling noises, vocalizations, or even breathing sounds (Gröger and Wiegrebe, 2006). Because lower frequencies attenuate less over distance than higher frequencies, extending sensitivity into the lower frequencies would be useful to a bat that is detecting and locating prey by the sounds they generate. Thus it is not surprising that several other gleaning bats are also thought to detect low frequencies. The Frog-eating bat (Trachops cirrhosus) and the Indian false vampire bat (Megaderma lyra) are both reported to hear low frequencies and to use these to detect and identify prey that includes small vertebrates. However, these reports must be considered tentative because T. cirrhosus estimates were based on unconditioned responses (Ryan et al., 1983), and the thresholds for M. lyra may have been compromised because the bats were required to localize the pure tones in order to indicate detection (Schmidt et al., 1983–1984). The Pallid bat, Antrozous pallidus, also listens for the sounds of prey and is thought to hear 3 kHz and perhaps lower frequencies (Fuzessery et al., 1993), but a complete operant audiogram is not yet available for comparative analysis.
Figure 4.
Pure-tone audiograms of four Phyllostomidae (Heffner et al., 2003; Koay et al., 2002, 2003). Note that the Common vampire bat, D. rotundus, hears much lower frequencies than the others. Because head size is a known correlate of hearing, in particular high-frequency hearing, interaural distance (in microseconds) is given for a typical individual of each species and provides an indication of their relative sizes.
Figure 5.
Audiograms of the Common vampire bat (D. rotundus) compared to behavioral audiograms for bats in other families. Note that the Common vampire hears lower frequencies than any of the other bats so far tested, even the Egyptian fruit bat, R. aegyptiacus, and the non-echolocators, E. helvum and C. brachyotis, in Yinpterochiroptera. Data from Dalland, 1965; Heffner et al., 2006; Koay et al., 1997, 1998; Long and Schnitzler, 1975; Wenstrup, 1984.
The shape of the low-frequency portion of the audiogram of D. rotundus is unusual, with its two discontinuities that appear to be reduced sensitivity at 2.36 and 5 kHz (although they could also be interpreted as peaks of sensitivity at 2 and 4 kHz). These discontinuities do not result from irregularities in a single individual or random variation among individuals, but rather appeared in each bat at the same frequencies and remained stable with repeated testing. The discontinuities occur outside the area of sensitivity commonly seen in other bats as shown in Figures 4 and 5. One interpretation is that additional low-frequency sensitivity is grafted on to a more typical audiogram, and the presence of two discontinuities suggests the possibility of two evolutionary events that incrementally expanded low-frequency hearing. This unusual configuration of sensitivity may indicate that special mechanisms have been recruited to serve the low-frequency hearing of this species and it may be worthwhile to explore their cochlear anatomy and physiology for special mechanisms to extend their hearing to lower frequencies. However, reports so far indicate that vampire bats have relatively small cochleae without unusual specializations (Habersetzer and Storch, 1992).
One possible consequence of the extended low-frequency hearing of Common vampire bats compared to other bats is that they may have added the ability to use a temporal code for pitch. It seems likely that temporal coding for pitch perception is restricted in mammals to frequencies below 5 kHz, possibly much below (Møller, 2000; Rose et al., 1967; Taberner and Liberman, 2005; cf. Heffner et al., 2001b), and most bats have little or no hearing in that range. A direct comparison of auditory nerve synchrony below 5 kHz in bats that have at least some hearing below 5 kHz could provide insight into the nature of pitch coding and its mechanisms in mammals. For example, the megachiropteran (Yinpterochiroptera), Eidolon helvum, and the microchiropteran (Yangochiroptera), P. hastatus and possibly M. lyra, hear below about 1.8 kHz and the synchrony of responses in their auditory nerve to frequencies below 5 kHz might make interesting comparisons to responses in Common vampire bats to the same frequencies.
In the higher-frequency portion of the hearing range, peaks of sensitivity occur in every species of bat tested so far (Figures 4 and 5)—indeed they are common throughout mammals (e.g., Heffner et al., 2001a, 2006; Koay et al., 2003). The dominant harmonics of the echolocation calls are usually in the frequency range of these peaks, as they are for Common vampire bats (Figure 1), and the peaks are usually attributed to the filtering characteristics of the pinnae (e.g., Koay et al., 2003; Kuc, 2009; Macias et al., 2006). Indeed, these high-frequency peaks in sensitivity can often be shifted by slight changes in the elevation of the loudspeakers relative to the pinnae (Heffner et al., 2003; Koay et al., 2003). The peak of sensitivity in the Common vampire audiogram at 71 kHz appears typical and probably reflects the role of pinna cues in sound localization and echolocation.
Finally, Common vampire bats are more sensitive than the other bats tested so far. They can detect tones of 5 dB or less over a two-octave range (between 10–40 kHz). It is not surprising that the communication calls, especially between mothers and pups, are within this frequency range (Schmidt, 1972). This excellent sensitivity is also likely to be useful in homing in on the rustling and perhaps breathing sounds of their large prey.
4.3. Comparisons with mammals
4.3.1. High-frequency hearing
Small mammals with small heads and pinnae are under selective pressure to hear high frequencies if they are to make use of interaural intensity or spectral differences for sound localization, and the correlation supporting this idea is shown in Figure 6. Common vampire bats fall among the cluster of other echolocating mammals and support the hypothesis that the advantage of hearing wavelengths short enough to be shadowed by the head and pinnae is an important factor in high-frequency hearing. Indeed, the only mammals without high-frequency hearing—those whose hearing remains restricted to the range of most birds and probably reptiles—are species that live entirely underground and do not localize sound at all, shown as triangles in Figure 6 (cf., Heffner and Heffner, 1993). Excluding these non-localizing subterranean species, the correlation between functional interaural distance and high-frequency hearing remains strong for the sample of 68 published species now available (r = −.789, p < .0001).
Figure 6.
Relation between the highest frequency audible at 60 dB and functional interaural distance, measured as the time required for a sound to travel from one ear to the other. Star indicates vampire bat reported here, filled circles represent echolocating bats and squares indicate non-echolocating and Egyptian fruit bats. Two regression lines are drawn to illustrate the influence of excluding echolocators from the relationship—echolocators influence the slope of the line but the strong correlation between high-frequency hearing and interaural distance remains. (Aj-Artibeus jamaicensis, Cb-Cynopterus brachyotis, Cp-Carollia perspicillata, Dr-Desmodus rotundus, Ef-Eptesicus fuscus, Eh-Eidolon helvum, Ml-Myotis lucifugus, Nl-Noctilio leporinus, Ph-Phyllostomus hastatus, Ra-Rousettus aegyptiacus, Rf-Rhinolophus ferrumequinum (for references, see Table 1). Some familiar species are labeled for reference. Audiograms for most of the species in this and subsequent figures are available in tabular form on the Internet (www.utoledo.edu/psychology/animalhearing/) or by contacting the authors.
The Common vampire bat, with an interaural distance of only 61 μs, falls among the other small species, including most bats, that hear very high frequencies. We have argued that any extension of mammalian hearing above about 10 kHz is probably due to selective pressure to use spectral cues for passive localization (Heffner and Heffner, 2008; Heffner et al., 2003). However, some of the extended high-frequency hearing of bats may be attributable to additional pressure for precision in echolocation. Indeed, is has become apparent that all the echolocators in Figure 6 lie above the regression line, including a porpoise, Tursiops truncatus, whose interaural distance is functionally small due to the faster speed of sound in water and to a shorter direct route through the head, from bulla to bulla, for underwater sound. To examine whether echolocators, or perhaps all bats, have enhanced high-frequency hearing beyond that expected for passive localization, we generated a second regression line based only on species that do not echolocate (and excluding non-echolocating bats). We then compared the observed high-frequency hearing limits of bats to those predicted based on the regression line for non-echolocators. Table 1 reveals that the mean increase in high-frequency hearing beyond that expected for passive sound localization, and that might therefore be attributable to echolocation, is 0.7 octaves. Notably, neither the non-echolocating bats, E. helvum and C. brachyotis, nor R. aegyptiacus that echolocates using tongue clicks, deviates from the regression line established by other non-echolocating mammals (mean deviation 0.029 octaves). Thus, our current estimate of the increase in extent of high-frequency hearing that resulted from the evolution of echolocation is about 0.7 octaves (slightly greater than an earlier estimate based on a smaller sample; Heffner et al., 2003). This is not an essential feature of all bats, but applies only to those that echolocate using laryngeal sonar calls. A likely explanation is the advantage of increased high-frequency hearing for echolocation precision.
Table 1.
Echolocators hear higher than similar sized non-echolocating bats.
| Species | Predicted12 High-frequency Hearing Limit in kHz | Observed High-frequency Hearing Limit in kHz | Excess High-frequency Hearing in Octaves |
|---|---|---|---|
| Laryngeal Echolocators | |||
| Big Brown Bat1 Eptesicus fuscus |
79.66 | 105.0 | 0.398 |
| Horseshoe Bat2 Rhinolophus ferrumequinum |
76.94 | 103.0 | 0.421 |
| Little Brown Bat3 Myotis lucifugus |
85.63 | 115.0 | 0.425 |
| Common Vampire Bat4 Desmodus rotundus |
74.09 | 112.9 | 0.608 |
| Fish-catching Bat5 Noctilio leporinus |
65.11 | 111.0 | 0.770 |
| Greater Spear-nosed bat6 Phyllostomus hastatus |
60.18 | 105.0 | 0.803 |
| Short-tailed Fruit Bat7 Carollia perspicillata |
81.47 | 150.0 | 0.881 |
| Dolphin8 Tursiops truncatus |
68.72 | 136.0 | 0.985 |
| Jamaican Fruit Bat9 Artibeus jamaicensis |
64.57 | 130.0 | 1.010 |
| Mean deviation of echolocators | 0.700 | ||
| Non-echolocators | |||
| Straw-colored Fruit Bat10 Eidolon helvum |
54.05 | 41.3 | −0.388 |
| Dog-faced Fruit Bat10 Cynopterus brachyotis |
65.38 | 70.0 | 0.098 |
| Egyptian Fruit Bat11 Rousettus aegyptiacus |
55.63 | 64.0 | 0.202 |
|
| |||
| Mean deviation of non-echolocating bats | −0.029 | ||
current report
Koay et al., 1998 (note that this Pteropodidae echolocates using paired tongue clicks, thought to be secondarily acquired)
Regression equation: log (high-frequency hearing limit) = 2.52 − (0.3642)(log interaural distance in μs)
4.3.2. Low-frequency hearing
Although D. rotundus does not hear nearly as low as the 100 Hz suggested by cochlear potentials (Vernon and Peterson, 1966), it does have unusually good low-frequency hearing compared to other bats. To gain perspective on its low-frequency hearing relative to the broader range of mammals as a whole, a quick overview of mammalian low-frequency hearing is appropriate. We have operationally defined the low-frequency hearing limit as the lowest frequency audible at an intensity of 60 dB SPL; few published audiograms test beyond this level due to the difficulty of producing very high levels necessary for testing without distortion. The low-frequency hearing limits thus defined range in mammals from 17 Hz in the elephant to 10.3 kHz in the Little brown bat (M. lucifugus; Dalland, 1965; Heffner and Heffner, 1982)—a span of 9 octaves, far exceeding the 4.7-octave range of variation of high-frequency hearing. Even excluding bats, the low-frequency hearing limits among other mammals extend from 17 Hz to 3.6 kHz (Elegant fat-tailed opossum; Frost and Masterton, 1994). However the most remarkable aspect of low-frequency hearing in mammals is that it is bimodally distributed (Heffner et al., 2001a). The distribution of low-frequency hearing limits can be seen in Figure 7. Mammals fall into two groups: Those with low-frequency hearing limits between 17–125 Hz, and a smaller group with low-frequency hearing limits between 500 Hz and 10.3 kHz. Between these two groups is a range of nearly two octaves containing only laboratory rats, the subterranean pocket gopher, and amphibious fur seal (tested in air). The implications of this unusual distribution have been discussed previously (Heffner et al., 2001a; Heffner et al., 2003) but in the intervening decade no compelling evolutionary or physiological explanation has surfaced for the bimodal distribution of low-frequency hearing.
Figure 7.
Distribution of behaviorally determined lowest frequency audible at 60 dB SPL among mammals. Bats are indicated by gray shading. All species with limited low-frequency hearing are named; examples of species with good low-frequency hearing are given for comparison. Bin widths are one-half octave.
So far, all the bats whose hearing has been tested, including the Common vampire bat reported here, fall into the group with poor low-frequency hearing. However, the Common vampire bat is clearly unusual as its 716-Hz hearing limit is at the low end of this group. It is noteworthy that nearly all the species with limited low-frequency hearing are small—the Virginia opossum (Didelphis virginiana) being the only species weighing more than 1 kg. However the lower group with extended low-frequency hearing contains not only large species, but also sixteen small species weighing less than 1 kg—with some as small as 18 g (naked mole rats). These small species represent several orders of mammals, including rodents, carnivores, and primates. This illustrates that neither simple body weight, nor even functional head size, is a good predictor of low-frequency hearing among mammals (Heffner et al., 2001a) and that a large head is not necessary for good low-frequency hearing.
The pattern of low-frequency hearing among mammals, and particularly among bats, forces us to ask why one group of mammals does not hear low frequencies (i.e., below about 500 Hz) when those frequencies are audible to every other vertebrate group, from fish to birds, as well as to the majority of mammals. Is the information provided by those frequencies not useful to some mammals? Is there some cost to hearing those low frequencies that is too great for some species? So far, no species that hears above 80 kHz can also hear below 500 Hz (although the domestic cat, with high-frequency hearing up to 79 kHz, comes very close; Heffner and Heffner, 1985). It is tempting to suggest that hearing these extraordinarily high frequencies might be incompatible with hearing low frequencies and that the advantage conferred by enhanced high-frequency hearing for echolocation was worth giving up low-frequency sensitivity. However, even this possibility is contradicted by a few small rodents (non-echolocators) that hear above 80 kHz and that forego low-frequency hearing without any of the additional advantage of extraordinary high-frequency hearing being apparent. These questions make it especially important to pursue behavioral hearing tests with echolocating bats suspected of hearing low frequencies, such as T. cirrhosus, A. pallidus, and other gleaning bats (Ryan et al., 1983; Fuzessery, 1994). The wide variation in bats, not yet fully explored, suggests bats may have many more insights to provide about both the mechanisms and evolution of mammalian hearing.
Highlights.
We trained Common vampire bats, Desmodus rotundus, to respond whenever they heard a tone
Thresholds were obtained at 30 frequencies from 250 Hz to 116 kHz
At a sound pressure level of 60 dB, D. rotundus can hear from 716 Hz to 113 kHz
D. rotundus is more sensitive to low frequencies than any other bat tested behaviorally but does not hear low frequencies as well as most mammals
Echolocating bats hear approximately .7 octave higher than predicted by selective pressure for passive sound localization
Acknowledgments
Supported by NIH R15-DC009321
We thank the Milwaukee County Zoo for the use of these bats for behavioral research. We also thank Steve Wing for his very helpful manual for the maintenance and husbandry of Common vampire bats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gimseong Koay, Email: Gim_Koay@yahoo.com.
Henry E. Heffner, Email: Henry.Heffner@utoledo.edu.
References
- Barnard S. Diet and Feeding - Environment and Housing. Vol. 3. Logos Press; 2011. Bats in Captivity. [Google Scholar]
- Dalland JI. Hearing sensitivity in bats. Science. 1965;150:1185–1186. doi: 10.1126/science.150.3700.1185. [DOI] [PubMed] [Google Scholar]
- Frost SB, Masterton RB. Hearing in primitive mammals: Monodelphis domestica and Marmosa elegans. Hear Res. 1994;76:67–72. doi: 10.1016/0378-5955(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM. Response selectivity for multiple dimensions of frequency sweeps in the pallid bat inferior colliculus. J Neuropysiol. 1994;72:1061–1079. doi: 10.1152/jn.1994.72.3.1061. [DOI] [PubMed] [Google Scholar]
- Fuzessery ZM, Buttenhoff P, Andrews B, Kennedy JM. Passive sound localization of prey by the pallid bat (Antrozous p. pallidus) J Comp Physiol A. 1993;171:767–777. doi: 10.1007/BF00213073. [DOI] [PubMed] [Google Scholar]
- Greenhall RM, Joermann G, Schmidt U. Desmodus rotundus. Mammalian Species. 1983;202:1–6. [Google Scholar]
- Greenhall AM, Schmidt U. Natural history of vampire bats. CRC Press, Inc; Boca Raton, FL: 1988. [Google Scholar]
- Gröger U, Wiegrebe L. Classification of human breathing sounds by the common vampire batDesmodus rotundus. BMC Biol. 2006;4:18. doi: 10.1186/1741-7007-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habersetzer J, Storch G. Cochlea size in extant chiroptera and middle eocene microchiropterans from Messel. Naturwis. 1992;79:462–466. [Google Scholar]
- Heffner HE, Heffner RS. Conditioned avoidance. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Birkhauser; Basel: 1995. pp. 73–87. [Google Scholar]
- Heffner HE, Heffner RS. High-frequency hearing. In: Dallos P, Oertel D, Hoy R, editors. The Senses: A Comprehensive Reference Vol. 3 Audition. Elsevier; The Netherlands: 2008. pp. 55–60. [Google Scholar]
- Heffner RS, Heffner HE. Hearing in the elephant: Absolute thresholds, frequency discrimination, and sound localization. J Comp Physiol Psychol. 1982;96:926–944. [PubMed] [Google Scholar]
- Hefner RS, Heffner HE. Hearing range of the domestic cat. Hear Res. 1985;19:85–88. doi: 10.1016/0378-5955(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. J Comp Neurol. 1993;331:418–433. doi: 10.1002/cne.903310311. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Audiograms of five species of rodents: Implications for the evolution of hearing and the perception of pitch. Hear Res. 2001a;157:138–152. doi: 10.1016/s0378-5955(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Sound localization in a new-world frugivorous bat, Artibeus jamaicensis: Acuity, use of binaural cues, and relationship to vision. J Acoust Soc Am. 2001b;109:412–421. doi: 10.1121/1.1329620. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Hearing in American leaf-nosed bats. III: Artibeus jamaicensis . Hear Res. 2003;184:113–122. doi: 10.1016/s0378-5955(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Hearing in large (Eidolon helvum) and small (Cynopterus brachyotis) non-echolocating fruit bats. Hear Res. 2006;221:17–25. doi: 10.1016/j.heares.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Johnson CS. Sound detection thresholds in marine mammals. In: Tavolga WN, editor. Marine Bio-Acoustics. Vol. 2. Pergamon Press; New York: 1967. pp. 247–260. [Google Scholar]
- Jones G, Teeling EC. The evolution of echolocation in bats. Trends Ecol Evol. 2006;21:149–156. doi: 10.1016/j.tree.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kalko EKV, Condon MA. Echolocation, olfaction and fruit display: How bats find fruit of flagellichorous cucurbits. Funct Ecol. 1998;12:364–372. [Google Scholar]
- Koay G, Heffner HE, Heffner RS. Audiogram of the big brown bat (Eptesicus fuscus) Hear Res. 1997;105:202–210. doi: 10.1016/s0378-5955(96)00208-0. [DOI] [PubMed] [Google Scholar]
- Koay G, Heffner RS, Heffner HE. Hearing in a megachiropteran fruit bat, Rousettus aegyptiacus. J Comp Psychol. 1998;112:371–382. doi: 10.1037/0735-7036.112.4.371. [DOI] [PubMed] [Google Scholar]
- Koay G, Bitter KS, Heffner HE, Heffner RS. Hearing in American leaf-nosed bats. I: Phyllostomus hastatus. Hear Res. 2002;171:96–102. doi: 10.1016/s0378-5955(02)00458-6. [DOI] [PubMed] [Google Scholar]
- Koay G, Heffner RS, Bitter KS, Heffner HE. Hearing in American leaf-nosed bats. II: Carollia perspicillata . Hear Res. 2003;178:27–34. doi: 10.1016/s0378-5955(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Kuc R. Model predicts bat pinna ridges focus high frequencies to form narrow sensitivity beams. J Acoust Soc Am. 2009;125:3454–3459. doi: 10.1121/1.3097500. [DOI] [PubMed] [Google Scholar]
- Kurten L, Schmidt U. Thermoperception in the common vampire bat (Desmodus rotundus) J Comp Physiol. 1982;146:223–228. [Google Scholar]
- Kuwabara N, Bhatnagar KP. The superior olivary complex of the vampire bat, Desmodus rotundus (Chiroptera: Phyllostomidae) Acta Chiropterologica. 1999;1:81–92. [Google Scholar]
- Ljungblad DK, Scoggins PD, Gilmartin WG. Auditory thresholds of a captive Eastern Pacific bottle-nosed dolphin, Tursiops spp. J Acoust Soc Am. 1982;72:1726–1729. doi: 10.1121/1.388666. [DOI] [PubMed] [Google Scholar]
- Long GR, Schnitzler HU. Behavioral audiograms from the bat, Rhinolophus ferrumequinum. J Comp Physiol. 1975;100:211–219. [Google Scholar]
- Macias S, Mora EC, Coro F, Kossl M. Threshold minima and maxima in the behavioral audiograms of the bats Artibeus jamaicensis and Eptesicus fuscus are not produced by cochlear mechanics. Hear Res. 2006;212:245–250. doi: 10.1016/j.heares.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Møller AR. Hearing. Academic Press; New York: 2000. [Google Scholar]
- Neuweiler G. Evolutionary aspects of bat echolocation. J Comp Physiol A. 2003;189:245–256. doi: 10.1007/s00359-003-0406-2. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophys. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Tuttle MD, Barclay RMR. Behavioral responses of the frog-eating bat, Trachops cirrhosus, to sonic frequencies. J Comp Physiol. 1983;150:413–418. [Google Scholar]
- Schmidt S, Turke B, Volger B. Behavioural audiogram from the bat, Megaderma lyra. Myotis. 1983–1984;21–22:62–66. [Google Scholar]
- Schmidt U. Social calls of juvenile vampire bats (Desmodus rotundus) and their mothers. Zool Beitr. 1972;4:310–316. [Google Scholar]
- Schmidt U. Orientation and sensory functions in Desmodus rotundus. In: Greenhall AM, Schmidt U, editors. Natural history of vampire bats. CRC Press; Boca Raton, FL: 1988. pp. 143–166. [Google Scholar]
- Schmidt U, Schlegel P, Schweizer H, Neuweiler G. Audition in vampire bats, Desmodus rotundus. J Comp Physiol A. 1991;168:45–51. [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–569. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Vernon J, Peterson E. Hearing in the vampire bats, Desmodus rotundus murinus, as shown by cochlear potentials. J Aud Res. 1966;6:181–187. [Google Scholar]
- Wenstrup JJ. Auditory sensitivity in the fish-catching bat, Noctilio leporinus. J Comp Physiol A. 1984;155:91–101. [Google Scholar]
- Wing S. Manual for the husbandry of vampire bats. 2008 Unpublished document. [Google Scholar]
- Wotton JM, Haresign T, Simmons JA. Spatially dependent acoustic cues generated by the external ear of the big brown bat, Eptesicus fuscus. J Acoust Soc Am. 1995;98:1423–1445. doi: 10.1121/1.413410. [DOI] [PubMed] [Google Scholar]
- Wotton JM, Jenison RL. The combination of echolocation emission and ear reception enhances directional spectral cues of the big brown bat, Eptesicus fuscus. J Acoust Soc Am. 1997;101:1723–1733. doi: 10.1121/1.418271. [DOI] [PubMed] [Google Scholar]
- Wotton JM, Simmons JA. Spectral cues and perception of the vertical position of targets by the big brown bat, Eptesicus fuscus. J Acoust Soc Am. 2000;107:1034–1041. doi: 10.1121/1.428283. [DOI] [PubMed] [Google Scholar]
- Yovel Y, Geva-Sagiv M, Ulanovsky N. Click-based echolocation in bats: not so primitive after all. J Comp Physiol A. 2011;197:515–530. doi: 10.1007/s00359-011-0639-4. [DOI] [PubMed] [Google Scholar]