Abstract
Global cerebral ischemia is an important clinical problem with few effective treatments. The hippocampus, which is important for memory, is especially vulnerable during global ischemia. Brain-specific knockout of hypoxia inducible factor-1α (HIF-1α) has been shown to be protective in focal ischemia in vivo. 2-methoxyestradiol (2ME2) is a natural metabolite of estrogen that is known to inhibit HIF-1α. We tested 2ME2 in a rat model of global cerebral ischemia. Global ischemia was induced with the two-vessel occlusion model (2VO) which entailed hemorrhagic hypotension to a mean arterial pressure of 38–42 mmHg with simultaneous bilateral common carotid artery occlusion for 8 minutes. Sprague–Dawley rats (male, 280–350 g) were randomly assigned to three groups: global ischemia (GI, n=17), global ischemia with 2ME2 treatment (GI + 2ME2, n=17) and sham surgery (sham, n=12). 2ME2 treatment (15 mg/kg in 1% DMSO) was rendered 10 minutes after reperfusion. Rats in the GI and sham groups received similar doses of the DMSO solvent. Rats were killed 24 hours, 72 hours and 7 days after reperfusion. Quantitative CA1 hippocampal cell counts demonstrated significantly lower cell survival in the GI + 2ME2 group compared to either the GI or sham groups, in spite of a statistically significant reduction in HIF-1α by Western blotting analysis of the GI + 2ME2 group. We conclude that 2ME2 worsens outcomes after global ischemia in rats.
Keywords: Global ischemia, hypoxia inducible factor, 2-methoxyestradiol
INTRODUCTION
Global cerebral ischemia leads to apoptotic hippocampal cell loss and anterograde amnesia1,2. At present, there are few effective treatments for global cerebral ischemia3.
Hypoxia inducible factor-1 (HIF-1) is a heterodimeric transcription factor that is known to be up-regulated following ischemia4. HIF-1 consists of two subunits, one of which (β) is constitutively expressed, and the other of which (α) is regulated by hypoxia4. The precise role that HIF-1α plays after ischemia is somewhat controversial. Previous works have demonstrated that low concentrations of HIF-1α may be important for cell survival during hypoxia, while high levels may induce apoptosis after ischemia4. A brain-specific knockout of HIF-1α has recently been shown to lead to improved outcomes after focal ischemia4.
2-methoxyestradiol (2ME2) is an endogenous metabolite of estrogen that is known to inhibit HIF-1α in a dose-dependent manner5. In the present study, we tested 2ME2 in a rat model of global cerebral ischemia. We hypothesize that 2ME2 will improve outcomes after global cerebral ischemia by inhibiting HIF-1α expression.
MATERIALS AND METHODS
Institutional Animal Care and Use Committee approval was obtained for the global ischemia protocol. We used the two-vessel occlusion (2VO) model of global cerebral ischemia in rats described by Smith et al.6 with minor modification. Male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA; 280–350 g) were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection, and were intubated and ventilated with a small animal ventilator (Kent Scientific, Torrington, CT, USA). Temperature was controlled with a heating pad and lamp. The left femoral artery and vein were cannulated with PE-50 tubing. The arterial line was used for blood pressure monitoring, and the venous line was used for controlled hemorrhage. The common carotid arteries were exposed bilaterally. Controlled hemorrhage was undertaken by withdrawal of blood into a heparinized syringe until the mean arterial pressure (MAP) reached 40 mmHg. At this point, the common carotid arteries were clamped with microvasculature clamps for 8 minutes. MAP was maintained at 40 ± 2 mmHg during ischemia. The clamps were subsequently removed, and blood was reinfused. Operative sites were closed, and the animals were allowed to recover.
2ME2 (15 mg/kg, intraperitoneal injection, Sigma Aldrich, St Louis, MO, USA) was administered 10 minutes after global ischemia to rats in the treatment group. The dose of 2ME2 agreed with previously published in vivo doses5. 2ME2 was dissolved in a solution of 1% dimethyl sulfoxide (DMSO). Rats in the other groups received equivolume injections of the DMSO solution.
Nissl histology and immunohistochemistry followed standard protocols7, as did Western blotting8. For Western blot, bilateral hippocampal samples were isolated from rats in each group (n=4) 24 hours after global ischemia. Antibodies included rabbit anti-HIF-1α (Santa Cruz Biotech, Santa Cruz, CA, USA; sc-10790), goat anti-β-actin (Santa Cruz Biotech; sc-1616) and appropriate secondary antibodies (Jackson Immuno Research, West Grove, PA, USA). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was carried out with a kit (No. 11684795910, Roche, Indianapolis, IN, USA).
Animals from day 7 were killed for quantitative cell count. For sham group, n=5, for GI group, n=6, and for GI + 2ME2 group, n=4. Rat brains were exhaustively sectioned from bregma −3.3 to −4.5 mm, and ten sections were taken randomly for further analysis. These sections were stained with the nissl protocol, and morphologically normal neurons per high power fields (HPF) in the dorsal CA-1 region were counted by blinded investigator at ×100 magnification.
Differences between groups were compared by analysis of variance (ANOVA) supported by Sigma Stat (Systat Software, Richmond, CA, USA) (Figures 1 and 2). Chi-squared analysis was used for mortality comparison.
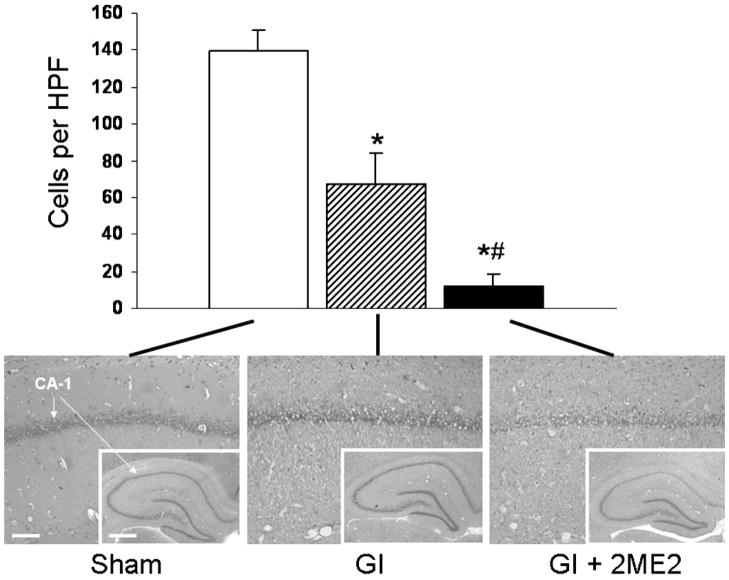
Figure 1.
Nissl histology. Quantitative nissl histology 7 days after global ischemia demonstrated a worse outcome in the GI + 2ME2 group than in the GI or sham group. This difference was statistically significant by ANOVA with Bonferroni correction, p<0.05. Scale bar=100 and 500 μm at low and high magnification, respectively
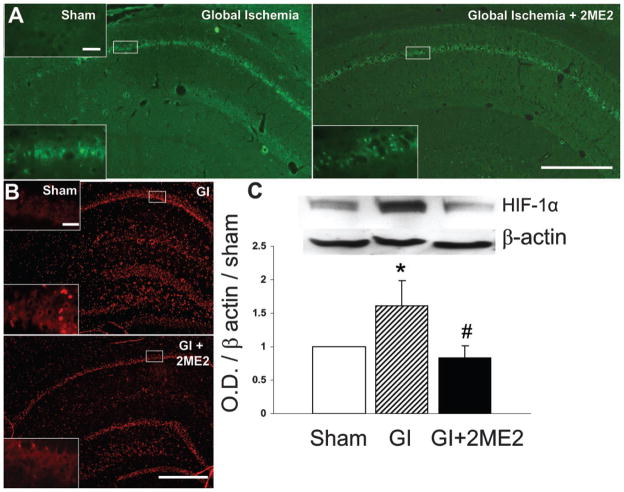
Figure 2.
Apoptosis and HIF-1α expression. (A) Apoptotic neurons (green) were noted in both the GI and GI + 2ME2 groups 72 hours after global ischemia. Scale bar=500 and 50 μm at low and high magnification, respectively. (B) Immunohistochemical stain for HIF-1α (red) demonstrated a qualitative reduction in the GI + 2ME2 treatment group 24 hours after ischemia. Scale bar=500 and 50 μm at low and high magnification, respectively. (C) Western blot of HIF-1α expression 24 hours after ischemia demonstrated a quantitative reduction in HIF-1α in the hippocampus of the GI + 2ME2 treatment group (p<0.05, ANOVA)
RESULTS
Mortality was 0% (0/12) in the sham group, 18% (3/17) in the GI group and 29% (5/17) in the GI + 2ME2 group. There was no statistically significant difference in mortality between GI and GI + 2ME2 groups by chi-squared analysis.
Nissl histology demonstrated significant cell loss in the CA-1 region of both the GI and GI + 2ME2 groups. Quantitative nissl analysis demonstrated a significantly worse CA-1 lesion in the GI + 2ME2 group than in the GI or sham group (Figure 1).
TUNEL stain demonstrated the presence of apoptotic neurons in the CA-1 region, which was qualitatively similar between the GI and GI + 2ME2 groups (Figure 2A). Immunohistochemical analysis of HIF-1α expression demonstrated less HIF-1α in the 2ME2 treatment group (Figure 2B), and this was supported quantitatively by Western blot (Figure 2C).
DISCUSSION
In this study, we found that 2ME2, a HIF-1α inhibitor, was associated with worse outcomes after global cerebral ischemia. Western blot analysis demonstrated that 2ME2 successfully lowered HIF-1α levels (Figure 2), although this was not associated with an improved outcome after global ischemia.
2ME2 exerts multiple intracellular effects, which include inhibition of HIF-1α as well as inhibition of polymerization of microtubules5. In the context of neoplastic disease, 2ME2 exerts a pro-apoptotic effect by suppression of Bcl-2 (Ref. 9), and it is possible that this effect of 2ME2 explains the worse outcome in the treated animals (Figure 1). Additionally, 2ME2 has been shown to be toxic to neurons in vitro10, with selective toxicity to dentate gyrus neurons in vivo9. It is possible that 2ME2 is also toxic to CA-1 neurons in the context of severe physiologic stress such as global ischemia.
We conclude that administration of 2ME2, an endogenous metabolite of estrogen, results in worse outcomes after global cerebral ischemia in rats.
Acknowledgments
This work was supported by NIH grants NS45694, HD43120, NS43338 and NS53407 to J. H. Zhang.
References
- 1.Allen JS, Tranel D, Bruss J, et al. Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara T, Lewen A, Noshita N, et al. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19:85–89. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- 3.Popp E, Bottiger B. Cerebral resuscitation: State of the art, experimental approaches, and clinical perspectives. Neurol Clin. 2006;24:73–87. doi: 10.1016/j.ncl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Helton R, Cui J, Scheel JR, et al. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005;25:4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricker JL, Chen Z, Yang XP, et al. 2-methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:8665–8673. doi: 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- 6.Smith ML, Bendek G, Dahlgren N, et al. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 7.Matchett G, Hahn J, Obenaus A, et al. Surgically induced brain injury in rats: The effect of erythropoietin. J Neurosci Methods. 2006;158:234–241. doi: 10.1016/j.jneumeth.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Shimamura N, Matchett G, Yatsushige, et al. Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke. 2006;37:1902–1909. doi: 10.1161/01.STR.0000226991.27540.f2. [DOI] [PubMed] [Google Scholar]
- 9.Picazo O, Azcoitia I, Garcia-Segura LM. Neuroprotective and neurotoxic effects of estrogens. Brain Res. 2004;990:20–27. doi: 10.1016/s0006-8993(03)03380-8. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa-Yagi Y, Nobou O, Yutaka I, et al. The endogenous estrogen metabolite 2-methoxyestradiol induces apoptotic neuronal death in vitro. Life Sci. 1996;58:1461–1467. doi: 10.1016/0024-3205(96)00116-6. [DOI] [PubMed] [Google Scholar]