Abstract
Background
The goal of this study was to determine whether antidonor antibodies directed against human leukocyte antigen (HLA) or endothelial cells (ECs) expressed antigens, including major histocompatibility complex class I chain-related antigens A (MICA) are associated with the diagnosis of antibody-mediated rejection (AMR) in heart transplant recipients.
Methods
We studied posttransplant antidonor HLA antibodies in 168 heart allograft recipients transplanted from October 2001 to December 2005. Among them, there were 37 AMR + patients and 131 age- and sex- matched AMR − controls. Sera were collected at the time of protocol biopsies and tested for the presence of HLA antibodies. Seventy-two of the 168 patients were genotyped for donor and recipient MICA alleles and were tested for the presence of anti-MICA antibodies. Thirty-one patients who never developed antibodies to HLA or MICA were further tested for anti-EC antibodies.
Results and Conclusions
Of 37 AMR + patients, 22 (60%) developed donor-specific antibodies (DSA) to HLA compared with 6 of 131(4%) AMR − patients (P<0.0001). Of the remaining 15 AMR + patients, 5 had anti-HLA antibodies that were not donor specific and 10 did not show any HLA antibodies. In the subgroup of 72 patients, all 19 AMR + patients had clearly demonstrable antibodies reactive with donor HLA, MICA or with nondonor-derived ECs, with 30% of them showed antibodies directed to non-HLA antigens. The incidence of transplant coronary artery disease was significantly higher in patients who had DSA to HLA and MICA compared with patients without DSA.
Keywords: Non-HLA antibody, MICA, Endothelial cell antibody, HLA antibody, Antibody-medicated rejection, Chronic rejection
The contribution of antibodies to acute and chronic cardiac allograft rejection has been increasingly recognized (1, 2) antibody-mediated rejection (AMR) in heart transplant recipients is often associated with higher mortality and development of accelerated transplant coronary artery disease (TCAD) that is the major complication after cardiac transplantation (3). Human leukocyte antigen (HLA) antigens are the major targets of allograft rejection. However, not all heart transplant recipients diagnosed with AMR develop antidonor HLA antibodies, suggesting that the humoral immune response to antigens expressed by the graft is not limited to anti-HLA antibodies. Among the candidates for important non-HLA target antigens are the major histocompatibility complex class I chain-related antigens A (MICA). These antigens are expressed on fibroblasts, epithelial cells, monocytes and endothelial cells (ECs), but are not normally expressed on lymphocytes; hence, donor-specific antibodies (DSA) to MICA are not detected by pretransplant crossmatch tests using lymphocytes as targets.
Alloantibodies against MICA have been associated with acute and chronic vascular rejection in solid organ transplants (4 –9) and can be cytotoxic to ECs in the presence of complement (10). However, most studies reporting a role for MICA in transplantation have been indirect and circumstantial because organ donors are not routinely typed for MICA and the donor specificity of the antibodies was not ascertained.
In addition to MICA antibodies, non-HLA antibodies to EC antigens have been reported to have a deleterious effect in solid organ transplantation. ECs lining the blood vessels of the organ are the most proximal targets of the host’s immune system during allograft rejection (11–15). Interestingly, many of the antiendothelial antibodies that have been identified are autoantibodies, including antibodies directed against vimentin, myosin, and angiotensin II type 1 receptor (16). It is speculated that EC damage caused by ischemia reperfusion injury or rejection may cause release of EC-specific antigens and result in production of antibodies to those antigens.
The aim of this retrospective study was to evaluate the role of posttransplant DSA to HLA, MICA and anti-EC antibodies on development of AMR in heart transplantation and to examine the impact of posttransplant DSA on the development of TCAD.
RESULTS
Patient Characteristics and Graft Outcome
The study comprised 168 heart allograft recipients transplanted during the period from October 2001 to December 2005 who consented to participate in this institutional review board approved research study. The characteristics of the patient population are summarized in Table 1. Of the 168 patients studied, 33 were diagnosed with AMR and 4 were diagnosed with both AMR and acute cellular rejection. The control group comprised 131 heart transplant recipients and included four patients diagnosed with acute cellular rejection. Multivariate analysis showed no association between diagnosis of AMR and recipient race, number of transplants and HLA mismatch. Four patients with pretransplant DSA experienced AMR compared with 1 of 131 DSA patients (P=0.008). This indicates that presensitization to donor HLA antigens is a risk factor for AMR. Patients with and without AMR had a similar 24-month median follow-up time. Among 154 patients who had a follow-up time over 6 months, 11 of 31 (33%) patients with AMR developed TCAD compared with 21 of 123 (18%) patients without AMR. A significantly higher incidence of TCAD was found in patients who developed AMR. The TCAD incident rate was 1.5 cases per month per 100 cases in patients with AMR compared with 0.7 cases per month per 100 cases in patients without AMR (P=0.05).
TABLE 1.
Study population demographics
| Non-AMR (n=131) | AMR (n=37) | P | |
|---|---|---|---|
| Age (yr), mean±SD | 55±13 | 52±14 | 0.26 |
| Gender (males/females) | 98/33 | 22/15 | 0.1 |
| Race (AA/non-AA) | 7/101 | 3/28 | 0.5 |
| Retransplants (primary/regrafts) | 117/14 | 32/5 | 0.17 |
| HLA ABDR MM (mean±SD) | 4.7±0.9 | 4.4±1.1 | 0.14 |
| Pre-TX DSA | |||
| Positive | 1 | 4 | |
| Negative | 130 | 33 | 0.008 |
| Median follow-up time (mo) | 24.2 | 24.1 | |
| TCAD incident rate (cases/mo/100 TX) | 0.7 | 1.5 | 0.05 |
AA, African American; HLA, human leukocyte antigen; MM, mismatch; TCAD, transplant coronary artery disease; DSA, donor-specific antibodies.
Twenty-two of the 37 AMR+ patients had posttransplant DSA to HLA antigens, compared with only 5 of 131 patients without AMR (P<0.0001, Table 2). Of the 15 AMR+ recipients without DSA, 5 had anti-HLA antibodies that were not donor specific and the remaining 10 did not show any HLA antibodies. In the AMR− group, 5 of 131 recipients displayed anti-HLA antibodies to antigens not present in the donor. Anti-HLA antibodies that were not directed against donor HLA had no significant association with AMR (P=0.3). Among the 22 AMR+ patients with posttransplant DSA to HLA, 12 had antibodies to HLA class I antigens only, 7 to class II antigens only, and 3 to both class I and class II antigens (Table 2).
TABLE 2.
Association between posttransplant HLA antibodies, MICA antibodies, anti-EC antibodies, and AMR
| Antibodies | AMR+ | AMR− | P |
|---|---|---|---|
| HLA DSA (n=168) | 22/37 | 6/131 | 0.0001 |
| Class I | 12 | 3 | |
| Class II | 7 | 3 | |
| Class I + II | 3 | 0 | |
| MICA DSA (n=72) | 5/19 | 1/53 | 0.01 |
| HLA+MICA (n=72) | 2/19 | 0/53 | |
| Anti-EC antibody (n=31′) | 3/3 | 7/28 | 0.01 |
HLA, human leukocyte antigen; AMR, antibody-mediated rejection; MICA, major histocompatibility complex class I chain-related antigens A; EC, endothelial cells; DSA, donor-specific antibodies.
To determine the relevance of DSA to MICA in AMR, we studied 72 of the 168 recipients who had both donor and recipient DNA available to perform MICA genotyping to establish the presence or absence of MICA DSA. Among these 72 recipients, 5 of 19 (25%) AMR+ patients had DSA to MICA compared with 1 of 53 (2%) patients without AMR (P<0.01, Tables 2 and 3). Two patients with AMR and 10 patients without AMR developed antibodies against MICA antigens not present in the donor. Antibodies to MICA antigens that were not donor specific were not associated with AMR (P=0.99). Interestingly, seven patients developed auto-antibodies against their own MICA antigens. One of these, seven patients developed AMR.
TABLE 3.
Association between posttransplant MICA antibodies and AMR
| Total | MICA antibody | AMR (n=19) | Non-AMR (n=53) | P |
|---|---|---|---|---|
| N=72 | MICA DSA | 5 | 1 | 0.01 |
| Non-DSA | 2 | 10 | 0.99 | |
| Negative | 12 | 42 |
AMR, antibody-mediated rejection; MICA, major histocompatibility complex class I chain-related antigens A; DSA, donor-specific antibodies.
Within the subgroup of 72 recipients tested for both HLA and MICA antibodies, 3 of 19 AMR+ patients did not have antibodies to HLA or MICA. To determine whether antibodies to EC antigens other than MICA or HLA play a role in AMR, sera from the 3 AMR+ patients and 28 AMR− patients were tested for antiendothelial IgG antibodies by flow cytometry. All 3 AMR+ patients and 7 of 28 (25%) patients without AMR had positive EC crossmatches (P=0.01, Table 2). These data suggest that anti-EC antibodies may also play a role in AMR. The 10 EC crossmatch positive sera were further tested for antivimentin antibodies, however, none were positive. Table 4 shows the combined relationship between posttransplant HLA, MICA, and EC antibodies and diagnosis of AMR in the subgroup of 72 recipients. In the AMR+ group, 11 had DSA to HLA, 2 had DSA to both HLA and MICA, 3 had DSA to MICA alone, and 3 patients had anti-EC antibodies. All 19 AMR + patients had demonstrable antibodies reactive with donor HLA, MICA, or with nondonor-derived ECs.
TABLE 4.
Incidence of HLA, MICA, and anti-EC antibodies in cardiac transplant recipients with and without AMR
| Categories | HLA DSA | MICA DSA | Both HLA/MICA | EC XM positive | Both DSA– EC– | Total |
|---|---|---|---|---|---|---|
| AMR+ | 11 | 3 | 2 | 3 | 0 | 19 |
| AMR− | 3 | 2 | 0 | 7 | 41 | 53 |
AMR, antibody-mediated rejection; HLA, human leukocyte antigen; MICA, major histocompatibility complex class I chain-related antigens A; DSA, donor-specific antibodies; EC, endothelial cells.
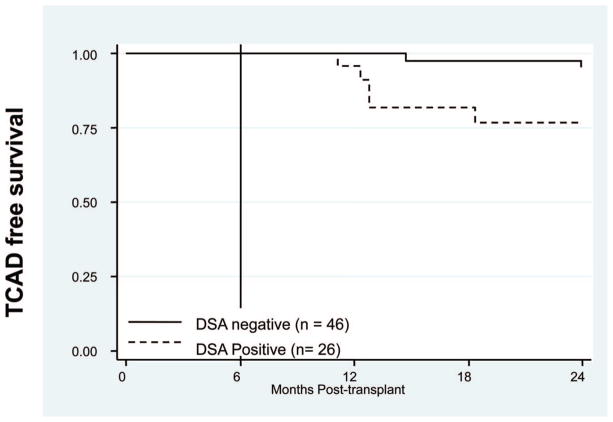
To study the long-term effect of DSA on graft survival, HLA and MICA DSA were correlated with TCAD in the 72 patients tested for HLA and MICA DSA. There was no significant difference in 1-year survival comparing patients with and without DSA; however, the 26 patients who had DSA had a 77% TCAD-free survival rate at 2 years posttransplant, compared with 96% for 46 patients without DSA. The incidence of TCAD was significantly higher among patients who had DSA to HLA or MICA (P<0.02, Fig. 1). Although patients producing HLA DSA alone had a higher incidence of TCAD, it did not reach statistical significance.
FIGURE 1.
Posttransplant human leukocyte antigen (HLA) and major histocompatibility complex class I chain-related antigens A (MICA) donor-specific antibodies (DSA) increases the probability of transplant coronary artery disease (TCAD). TCAD-free survival at 2 years posttransplant was significantly lower in the 26 patients with anti-donor HLA and MICA antibodies compared with 46 patients without DSA (P<0.02).
DISCUSSION
Although HLA antigens are the major targets of the alloimmune response causing graft rejection, we were able to detect DSA against donor HLA antigens in only 22 of 37 (59%) heart transplant recipients in this study who had AMR diagnosed by endomyocardial biopsy. These results suggest that antibodies against non-HLA antigens may also contribute to the development of AMR. The results of this study substantiate this conclusion and demonstrate that in addition to DSA to HLA, development of anti-EC antibodies including DSA to MICA, are associated with diagnosis of AMR in cardiac transplantation.
There is circumstantial evidence for an association between antibodies against MICA and graft dysfunction. MICA antibodies have been reported in renal and heart transplant patients whose grafts later failed, although donor specificity was not determined (4, 6). Zou et al. (10) demonstrated that MICA antibodies are capable of fixing complement and killing of Hela cells in vitro suggesting these antibodies can cause complement-mediated damage of the donor endothelium. In the subset of 72 patients where we could determine MICA genotypes for the donor and recipient, we showed a significant correlation between development of DSA to donor MICA antigens and AMR. In some cases, donor-specific MICA antibodies developed in the absence of anti-HLA antibodies suggesting that anti-MICA antibodies alone may induce rejection. However, we found no correlation between MICA antibodies that were not directed against donor MICA antigens and AMR. In addition, we found that approximately 10% of patients displayed autoantibodies to MICA and these antibodies were not associated with AMR. These findings underscore the importance of performing the MICA genotyping of the donor-recipient pair to assess the risk of AMR in recipients with antibodies to MICA. Smith et al. (17) have also reported that a small number of patients (about 8%) produce de novo anti-MICA antibodies after cardiac transplantation, some of which are donor specific. This group did not find an association between MICA antibodies and acute rejection or cardiac allograft vasculopathy, but AMR was not reported.
Among the six AMR+ patients without DSA to donor HLA or MICA antigens, five displayed antibodies reactive with EC, suggesting that EC antigens other than MICA can elicit humoral immune responses that may cause AMR. Non-HLA antibodies directed against vimentin (18), antiangiotensin II type 1 receptor (16, 19) have been reported to occur with AMR and to have a deleterious effect in solid organ transplants. We found no antivimentin antibodies among the 12 EC crossmatch-positive sera tested. Therefore, it will be important to continue to pursue the identity of the antibodies causing the positive EC crossmatch in patients with and without AMR. Protein microarrays have recently demonstrated potential to identify antibodies in patients with cancer that correlate with the state and progression of disease (20). Furthermore, protein arrays have been used to identify non-HLA antibodies directed against novel targets expressed on distinct compartments of the kidney in recipient of renal allografts (21).
Even in the short, 2-year, posttransplant follow-up, our results also showed a significant increase in TCAD among patients who developed DSA to HLA or MICA antigens, suggesting that antibodies directed against donor MICA antigens also play a role in chronic rejection. Antibody crosslinking of HLA antigens on the ECs and smooth muscle cells stimulates cell survival and proliferation which may lead to intimal thickening of the vessels of the allograft (22). Anti-HLA class I antibodies control EC survival and proliferation by transducing signals via the PI3 kinase/Akt signaling pathway (23) and inducing downstream activation of mammalian target of rapamycin complex 1 and 2 (24). Therefore, anti-HLA (and perhaps MICA) antibodies may contribute to the process of chronic allograft rejection by promoting EC and smooth muscle cell proliferation.
One limitation of our study was that pretransplant sera were not available to study the effect of MICA sensitization before transplantation and graft rejection. In addition, donor and recipient DNA samples were not available on all patients that accordingly resulted in a smaller study population for analysis. Another drawback is the ECs used in the flow cross-match were not derived from the transplant donor which may have limited our ability to detect DSA against polymorphic non-HLA antigens such as major histocompatibility complex class I chain-related antigens B. Currently, the isolation of donor ECs is labor intensive, costly and not suitable for diagnostic testing. Recently, a flow cytometry crossmatch method (XM-ONE) was developed to identify donor-specific anti-EC antibodies using donor endothelial precursor cells isolated from blood using beads carrying antibodies specific for the angiopoietin receptor, Tie-2 as target cells. In a prospective multicenter kidney transplant trial, Breimer et al. (25) reported that the presence of DSA to endothelial precursor cells was associated with acute renal allograft rejection. Similar to our findings, they showed that not all of the patients producing anti-EC antibodies are diagnosed with AMR. Thus, additional studies are required to understand the nature and clinical relevance of anti-EC antibodies.
In conclusion, we report that DSA to both HLA and MICA are increased in sera in heart transplant recipients diagnosed with AMR. Although donor HLA antigens were the most common target of AMR, donor MICA and other antigens expressed on ECs also seem to be relevant targets of the humoral immune response after cardiac transplantation. The results of this study have changed clinical practice at University of California, Los Angeles. In suspected cases of AMR, in the absence of HLA antibodies, recipient sera are tested for the presence of DSA to MICA, and for EC antibodies. This approach has aided in the diagnosis of AMR and assessment of risk for TCAD.
MATERIALS AND METHODS
Study Population
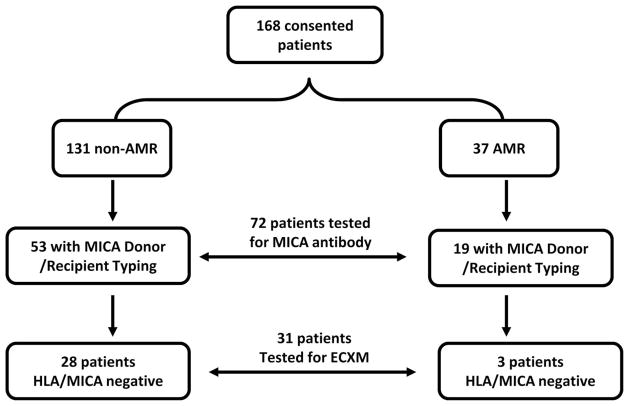
This retrospective, institutional review board-approved study comprised 168 heart allograft recipients transplanted from October 2001 to December 2005 who consented to participate in this research study. Inclusion in this study required the availability of blood samples collected at the time of protocol biopsies (1, 3, 6, and 12 months posttransplantation). The study group comprised 120 men and 48 women. Data gathered included patient demographics, donor demographics, and posttransplant outcome variables including diagnosis of rejection and TCAD. As illustrated in Figure 2, HLA antibodies were tested in all 168 recipients. MICA antibody studies were limited to 72 of the 168 recipients for whom DNA was available on both the donor and recipient for MICA genotyping. Because ECs express both HLA and MICA antigens and to avoid positive EC crossmatches caused by HLA or MICA antibodies, endothelial crossmatches were only performed on 31 of the 72 recipients who tested negative for antibodies to HLA or MICA.
FIGURE 2.
Human leukocyte antigen (HLA) antibodies were tested in all 168 institutional review board-consented recipients. Major histocompatibility complex class I chain-related antigens A (MICA) antibody studies were limited to 72 of the 168 recipients for whom DNA was available on both the donor and recipient for MICA genotyping. Anti-endothelial antibodies were performed on 31 of the 72 recipients who tested negative for antibodies to HLA or MICA study population.
All patients were started on triple-drug immunosuppression (tacrolimus, mycophenolate mofetil, and corticosteroids) after transplant. Cellular and AMR was diagnosed by endomyocardial biopsies according to the International Society for Heart and Lung Transplantation criteria (26) and as reported previously from our institution (3). Moderate or severe cellular rejection seen on endomyocardial biopsy (International Society for Heart and Lung Transplantation grade 2R or greater) and AMR with left ventricular dysfunction merit treatment. Treatment ranges from an oral prednisone bolus and taper for asymptomatic rejection to cytolytic therapy with antithymocyte globulin for hemodynamically compromising rejection. Coronary angiography was performed annually posttransplant. A diagnosis of TCAD was made when there was any new luminal stenosis more than or equal to 30% or significant distal pruning of the coronary arteries at annual angio-grams compared with the baseline angiogram.
HLA and MICA Typing
HLA typing was performed on all 168 donors and recipients. MICA typing was performed on a subset of 72 donor/recipient pairs for whom DNA samples were available. HLA-A, -B, -DRB1, -DQB1, and MICA donor and recipient typing were performed by LABType SSO DNA typing (One Lambda, Canoga Park, CA) according to the manufacturers’ specifications and examined with the Luminex 100 (Luminex, Austin, TX). Genotype determination and data analysis were performed using the HLA Visual software (One Lambda) according to the manufacturer’s instructions.
Evaluation of Anti-HLA Antibodies
Sera samples were analyzed for antibodies directed against HLA class I (A, B, C) and class II (DR, DQ, and DP) antigens using the Gen-Probe Luminex PRA and antibody specificity reagents (San Diego, CA). In brief, 5 μL of HLA class I or II antigen-coated Luminex beads were incubated with 20 μL of patient’s serum sample for 30 min. Unbound excess serum was removed by washing and the microparticles were stained with 100 μL phycoerythrin-conjugated goat antihuman immunoglobulin (Ig) G antibody and incubated in the dark on a rotating platform for 30 min. Particle florescence was assessed by Luminex 100 IS (Luminex, Austin, TX). Additional Single Antigen Antibody Identification Assays (One Lambda) were run on positive sera to confirm the antibody specificity assignment. Antibodies were considered positive if the median florescence intensity was more over 1000.
Evaluation of Anti-MICA Antibodies
MICA antibodies were determined using LABScreen assay by Luminex Technology, according to the manufacturer’s specifications (One Lambda, Inc.). Serum samples of heart-transplant subjects were tested against MICA alleles *001, *002, *004, *007, *009, *012, *017, *018, *019, and *027. MICA *027 and MICA*008 alleles share the same amino acidic sequence in the extracellular domains therefore antibodies to both MICA *027 and MICA*008 were referred as MICA*027 in this study. The fluorescent signal for each MICA-allele coated bead was measured using LABScan 100 Flow Cytometry and analyzed by HLA-Visual software (One Lambda, Inc.). Antibodies against different MICA alleles were considered positive if the median florescence intensity was more than 1000.
Cell Culture and Anti-EC Flow Cytometry Crossmatch
Primary human aortic ECs were isolated from the aortic rings of explanted donor hearts (27) or obtained from Cambrex. The cells were cultured in M199 medium supplemented with 20% (vol/vol) fetal bovine serum, penicillin-streptomycin (100 U/mL and 100 μg/mL, respectively; both from Invitrogen Life Technologies), sodium pyruvate (1 mM), heparin (90 μg/mL; Sigma-Aldrich), and EC growth supplement (20 μg/mL; Fisher Scientific). ECs from passages 7–8 were frozen and used in the EC flow crossmatch. A total of 2×105 ECs were incubated with 100 μL patient serum on ice for 30 min. ECs were washed three times and incubated with 50 μL of 1 of 400 diluted fluorescein isothiocyanate Affinipure F(ab′)2 fragment Goat Anti-Human IgG Fcγ fragment (Jackson ImmunoResearch Laboratories) for 30 min on ice, washed three times, and cell fluorescence was analyzed on a FACSCalibur flow cytometry using CellQuest Software (BD Biosciences). Gates for forward and side scatter measurements were set on EC, and a minimum of 10,000 events was acquired. The positive EC crossmatch threshold was set at two standard deviations (50 Median Channel Shift) above the mean of negative control serum tests. Antivimentin antibodies were assessed in the laboratory of Dr. Marlene Rose according to their previously described methods (28).
Statistical Analysis
Contingency tables were analyzed for differences in proportions among categorical variables between recipients with and without rejection using Fisher’s exact test. Differences in mean values of continuous variables (expressed as mean±standard deviation) were analyzed using Wilcoxon’s rank-sum test. Statistical significance was defined as a P value less than 0.05, allowing for multiple comparisons of main variables using Bonferroni’s procedure. All P values were two sided, and all estimates were performed using the STATA statistical software package (StataCorp, 2003, Stata Statistical Software, College Station, TX). Actuarial graft survival and freedom from TCAD was estimated using Kaplan-Meier analysis and statistical differences calculated with the log-rank statistic.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases Grant RO1 AI 42819 and NIH U01AI077821 and the National Heart Lung and Blood Institute Grant RO1 HL 090995 (E.F.R.).
Footnotes
There authors declare no conflict of interest.
Q.Z. participated in research design, performance of the research, and writing of the manuscript; E.F.R. participated in research design and writing of the manuscript; D.W.G. contributed analytic tools; J.M.C., J.K.P., J.K., and A.A. participated in writing of the manuscript; P.G. and M.L.R. participated in performance of the research; and M.C.F. participated in performance of the research and writing of the manuscript.
References
- 1.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 2.Uber WE, Self SE, Van Bakel AB, et al. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7:2064. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 3.Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: Risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 4.Mizutani K, Terasaki P, Rosen A, et al. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5:2265. doi: 10.1111/j.1600-6143.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 5.Panigrahi A, Gupta N, Siddiqui JA, et al. Post transplant development of MICA and anti-HLA antibodies is associated with acute rejection episodes and renal allograft loss. Hum Immunol. 2007;68:362. doi: 10.1016/j.humimm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Suarez-Alvarez B, Lopez-Vazquez A, Gonzalez MZ, et al. The relationship of anti-MICA antibodies and MICA expression with heart allograft rejection. Am J Transplant. 2007;7:1842. doi: 10.1111/j.1600-6143.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 7.Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 8.Zwirner NW, Marcos CY, Mirbaha F, et al. Identification of MICA as a new polymorphic alloantigen recognized by antibodies in sera of organ transplant recipients. Hum Immunol. 2000;61:917. doi: 10.1016/s0198-8859(00)00162-2. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Stastny P, Susal C, et al. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y, Mirbaha F, Lazaro A, et al. MICA is a target for complement-dependent cytotoxicity with mouse monoclonal antibodies and human alloantibodies. Hum Immunol. 2002;63:30. doi: 10.1016/s0198-8859(01)00349-4. [DOI] [PubMed] [Google Scholar]
- 11.Le Bas-Bernardet S, Hourmant M, Coupel S, et al. Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant. 2003;3:167. doi: 10.1034/j.1600-6143.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 12.Dunn MJ, Crisp SJ, Rose ML, et al. Anti-endothelial antibodies and coronary artery disease after cardiac transplantation. Lancet. 1992;339:1566. doi: 10.1016/0140-6736(92)91832-s. [DOI] [PubMed] [Google Scholar]
- 13.Derhaag JG, Duijvestijn AM, Damoiseaux JG, et al. Effects of antibody reactivity to major histocompatibility complex (MHC) and non-MHC alloantigens on graft endothelial cells in heart allograft rejection. Transplantation. 2000;69:1899. doi: 10.1097/00007890-200005150-00027. [DOI] [PubMed] [Google Scholar]
- 14.Deal JE, Rigden SP, Harmer AW, et al. Renal allograft failure and antibodies to epithelial cells. Lancet. 1992;339:941. doi: 10.1016/0140-6736(92)90993-d. [DOI] [PubMed] [Google Scholar]
- 15.Claas FH, Paul LC, van Es LA, et al. Antibodies against donor antigens on endothelial cells and monocytes in eluates of rejected kidney allografts. Tissue Antigens. 1980;15:19. doi: 10.1111/j.1399-0039.1980.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 16.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 17.Smith JD, Brunner VM, Jigjidsuren S, et al. Lack of effect of MICA antibodies on graft survival following heart transplantation. Am J Transplant. 2009;9:1912. doi: 10.1111/j.1600-6143.2009.02722.x. [DOI] [PubMed] [Google Scholar]
- 18.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Dechend R, Homuth V, Wallukat G, et al. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 20.Wulfkuhle JD, Speer R, Pierobon M, et al. Multiplexed cell signaling analysis of human breast cancer applications for personalized therapy. J Proteome Res. 2008;7:1508. doi: 10.1021/pr7008127. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Wadia P, Chen R, et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci USA. 2009;106:4148. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin YP, Korin Y, Zhang X, et al. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 24.Jin YP, Jindra PT, Gong KW, et al. Anti-HLA class I antibodies activate endothelial cells and promote chronic rejection. Transplantation. 2005;79(3 suppl):S19. doi: 10.1097/01.tp.0000153293.39132.44. [DOI] [PubMed] [Google Scholar]
- 25.Breimer ME, Rydberg L, Jackson AM, et al. Multicenter evaluation of a novel endothelial cell crossmatch test in kidney transplantation. Transplantation. 2009;87:549. doi: 10.1097/TP.0b013e3181949d4e. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Yeh M, Leitinger N, de Martin R, et al. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:1585. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 28.Mahesh B, Leong HS, McCormack A, et al. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]