Abstract
Long chain omega-3 fatty acids (FAs) are effective for reducing plasma triglyceride (TG) levels. At the pharmaceutical dose, 3.4 g/day, they reduce plasma TG by about 25-50% after one month of treatment, resulting primarily from the decline in hepatic very low density lipoprotein (VLDL-TG) production, and secondarily from the increase in VLDL clearance. Numerous mechanisms have been shown to contribute to the TG overproduction, but a key component is an increase in the availability of FAs in the liver. The liver derives FAs from three sources: diet (delivered via chylomicron remnants), de novo lipogenesis, and circulating non-esterified FAs (NEFAs). Of these, NEFAs contribute the largest fraction to VLDL-TG production in both normotriglyceridemic subjects and hypertriglyceridemic, insulin resistant patients. Thus reducing NEFA delivery to the liver would be a likely locus of action for fish oils (FO). The key regulator of plasma NEFA is intracellular adipocyte lipolysis via hormone sensitive lipase (HSL), which increases as insulin sensitivity worsens. FO counteracts intracellular lipolysis in adipocytes by suppressing adipose tissue inflammation. In addition, FO increases extracellular lipolysis by lipoprotein lipase (LpL) in adipose, heart and skeletal muscle and enhances hepatic and skeletal muscle β-oxidation which contributes to reduced FA delivery to the liver. FO could activate transcription factors which control metabolic pathways in a tissue specific manner regulating nutrient traffic and reducing plasma TG.
Keywords: fish oil, omega-3, plasma triglycerides, lipolysis, NEFA, eicosapentaenoic acid, docosahexaenoic acid
Understanding how fish oil (FO) reduces plasma triglycerides (TGs) is important not only because elevated TG is a cardiovascular risk factor, but also because it informs our view of basic lipid biology and can help in the development of new or improved pharmacologic approaches to treating hypertriglyceridemia. This review updates progress in understanding the FO mechanism of action with an emphasis on kinetic modeling and new information that has appeared regarding VLDL production. It focuses on how FO and its active ingredients, eicosapentaenoic acid (C20:5n3, EPA) and docosahexaenoic acid (C22:6n3, DHA) contribute to improved fatty acid (FA) trafficking; it addresses intricacies of the TG-lowering effect of FO such as how reduced hepatic production of TG-rich very low density lipoprotein (VLDL) occurs without causing steatosis.
1. FO effects on plasma TG
Omega-3 FAs have long been known to lower plasma TG [1-3] along with variety of other drugs such as fibrates, statins, thiazolidinediones, niacin, and metformin. Although the TG-lowering effects of FO are not evident at intakes typical of Western diets (about 130 mg/day) [4] they manifest at “pharmacologic” doses (i.e., >3 g/day of EPA+DHA) [5-13]. This is similar to another dietary component, niacin (vitamin B3), that is also lipid-lowering at supra-nutritional intakes [14]. The pharmaceutical preparation of omega-3 acid FAs (Lovaza, GlaxoSmithKine) provides EPA and DHA as acid ethyl esters, and the approved dose is 4, 1-g capsules per day which provides 1,860 mg of EPA and 1,500 mg of DHA for a total of 3.4 g omega-3 FAs/day. The TG-lowering effect of EPA+DHA has been demonstrated in numerous trials and 3-4 g/day of omega-3 FAs decrease plasma TG by about 30% (range 16-45%) [15]. The effect is dose dependent [16, 17] with the minimal effective dose being > 2 g/day [7]. EPA and DHA appear to be equally potent in lowering plasma TG [5] and are effective in multiple settings including type II diabetes (T2D) [6], metabolic syndrome [8], non-alcoholic fatty liver disease (NAFLD) [9], HIV-dyslipidemida [10], nephrotic syndrome [11] and hemodialysis [12, 13] populations.
EPA and DHA belong to a unique group of nutri-pharmaceutical agents that are already present in tissues prior to use. In fact, the endogenous level of EPA and DHA (e.g., red blood cells EPA+DHA, or the omega-3 index [18]) is itself a biomarker for cardiovascular disease risk [19]. No studies to date have examined whether the magnitude of TG-reduction is a function of the baseline omega-3 index. If the response to therapy indeed depends on the baseline omega-3 index, it would suggest that omega-3 intervention studies should stratify for baseline omega-3 levels, or better, subjects should be titrated to an optimal omega-3 index instead of using a single dosing regimen.
In addition to their TG lowering effects, FO also have strong evidence for risk reduction for a number of CVD-related endpoints including primary prevention of major coronary events [20], secondary prevention of death or non-fatal MI [21], and all-cause mortality in heart failure subjects [22]. The role of TG lowering in risk reduction is not clear, however. In the Japan EPA-lipid intervention study [20], statin-treated subjects with high TG (>150 mg/dL) and low HDL cholesterol (<40 mg/dL) were at 71% greater risk for major coronary events compared to patients without this pattern. Monotherapy with EPA resulted in a 5% reduction in plasma TG but a 53% reduction in risk for major coronary events in EPA group compared to control group meaning that the reduction in risk occurred independent of a reduction in TGs [23]. While the link between FO-induced risk reduction vs. TG reduction is not clearly established, there remains a strong epidemiological link between elevated TGs and coronary artery disease [24, 25] and although not yet unambiguously demonstrated in a randomized trial, it seems likely that the TG-lowering has cardiovascular benefit.
2. The underlying causes of hypertriglyceridemia and the effects of FO on lipoprotein metabolism
TG are transported through the body by all classes of lipoprotein particles, with VLDL and chylomicrons being the primary TG-bearing lipoproteins. Concentrations of plasma lipoproteins are established by the balance between their rate of appearance in plasma, or production, and by their rate of removal from the plasma, or clearance. Thus, elevations in plasma TG could represent either an increase in VLDL-TG production that overcomes peripheral clearance or from a decrease in clearance without a compensatory decrease in production. The former has been demonstrated in genetic dyslipidemias such as familial hypercholesterolemia [26, 27], familial combined hyperlipidemia [28, 29], but also in more common contexts such as obesity [30] and insulin resistance [31-34] with overproduction being confined primarily to VLDL1 (the largest VLDL particles). Among subjects undergoing hemodialysis, elevated TG is also caused by overproduction of VLDL apoB [35], however in the presence of albuminuria, i.e. the nephrotic syndrome, high TGs result from reduced clearance [36]. Thus, while increased production is the most common cause of hypertriglyceridemia, reduced clearance is sometimes involved. Therefore, tracer kinetic studies of lipoprotein metabolism in humans are important to understanding the effects of FO on plasma TG concentrations.
The studies to date are summarized in Table 1. Collectively the results of tracer kinetic studies indicate that omega-3 FAs reduce VLDL-TG production and likely increase TG clearance.
Table 1.
Human tracer kinetic studies of the effects of FO treatment on lipoprotein synthesis and clearance rates
| Study | N | Tracer | omega-3 (g/d) | Duration (weeks) | Baseline plasma TG (mg/dL) a | % change in fasting plasma TG | Kinetic Parameter (% change) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Production rate | p-value | FCR | p-value | |||||||
| Change-from-baseline studies | ||||||||||
| Nestel37 | 6 | 125I apoB | 19-29 b | 2-3.5 | 277+/-131 b | -59% b | -65% b | 0.002 b,c | 26% b | 0.09 b,c |
| 5 | 3H-glycerol | 19-23 b | 2-3.5 | 314+/-153 b | -68% b | -68% b | 0.005 b,c | 78% b | 0.50 b,c | |
| Sanders38 | 5 | 3H-glycerol | 4.6 d | 4 | 1136+/-532 | -43% b | -39% b | 0.03 b,c | 6% b | 0.36 b,c |
| Harris39 | 10 | 3H-glycerol | 10-17 | 3-5 | 442+/-99 | -66% | -45% | <0.005 | 65% | <0.005 |
| Bordin40 | 10 | 13C-leucine | 3 | 4 | 97+/-11 | -22% | -29% | 0.012 | 14% | 0.09 |
| Fisher41 | 5 | 3H-leucine | ~9 | 1 | 591+/-188 | -66% | -43% | 0.01 | 5% e | n.s. |
| Randomized, controlled trials | ||||||||||
| Park42 | 33 | 3H-triolein | 4 f | 4 | 82+/-5 b | -9% | n.a. | n.a. | ~30% g | <0.05 |
| Chan43 | 48 | 2H-leucine | 3.4 | 6 | 177+/-30 | -28% h | -22% h | 0.002 | -4% h | n.s. |
| conversion from VLDL to IDL: | 55% h | 0.002 | ||||||||
| Chan 44 | Cholesteryl 24i | 13C-oleate | 3.4 | 6 | 177+/-30 | -25% | -32% | <0.05 | -4% | n.s. |
| conversion from VLDL to IDL: | 49% | <0.05 | ||||||||
Mean+/-SEM;
re-calculated from original tables;
paired ANOVA on lg-transformed data;
EPA+DHA;
EPA or DHA ethyl esters;
estimated from slope;
adjusted effect size applied to FO group;
substudy of reference 43
Abbreviations: FCR - fractional catabolic rate; IDL - intermediate density lipoprotein; VLDL - very low density lipoprotein; n.a. - not applicable; n.s. - not significant; TG - triglyceride
2.1 Change from baseline studies
Tracer studies have consistently demonstrated that FO reduces VLDL production. Early studies employed large FO doses, included normotriglyceridemic with hypertriglyceridemic (HTG) subjects, and employed bolus injections of radiolabeled tracers. While they captured a large range of subject types, randomized controlled designs were not employed. Nestel et al treated five normal and two HTG subjects, with FO at high dose of ~30% of daily energy needs and found a 65-68% reduction in VLDL production rate measured by either the plasma kinetics of 125I-ApoB or 3H-glycerol tracers [37]. The estimated increase in fractional catabolic rate (FCR; the fraction of the pool cleared per unit of time) of 78% was not significant and was attributed to the smaller apoB pool. When measuring FCR, complications can occur such as here, where even a large effect size is not significant indicating difficult detection and large variance. Further, a decreased pool size due to reduced production can itself increase FCR, making FCR increases a secondary consequence of reduced production. In such cases, reductions in production rate lower plasma TG concentrations below levels which saturate clearance and so FCR is also increased while absolute clearance is unchanged. Sanders et al traced glycerol-labeled TG in 5 severely HTG males (mean baseline TG=1136 mg/dL) given 4.6 g EPA+DHA/day and showed a decrease in TG production rate but no change in FCR [38]. Harris et al used the same approach in 10 HTG subjects (mean baseline TG=442 mg/dL) receiving >10 g omega-3 FA/day (which lowered plasma TG by 66%) found a 45% decrease in TG-production rate along with a 65% increase in FCR [39]. Bordin et al traced apoB in 10 normotriglyceridemic subjects given 3 g omega-3 FA/day and found apoB production was decreased by 29% [40]. They also observed a 14% increase in FCR, however concluded (with a p=0.09) it was not increased. Finally, in a study tracing apoB production in 5 subjects with T2D and mean TG levels of 591 mg/dL using approximately 9 g omega-3 FA/day [41], reductions of 66% in plasma TG were observed. This study using 5 subjects, employed a complex model of apolipoprotein transfer to conclude that apoB synthesis was greatly reduced by FO, primarily by a shifting the initial appearance of apoB in plasma from the VLDL to the IDL and LDL fractions.
2.2 Randomized controlled trials
More recently, two larger randomized controlled trials using doses at or near the approved pharmaceutical dose have improved the parameter estimates of FO effects on VLDL and chylomicron TG kinetics. In the first trial, Park et al [42] measured the effects of 4 g/day EPA or DHA on postprandial TG in 33 normotriglyceridemic males tracing 3H-triolein administered as a lipid emulsion. This study demonstrated a 15% reduction in chylomicron TG half-life which was associated with a 30% increase in circulating (i.e., not heparin released) lipoprotein lipase (LpL) activity. In another randomized, placebo controlled trial, Chan et al measured the effect of 3.4 g/day of EPA+DHA and atorvastatin (40 mg/day) in a 2×2 factorial trial in 48 obese, insulin resistant males with mean plasma TG of 177 mg/dL. Using an apoB tracer (2H-leucine) they demonstrated a 22% decrease in VLDL-apoB synthesis [43] and no change in the FCR of VLDL apoB. They did, however, report a 1.5-fold increase in the fraction of VLDL converted to IDL (from 0.29 to 0.43) as opposed to direct clearance from the plasma. Since this study traced apoB, not TG directly, transfer from VLDL to intermediate density lipoprotein (IDL) corresponds to improved rates of lipolytic delipidation of the apoB-containing lipoprotein particle. A subset of subjects in the Chen et al study, specifically the placebo and FO groups, also received cholestryl 13C-oleate tracer of VLDL [44], and like the apoB tracer, this sub-study showed a significant reduction in VLDL production rate with no change in FCR and greater fractional transfer to IDL.
In summary, regardless of the cause of hypertriglyceridemia, the number of subjects, the tracer used, or the methodology used to model the effects, reduced hepatic VLDL-TG production is consistently demonstrated as a cause for the reduction of plasma TGs by FO. In studies using a dose of 3-4 g EPA/DHA per day, the size of the FO-induced reduction in synthesis is about -30%, which is large enough to explain most of the TG-lowering effect in these subjects with mild HTG. This represents an important action of FO since most hypertriglyceridemias are largely caused by increased hepatic secretion of VLDL-TG. Secondarily, FO may also induce an increase in clearance which would contribute to the FO effect.
3. Effects of FO on VLDL production
As described above, a reduced rate of FA incorporation into VLDL is a major effect of FO. In this regard, recent advances in our understanding of how hepatocytes obtain FAs for VLDL production are useful in identifying those effects which are physiologically relevant and where FO may be acting in a tissue specific manner. Hepatic VLDL production coordinates apolipoprotein synthesis with lipid synthesis in a multistep process. The FAs used in hepatic TG synthesis can be derived from at least three sources: 1) the diet (i.e. chylomicron/remnant); 2) de novo lipogenesis; 3) circulating non-esterified FAs (NEFAs).
FAs derived from de novo lipogenesis and/or diet are first stored in hepatocyte lipid stores (as TGs) whereas NEFAs can be directly incorporated into VLDL-TG [45]. Vedala et al used stable isotopes to measure the relative contributions of these three FA sources to overall VLDL-TG in lean normolipidemic, non-diabetic obese, and diabetic obese subjects [46]. They demonstrated that NEFA are the major source of FAs for VLDL-TG regardless of metabolic state and the contribution of each FA source to the VLDL-TG pool differs in patients with type-2 diabetes versus simple obesity. This study offers an opportunity to evaluate the maximal contribution that each source could, in theory, make to the FO effect of reducing TG production. Other studies confirm the primary contribution of NEFA to hepatic TG production [47], emphasizing that changes in NEFA flux to the liver would be necessary to reduce production of VLDL-TG. In table 2, we estimate the TG levels that would result if the contribution of each component to VLDL-TGs were eliminated using the parameters given by Vedala et al (independent of changes in clearance mechanisms). Notably, completely eliminating the contributions of de novo lipogenesis or diet would reduce TGs by no more than 13% even among diabetics with HTG. Therefore, FO interference with these two pathways could not realistically contribute to reduced synthesis in the short term, regardless of changes in the biology or regulation of enzymes, and the best explanation for why FOs reduce VLDL-TG production is via an effect on NEFA delivery to the liver.
Table 2.
Effect of eliminating FA sources on plasma TG
| TG precursor | control | HTG | HTG + T2D | |||
|---|---|---|---|---|---|---|
| TG (mg/dL) | % reduction | TG (mg/dL) | % reduction | TG (mg/dL) | % reduction | |
| All sources | 62 | - | 179 | - | 191 | - |
| Eliminate: | ||||||
| NEFA | 3 | -95% | 32 | -82% | 43 | -78% |
| de novo lipogenesis | 60 | -3% | 153 | -15% | 171 | -10% |
| Diet | 60 | -3% | 173 | -3% | 167 | -13% |
Vedala et al[46] studied the relative contribution of various FA sources to VLDL-TG. They report the plasma TG and the absolute contribution of each FA source to VLDL-TG synthesis, which was measured during tracer kinetics study and reported in g/day. We used the reported parameters to estimate the reduction in serum TG that would correspond to the elimination of each FA source, assuming no change in TG clearance.
4. FA trafficking
FAs are transported throughout the body in esterified and non-esterified forms. FA trafficking (the production, transport and delivery of FAs throughout the body) is a regulated process that distributes FA between different tissues compartments. VLDL-TG and NEFA are each important components of FA trafficking in vivo (illustrated in Fig. 1). VLDL are essentially targeted, one-way transporters of FAs to tissues which consume large amounts of FAs by expressing LpL –for energy storage in the case of adipocytes, and for energy generation in the case of skeletal muscle and the heart. On the other hand, NEFA distribute into a single plasma pool which exchanges FAs with all cell types. The FA transporters generally control facilitated uptake of NEFA by different tissues [48, 49] however small amounts can cross cell membranes by slow diffusion termed ‘flip-flop’ [50]. NEFA concentrations play an important role in establishing plasma TG levels [51] and three factors contribute to NEFA concentrations in plasma: 1) the rate of NEFA sequestration by adipose and other tissues; 2) the rate of FA release from the adipocyte TG stores by hormone sensitive lipasei (HSL); 3) and the FAs released from circulating lipoproteins by LpL localized to the vascular endothelium, commonly termed ‘spillover’ [47, 52].
Figure 1. FA transport and FO induced changes.
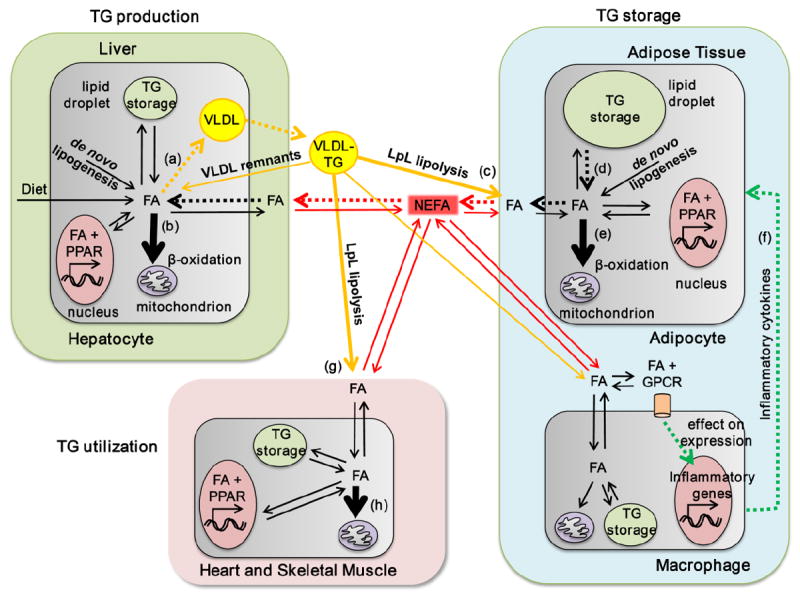
FAs are transported throughout the body in two major forms: esterified in TG carried by VLDL (VLDL-TG) and non-esterified carried by serum albumin (NEFA). From these two pools FA are distributed to different tissues depending on energy requirements and hormonal status. In the tissues, FA are used for ATP production via β-oxidation, re-esterification in TG for energy storage, incorporation into lipids composing cell membranes, and production of signaling molecules. Non-essential FA can also be de novo synthesized from other carbon sources. The liver (top left) acts as a major site for FA distribution, processing FAs from all sources: diet, de novo lipogenesis, circulating NEFA and VLDL remnants, and producing VLDL-TG which are secreted into the circulation. Adipose tissue (right) is the main storage depot for FA (as TG storage in lipid droplets). Adipocyte uptake of FAs from the circulation occurs via LpL lipolysis of VLDL-TG. Adipocytes largely contribute to the plasma NEFA pool by regulated release of FA from lipid droplets via HSL-lipolysis. Adipose tissue macrophages (bottom right) regulate intracellular lipolysis in adipocytes by secreting inflammatory cytokines. Heart and skeletal muscle (bottom left) obtain FAs from the NEFA pool and from the VLDL-TG pool via LpL-lipolysis. β-oxidation plays a major role in FA disposal in the heart and skeletal muscle. In hepatocytes, FO (a) down-regulates VLDL production and (b) up-regulates β-oxidation; in adipocytes, FO (c) increases FA uptake from LpL lipolysis of plasma TG, (d) decreases intracellular lipolysis in adipocytes, and (e) increases β-oxidation; FO also (f) reduces the secretion of pro-inflammatory cytokines from adipose tissue macrophages; in heart and skeletal muscle, FO (g) up-regulates LPL lipolysis of plasma TG and (h) β-oxidation. Pathways enhanced by FO are indicated by bold arrows, and those reduced by FO are indicated by dotted arrows. Abbreviations: FA – fatty acid, GPCR – g-protein coupled receptor; LpL – lipoprotein lipase; NEFA – non-esterified fatty acid; PPAR – peroxisome proliferator-activated receptor; VLDL – very-low density lipoprotein.
5. Fish oil and NEFA
We were able to identify only one randomized controlled trial examining the effects of FO on NEFA as a primary endpoint [53]. In this study of 20 healthy medical students, large amounts of FO (30g/day) were administered in the diet over 7 days. Based on their mean HOMA score of 1.8 (calculated from reported glucose and insulin values), the subjects were insulin sensitive, and FO had no effect on either fasting glucose or insulin. While the subjects were normotriglyceridemic at baseline (84 mg/dL), they had a 36% decrease in TG, and their fasting NEFA decreased by practically the same amount, 37%, an amount sufficient to reduce VLDL-TG synthesis. Using Vedala’s model [46], and assuming no change in clearance and that NEFA levels are proportional to production rates, we calculated that the 37% reduction in NEFA would translate into a 31% reduction in plasma TG which is nearly that observed. Unfortunately, the large FO dose (corresponding to ~9g/day omega-3 FA) and the type of subjects studied (healthy, insulin sensitive, and normotriglyceridemic) does not allow for straightforward extrapolation to insulin resistant, HTG subjects given pharmaceutical doses of omega-3 FAs (e.g. 3.4 g/day). In obese or insulin resistant subjects, a reduction of only 13% in NEFA levels would produce a 20% decrease in plasma TG, and given the relatively high coefficient of variation for NEFA (15-30% depending on the study population [54, 55] large sample sizes would be required to detect a meaningful effect of omega-3 FAs on NEFA. Thus, either larger trials powered on a NEFA endpoint, or more sensitive measures of NEFA metabolism are required to determine if changes in NEFA flux are the primary contributor to the omega-3 FAs induced reduction in VLDL-TG synthesis.
Other trials reporting the effects of omega-3 FAs on NEFA as secondary endpoints in more appropriate populations do exist. While significant reductions are not consistently reported, the observed changes have been large enough to explain the reduced TG levels. For example, in a randomized controlled trial of severely HTG subjects (N=40) treated with 3.4g/day of omega-3 FAs for six weeks, Pownal et al found a mean decrease in NEFA from 0.86 to 0.66 mmol/L. This effect was significant in the FO arm, but not when compared to placebo (0.89 to 0.85 mmol/L). Such an effect size could account for about 75% of the observed reduction plasma TG levels [56], again assuming no change in clearance. Bordin et al showed a 25% decrease in NEFA from 0.45 to 0.33 mmol/L in a normotriglyceridemic population [40] which, based on their reported kinetic parameters, corresponds to a 22% drop in plasma TG. Healthy men exposed to high NEFA by infusion of intralipid with heparin responded by increasing hepatic VLDL synthesis by 35% and somewhat surprisingly, by increasing intestinal chylomicron synthesis by 70% [57]. Notably, we found two trials in which there were no reported FO induced changes in fasting NEFA concentrations. The OPTILIP trial was designed to deliver specific ratios of omega-6 and omega-3 FAs and so delivered a sub-pharmacologic dose of EPA and DHA (~1.6 g/day). Although this trial found no change in NEFA, the intervention produced only a 15% decrease in fasting TG, and so the corresponding NEFA decrease could have been even smaller [58]. Given the study’s coefficient of variation for NEFA (15%), an effect size of this small magnitude was unlikely to be observed. In another trial studying subjects undergoing hemodialysis, there was no decrease in the rate of appearance of NEFA or the NEFA levels, however baseline NEFA levels were very low [13].
6. FO improves TG clearance
Of the tracer-label studies (Table 1), two studies identified an effect on clearance as an increased FCR (i.e., the fraction per unit time leaving the VLDL compartment) [39, 42] or as fractional transfer from VLDL to IDL [42]. A third, smaller study reported an effect nearing significance [40]. The effect of FO on TG clearance may be enhanced in the post-prandial state. Park et al traced 3H-triolein-labled chylomicron-like particles in the post-prandial state and found both increased clearance and a corresponding increase in non-heparin-stimulated LpL activity in plasma [42]. The rate limiting step of VLDL-TG clearance is LpL, not only as lipolytic enzyme but also as a ligand to capture and bind VLDL to the endothelial proteoglycans [59, 60]. LpL is synthesized by mesenchymal cells behind the endothelial barrier and then transported to the luminal endothelial surface and attached to heparan sulfate where it first captures the circulating particle in the glycocalyx [59]. Interestingly, endothelial binding can, in and of itself, nearly normalize clearance even in animal models where the endothelium has no LpL-lipolytic capacity [61]. LpL-mediated lipolysis and cellular uptake of the released FAs follows endothelial binding. CD36 facilitates the uptake of FAs from TG-rich lipoproteins and their remnants following LpL-lipoysis [62]. During the postprandial state, binding of chylomicron-like particles to the endothelium is 60% greater in subjects given 4 g/day EPA or DHA than in those given placebo [63]. Circulating LpL activity is a surrogate for LpL-lipolytic capacity in humans, and it is also higher after EPA or DHA treatment suggesting that the increased binding could be the result of increased bridging activity between particles and LpL [63]. The high NEFA in HTG increases VLDL apoC-III [57], which is an inhibitor of LpL-lipolysis, and FO blocks the accumulation of apoC-III on VLDL [64], potentially increasing LpL-lipolysis.
FO treatment may also direct FAs away from the liver to adipose tissue. In adipose tissue of subjects with atherogenic lipid profiles, LpL mRNA is increased by 55% as is post-heparin lipase, and these changes correlate with an improvement in clearance of post-prandial TG [65]. In fasting rats, FO appears to direct TG towards skeletal muscle (and away from the liver) by increasing the relative expression of skeletal muscle LpL [66]. Adipose tissue is responsible for a large portion of post-prandial TG removal and thus, while increased clearance of TG may contribute less to the overall reduction in TGs than decreased synthesis, it still contributes to a beneficial shift in FA trafficking.
7. Transcription factors regulating FA metabolism & the effects of FO
Changes in transcription of several nuclear receptors are reported to mediate the TG-reducing effects of FO: sterol regulatory element binding proteins (SREBP), liver X receptor-alpha (LXRα), retinoid X receptor alpha (RXRα), farnesoid X receptor (FXR), and peroxisome proliferator-activated receptors (PPARs), and each play prominent roles in controlling lipid metabolism.
The SREBPs are transcription factors that regulate expression of lipid synthesizing enzymes, including fatty acid and TG synthesis, and SREBP-1c, the primary hepatic activator of lipogenesis [67]. It is itself under the control of the Liver LXRα which binds to the LXR response element as a heterodimer with RXRα and activates expression of SREBP-1c. FOs prevent activation of SREBP-1c expression by inhibiting LXRα/RXRα binding to the LXR response element located in the promoter regions of SREBP-1c gene [68], and this is reflected in reduced mRNA levels of SREBP-1c in animals fed FO diets [69]. FOs also act directly on SREBP-1c protein to inhibit its maturation [70]. Since omega-6 FAs have similar effects on SREBP-1c but do not affect plasma TG levels [71], the reduction in SREBP-1c may be a response to the reduced FA flux rather than the cause.
FXR is expressed in the liver and small intestines and forms dimers with the RXR receptor subunits. It controls lipoprotein metabolism by stimulating expression of apolipoproteins, especially apoC-II [72] which is an activator of LpL. Since DHA is an activator of FXR [73], some effects of FO could be mediated through this receptor, however as with the SREBP-1c/LXRα/RXRα axis, omega-6 polyunsaturated FAs (PUFAs) are more potent activators [73] making the omega-3 activation of this pathway a less likely explanation of their TG-lowering effect. Other transcription factors of interest in mediating these adaptations include hepatocyte nuclear factor-α (HNF4α), a central regulator of enzymes controlling lipid metabolism [74] and is decreased by FO [75].
The most consistent effect of FO in animal studies is the activation PPARs [76]. PPARs are ligand-activated transcription factors that regulate the expression of enzymes and proteins involved in energy homeostasis: PPARα increases fatty acid oxidation in the liver, adipose, heart and skeletal muscle; PPARγ promotes adipocyte storage of FAs as TG; PPARβ/δ ubiquitously induces β-oxidation of fatty acids and along with other FAs, EPA and DHA are PPAR ligands [77]. PUFA metabolites, such as eicosanoids and more generally oxylipins, are potent activators of PPARs. Oxylipins are produced via the action of cyclooxygenase (COX) [78], lipooxygenase (LOX) [79, 80] or cytochrome p450 (CYP) [81, 82] activity on PUFAs, and can be more potent PPAR agonists than their parent FAs [83]. Many are acylated into VLDL-glycerolipids and released by LpL [84], and LpL-lipolysis of VLDL activates PPARα [85] demonstrating that VLDL are a source of non-paracrine PPAR activators. Further, FO therapy increases the plasma levels of the EPA and DHA-derived oxylipins which are analogs of the arachidonate oxylipins in plasma glycerolipids [86] and activation of PPARγ by 4-hydroxydocosahexaenoic acid (4-HDoHE; or 4-HDHA), an oxylipin derived from DHA, has been documented [87]. In many cases, EPA- and DHA-derived oxylipins have greater potency than their arachidonate analogs, especially among epoxygenated fatty acids [88], and such a scenario would provide a basis for greater PPAR activation in tissues with abundant FO.
8. Tissue specific effects of FO and the regulation of TG and FA trafficking
Plasma TG levels are merely a static snapshot of FA trafficking between tissues, so the kinetic contributions of individual tissues must be considered. These effects are discussed below.
8.1 Liver
The most consistent plasma TG-lowering effect of FO is the reduction of VLDL-TG production as demonstrated by all tracer kinetic studies [37-44] (see discussion above). VLDL assembly is a complex process in which the synthesis of apoB is coordinated with hepatocyte TG synthesis [89]. FO inhibits assembly and secretion of VLDL-TG and apoB100 from cultured hepatocytes (Fig. 1a) [90, 91]. EPA-coenzyme A (CoA) directly reduces TG synthesis via inhibition of the diacylglycerol acytransferase (DGAT) activity and reduces esterification of 1,2-diacylglycerol in rat liver microsomes [92]. Additionally, synthesis of apoB is affected by dietary omega-3 FA. A recent study showed that chylomicron remnant-like particles enriched with omega-3 PUFAs reduce the expression of HNF-4alpha protein and the expression of mRNA for HNF-4alpha target genes, including apoB and the microsomal TG transfer protein [75]. It has been shown that peroxide derivatives of EPA and DHA can stimulate degradation of apoB-100 thus reducing VLDL-TG secretion [93]. Moreover, omega-3 PUFA up-regulate β-oxidation in hepatocytes [91, 94] (Fig. 1b) thus reducing the pool of FA available for TG synthesis.
Excessive accumulation of TG in the liver leads to NAFLD, a typical co-morbidity of HTG [95, 96]. However NAFLD is ameliorated with FO supplementation in humans [97, 98] and in animal models of liver steatosis [99, 100], and these effects of FO are consistent with a reduced intracellular pool of free FAs and increased β-oxidation.
8.2 Adipose tissue
FO affects the primary function of adipose tissue, fat storage. FO supplementation reduces adiposity in animals fed high fat diets [101-105] despite stimulating FA uptake due to increased expression of LpL (Fig. 1c) and of CD36, the major FA membrane transporter [106, 107]. Similarly, although FO upregulates postprandial LpL expression in human adipose tissue [108], suggestive of increased FA uptake by adipose, no effect on body weight has been seen in multiple studies. Indeed, a few small clinical trials have reported weight loss [109-111]. Reduced fat mass, to the extent that it occurs, could be explained mechanistically by mitochondrial biogenesis and increased β-oxidation (Fig. 1e), concurrent with reduced de novo lipogenesis in white adipose tissue [102, 109].
Adipose tissue is the primary source of plasma NEFA, and there is good evidence from animal studies that FO can reduce NEFA output to the circulation by reducing HSL-mediated intracellular lipolysis (Fig. 1d). In a rat study comparing lard to lard+FO diets, FO lowered plasma NEFA (0.15 vs. 0.28 μmol/L) and lowered basal intracellular lipolysis by 50% [112]. Chronic low-grade adipose tissue inflammation in obesity and insulin resistant states is emerging as an important activator of HSL-mediated lipolysis in adipocytes [113-115]. TNF-α and serum amyloid A secreted in adipose tissue induce intracellular lipolysis [116-119], while IL-6 appears to increase the lipolytic response to adrenergic stimulus [120]. TNF-α also reduces the activity of LpL in cultured adipocytes [121, 122], increasing the gradient for FA efflux from adipocytes. In vitro studies using cultured adipocytes have shown that FO counteracts the effects of TNF-α and IL-6 on HSL-lipolysis [123], and adipocytes appear to have improved function with DHA treatment as evidenced by increased adiponectin production [124].
Inflammatory cytokines (Fig. 1f) are primarily produced in adipose tissue by infiltrating immune cells, specifically by adipose tissue macrophages (ATM). ATM secrete cytokines into the extracellular space where these inflammatory molecules find their receptors on the surface of adipocytes. Administration of high amounts of oral EPA (1g/kg/day) in rats prevented TNF-α gene over-expression in adipose tissue induced by high fat diet [125]. The rate of ex vivo intracellular lipolysis in adipocytes isolated from these FO-treated and control rats, however, was similar implying that when present, EPA and DHA modify the response of adipocytes to activated ATM. Therefore, up-regulation of HSL-mediated intracellular lipolysis likely requires paracrine factors secreted by other cells residing in adipose tissue. A recent genetic study in mice identified a plausible mechanism for how FO might down-regulate an inflammatory cytokine secretion from ATM [126]. According to the study, DHA can act as extracellular ligand for GPR120, a G-protein coupled receptor up-regulated on ATM by high fat diet. Activation of GPR120 by DHA attenuated the expression of pro-inflammatory cytokines in adipose tissue. The attenuation of pro-inflammatory cytokine expression was abrogated in GPR120 deficient mice. Moreover, the effect of DHA on cytokine expression was intrinsic to immune cells because it was not effective in irradiated wild type mice reconstituted with GPR120-deficient bone marrow. Although adipocyte lipolysis was not reported in this study, it is reasonable to suggest that the reduced expression of proinflammatory cytokines via activation of GPR120 might lead to paracrine down-regulation of intracellular lipolysis and the reduction of FA efflux from adipocytes. However, the reported effects of DHA on GPR120 may not be specific to omega-3 PUFAs since palmitoleic acid exhibited nearly the same activity on some metrics. Reduction of NEFA efflux by adipose tissue reduces the availability of FA as a substrate for TG synthesis and VLDL production in hepatocytes.
8.3 Heart and Skeletal Muscle
Heart and skeletal muscles are the primary sites for FA utilization, and LpL is highly expressed in both tissues [127]. In both tissues, LpL activity is enhanced by FO [128]. These results suggest that increased removal of TG by heart and skeletal muscle contributes to the plasma TG lowering effect (Fig. 1g). The uptake of FAs released from VLDL via LpL-lipolysis appears to be more efficient in skeletal muscle than in adipose tissue, suggesting less spillover of FAs into the NEFA pool. FO also increases the expression of genes regulating β-oxidation in skeletal muscle [129] (Fig. 1h). Moreover, the FO-induced increase in plasma adiponectin is likely to have a systemic effect on skeletal and cardiac muscle increasing TG hydrolysis, FA uptake and β-oxidation [130]. As a result cardiac and skeletal muscles could facilitate the TG-lowering effects of FO by upregulating FA uptake and oxidation.
9. Conclusion
There is uniform support for decreased production of TG by FO. The randomized controlled studies also demonstrate an increase in clearance. Based on the fact that NEFA are by far the primary source of FAs for VLDL-TG production and that FO reduces plasma NEFA, this could be the primary mechanism for the hypotriglyceridemic effect. Plasma NEFA are largely the result of intracellular HSL lipolysis in the adipocyte which FO counteracts by suppressing adipose tissue inflammation and increasing FA uptake in adipose, heart and skeletal muscle. In addition, a systemic increase in β-oxidation provides a sink for FA disappearance and contributes to decreased hepatocyte availability of FAs for VLDL-TG production.
Highlights.
Pharmaceutical long chain omega-3 fatty acids effectively reduce plasma triglyceride levels.
Fish oil reduces the rate of VLDL synthesis in the liver.
Reduced NEFA delivery to the liver is a likely locus of action for fish oils.
Fish oil counteracts the lipolytic release of NEFA from adipose tissue by suppressing inflammation.
Fish oil increases FA uptake and β-oxidation in adipose, heart and skeletal muscle.
Abbreviations
- 4-HDoHE
4-hydroxydocosahexaenoic acid
- ATM
dipose tissue macrophages
- CoA
coenzyme A
- COX
cyclooxygenase
- CYP
cytochrome p450
- DGAT
diacylglycerol acytransferase
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FXR
farnesoid X receptor
- FA
fatty acid
- FO
fish oils
- FCR
fractional catabolic rate
- HNF4α
hepatocyte nuclear factor-α
- HSL
hormone sensitive lipase
- HTG
hypertriglyceridemic
- IDL
intermediate density lipoprotein
- LOX
lipoxygenase
- LpL
lipoprotein lipase
- LXRα
liver X receptor alpha
- NAFLD
non-alcoholic fatty liver disease
- NEFA
non-esterified fatty acid
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acid
- RXRα
retinoid X receptor alpha
- SREBP
sterol regulatory element binding protein
- TG
triglyceride
- T2D
type II diabetes
- VLDL
very low density lipoprotein
Footnotes
Disclosures:
GCS receives research funding from GlaxoSmithKline and from the California Walnut Commission for research related to omega-3 fatty acids and/or triglyceride metabolism. GCS and WSH receive speakership honoraria from GlaxoSmithKline.
It is worth pointing out that within the diabetes literature, the term ‘lipolysis’ usually refers to intracellular lipolysis, while in the lipoprotein literature it refers to extracellular lipolysis on the vascular endothelium from TGbearing lipoproteins. These two types of lipolysis have opposing effects. LpL-mediated intravascular (periendothelial) lipolysis of lipoprotein TG would increase the local FA concentration, favoring FA flux into LpLexpressing tissues, including adipose. On the other hand, intracellular (i.e., hormone sensitive lipase-mediated in the adipocyte) lipolysis would increase intracellular FAs concentrations, thus promoting FAs efflux from the adipocyte. In the interest of clarity, here we will use two more descriptive terms: extracellular lipolysis refers to the former and intracellular lipolysis refers to the latter.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imaichi K, Michaels GD, Gunning B, Grasso S, Fukayama G, Kinsell LW. Studies with the use of fish oil fractions in human subjects. Am J Clin Nutr. 1963;13:158–168. doi: 10.1093/ajcn/13.3.158. [DOI] [PubMed] [Google Scholar]
- 2.von Lossonczy TO, Ruiter A, Bronsgeest-Schoute HC, van Gent CM, Hermus RJ. The effect of a fish diet on serum lipids in healthy human subjects. Am J Clin Nutr. 1978;31:1340–1346. doi: 10.1093/ajcn/31.8.1340. [DOI] [PubMed] [Google Scholar]
- 3.Kinsell LW, Michaels GD, Walker G, Visintine RE. The effect of a fish-oil fraction on plasma lipids. Diabetes. 1961;10:316–319. doi: 10.2337/diab.10.4.316. [DOI] [PubMed] [Google Scholar]
- 4.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–252. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 6.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, Guerre-Millo M, Rizkalla SW. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–1679. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 7.Park Y, Harris WS. Dose-response of n-3 polyunsaturated fatty acids on lipid profile and tolerability in mildly hypertriglyceridemic subjects. J Med Food. 2009;12:803–808. doi: 10.1089/jmf.2008.1250. [DOI] [PubMed] [Google Scholar]
- 8.Isley WL, Miles JM, Harris WS. Pilot study of combined therapy with omega-3 fatty acids and niacin in atherogenic dyslipidemia. J Clin Lipidol. 2007;1:211–217. doi: 10.1016/j.jacl.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40:194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Metroka CE, Truong P, Gotto AM., Jr Treatment of HIV-associated dyslipidemia: a role for omega-3 fatty acids. AIDS Read. 2007;17:362–364. 367–373. [PubMed] [Google Scholar]
- 11.Hall AV, Parbtani A, Clark WF, Spanner E, Huff MW, Philbrick DJ, Holub BJ. Omega-3 fatty acid supplementation in primary nephrotic syndrome: effects on plasma lipids and coagulopathy. J Am Soc Nephrol. 1992;3:1321–1329. doi: 10.1681/ASN.V361321. [DOI] [PubMed] [Google Scholar]
- 12.Rasic-Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Sobajic S, Djuric I, Ristic D. Effects of N-3 PUFAs supplementation on insulin resistance and inflammatory biomarkers in hemodialysis patients. Ren Fail. 2007;29:321–329. doi: 10.1080/08860220601184092. [DOI] [PubMed] [Google Scholar]
- 13.Delarue J, Guillodo MP, Guillerm S, Elbaz A, Marty Y, Cledes J. Fish oil attenuates adrenergic overactivity without altering glucose metabolism during an oral glucose load in haemodialysis patients. Br J Nutr. 2008;99:1041–1047. doi: 10.1017/S0007114507843534. [DOI] [PubMed] [Google Scholar]
- 14.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- 15.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Musa-Veloso K, Binns MA, Kocenas AC, Poon T, Elliot JA, Rice H, Oppedal-Olsen H, Lloyd H, Lemke S. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid dose-dependently reduce fasting serum triglycerides. Nutr Rev. 2010;68:155–167. doi: 10.1111/j.1753-4887.2010.00272.x. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS. n-3 fatty acids and lipoproteins: comparison of results from human and animal studies. Lipids. 1996;31:243–252. doi: 10.1007/BF02529870. [DOI] [PubMed] [Google Scholar]
- 18.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS. The omega-3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–417. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 21.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 22.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS) Atherosclerosis. 2008;200:135–140. doi: 10.1016/j.atherosclerosis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Assmann G, Schulte H. Role of triglycerides in coronary artery disease: lessons from the Prospective Cardiovascular Munster Study. Am J Cardiol. 1992;70:10H–13H. doi: 10.1016/0002-9149(92)91084-h. [DOI] [PubMed] [Google Scholar]
- 25.Assmann G, Schulte H, Cullen P. New and classical risk factors--the Munster heart study (PROCAM) Eur J Med Res. 1997;2:237–242. [PubMed] [Google Scholar]
- 26.Cummings MH, Watts GF, Umpleby M, Hennessy TR, Quiney JR, Sonksen PH. Increased hepatic secretion of very-low-density-lipoprotein apolipoprotein B-100 in heterozygous familial hypercholesterolaemia: a stable isotope study. Atherosclerosis. 1995;113:79–89. doi: 10.1016/0021-9150(94)05430-q. [DOI] [PubMed] [Google Scholar]
- 27.Zulewski H, Ninnis R, Miserez AR, Baumstark MW, Keller U. VLDL and IDL apolipoprotein B-100 kinetics in familial hypercholesterolemia due to impaired LDL receptor function or to defective apolipoprotein B-100. J Lipid Res. 1998;39:380–387. [PubMed] [Google Scholar]
- 28.Cortner JA, Coates PM, Bennett MJ, Cryer DR, Le NA. Familial combined hyperlipidaemia: use of stable isotopes to demonstrate overproduction of very low-density lipoprotein apolipoprotein B by the liver. J Inherit Metab Dis. 1991;14:915–922. doi: 10.1007/BF01800473. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesan S, Cullen P, Pacy P, Halliday D, Scott J. Stable isotopes show a direct relation between VLDL apoB overproduction and serum triglyceride levels and indicate a metabolically and biochemically coherent basis for familial combined hyperlipidemia. Arterioscler Thromb. 1993;13:1110–1118. doi: 10.1161/01.atv.13.7.1110. [DOI] [PubMed] [Google Scholar]
- 30.Cummings MH, Watts GF, Pal C, Umpleby M, Hennessy TR, Naoumova R, Sonksen PH. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in obesity: a stable isotope study. Clin Sci (Lond) 1995;88:225–233. doi: 10.1042/cs0880225. [DOI] [PubMed] [Google Scholar]
- 31.Cummings MH, Watts GF, Umpleby AM, Hennessy TR, Naoumova R, Slavin BM, Thompson GR, Sonksen PH. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia. 1995;38:959–967. doi: 10.1007/BF00400586. [DOI] [PubMed] [Google Scholar]
- 32.Pont F, Duvillard L, Florentin E, Gambert P, Verges B. Early kinetic abnormalities of apoB-containing lipoproteins in insulin-resistant women with abdominal obesity. Arterioscler Thromb Vasc Biol. 2002;22:1726–1732. doi: 10.1161/01.atv.0000032134.92180.41. [DOI] [PubMed] [Google Scholar]
- 33.Kissebah AH, Alfarsi S, Adams PW. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in man: normolipemic subjects, familial hypertriglyceridemia and familial combined hyperlipidemia. Metabolism. 1981;30:856–868. doi: 10.1016/0026-0495(81)90064-0. [DOI] [PubMed] [Google Scholar]
- 34.Kissebah AH, Alfarsi S, Evans DJ, Adams PW. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in non-insulin-dependent diabetes mellitus. Diabetes. 1982;31:217–225. doi: 10.2337/diab.31.3.217. [DOI] [PubMed] [Google Scholar]
- 35.Prinsen BH, Rabelink TJ, Romijn JA, Bisschop PH, de Barse MM, de Boer J, van Haeften TW, Barrett PH, Berger R, de Sain-van der Velden MG. A broad-based metabolic approach to study VLDL apoB100 metabolism in patients with ESRD and patients treated with peritoneal dialysis. Kidney Int. 2004;65:1064–1075. doi: 10.1111/j.1523-1755.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 36.de Sain-van der Velden MG, Kaysen GA, Barrett HA, Stellaard F, Gadellaa MM, Voorbij HA, Reijngoud DJ, Rabelink TJ. Increased VLDL in nephrotic patients results from a decreased catabolism while increased LDL results from increased synthesis. Kidney Int. 1998;53:994–1001. doi: 10.1111/j.1523-1755.1998.00831.x. [DOI] [PubMed] [Google Scholar]
- 37.Nestel PJ, Connor WE, Reardon MF, Connor S, Wong S, Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J Clin Invest. 1984;74:82–89. doi: 10.1172/JCI111422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders TA, Sullivan DR, Reeve J, Thompson GR. Triglyceride-lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis. 1985;5:459–465. doi: 10.1161/01.atv.5.5.459. [DOI] [PubMed] [Google Scholar]
- 39.Harris WS, Connor WE, Illingworth DR, Rothrock DW, Foster DM. Effects of fish oil on VLDL triglyceride kinetics in humans. J Lipid Res. 1990;31:1549–1558. [PubMed] [Google Scholar]
- 40.Bordin P, Bodamer OA, Venkatesan S, Gray RM, Bannister PA, Halliday D. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur J Clin Nutr. 1998;52:104–109. doi: 10.1038/sj.ejcn.1600522. [DOI] [PubMed] [Google Scholar]
- 41.Fisher WR, Zech LA, Stacpoole PW. Apolipoprotein B metabolism in hypertriglyceridemic diabetic patients administered either a fish oil- or vegetable oil-enriched diet. J Lipid Res. 1998;39:388–401. [PubMed] [Google Scholar]
- 42.Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res. 2003;44:455–463. doi: 10.1194/jlr.M200282-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Chan DC, Watts GF, Barrett PH, Beilin LJ, Redgrave TG, Mori TA. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes. 2002;51:2377–2386. doi: 10.2337/diabetes.51.8.2377. [DOI] [PubMed] [Google Scholar]
- 44.Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr. 2003;77:300–307. doi: 10.1093/ajcn/77.2.300. [DOI] [PubMed] [Google Scholar]
- 45.Hellerstein MK, Christiansen M, Kaempfer S, Kletke C, Wu K, Reid JS, Mulligan K, Hellerstein NS, Shackleton CH. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J Clin Invest. 1991;87:1841–1852. doi: 10.1172/JCI115206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res. 2006;47:2562–2574. doi: 10.1194/jlr.M600200-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 48.Bonen A, Luiken JJ, Glatz JF. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem. 2002;239:181–192. [PubMed] [Google Scholar]
- 49.Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids. 2010;82:149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 50.Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids. 2006;75:149–159. doi: 10.1016/j.plefa.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miles JM, Park YS, Walewicz D, Russell-Lopez C, Windsor S, Isley WL, Coppack SW, Harris WS. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes. 2004;53:521–527. doi: 10.2337/diabetes.53.3.521. [DOI] [PubMed] [Google Scholar]
- 53.Dagnelie PC, Rietveld T, Swart GR, Stijnen T, van den Berg JW. Effect of dietary fish oil on blood levels of free fatty acids, ketone bodies and triacylglycerol in humans. Lipids. 1994;29:41–45. doi: 10.1007/BF02537089. [DOI] [PubMed] [Google Scholar]
- 54.Abate N, Chandalia M, Snell PG, Grundy SM. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab. 2004;89:2750–2755. doi: 10.1210/jc.2003-031843. [DOI] [PubMed] [Google Scholar]
- 55.Vistisen B, Hellgren LI, Vadset T, Scheede-Bergdahl C, Helge JW, Dela F, Stallknecht B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol. 2008;58:61–68. doi: 10.1530/EJE-07-0493. [DOI] [PubMed] [Google Scholar]
- 56.Pownall HJ, Brauchi D, Kilinc C, Osmundsen K, Pao Q, Payton-Ross C, Gotto AM, Jr, Ballantyne CM. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143:285–297. doi: 10.1016/s0021-9150(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 57.Pavlic M, Valero R, Duez H, Xiao C, Szeto L, Patterson BW, Lewis GF. Triglyceride-rich lipoprotein-associated apolipoprotein C-III production is stimulated by plasma free fatty acids in humans. Arterioscler Thromb Vasc Biol. 2008;28:1660–1665. doi: 10.1161/ATVBAHA.108.169383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffin MD, Sanders TA, Davies IG, Morgan LM, Millward DJ, Lewis F, Slaughter S, Cooper JA, Miller GJ, Griffin BA. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: the OPTILIP Study. Am J Clin Nutr. 2006;84:1290–1298. doi: 10.1093/ajcn/84.6.1290. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 60.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 61.Shearer GC, Stevenson FT, Atkinson DN, Jones H, Staprans I, Kaysen GA. Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int. 2001;59:179–189. doi: 10.1046/j.1523-1755.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 62.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park Y, Jones PG, Harris WS. Triacylglycerol-rich lipoprotein margination: a potential surrogate for whole-body lipoprotein lipase activity and effects of eicosapentaenoic and docosahexaenoic acids. The American journal of clinical nutrition. 2004;80:45–50. doi: 10.1093/ajcn/80.1.45. [DOI] [PubMed] [Google Scholar]
- 64.Swahn E, von Schenck H, Olsson AG. Omega-3 Ethyl Ester Concentrate Decreases Total Apolipoprotein CIII and Increases Antithrombin III in Postmyocardial Infarction Patients. Clin Drug Investig. 1998;15:473–482. doi: 10.2165/00044011-199815060-00003. [DOI] [PubMed] [Google Scholar]
- 65.Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, Griffin BA. Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J Lipid Res. 2002;43:979–985. [PubMed] [Google Scholar]
- 66.Baltzell JK, Wooten JT, Otto DA. Lipoprotein lipase in rats fed fish oil: apparent relationship to plasma insulin levels. Lipids. 1991;26:289–294. doi: 10.1007/BF02537139. [DOI] [PubMed] [Google Scholar]
- 67.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshikawa T, Shimano H, Yahagi N, Ide T, Amemiya-Kudo M, Matsuzaka T, Nakakuki M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Takahashi A, Sone H, Osuga Ji J, Gotoda T, Ishibashi S, Yamada N. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J Biol Chem. 2002;277:1705–1711. doi: 10.1074/jbc.M105711200. [DOI] [PubMed] [Google Scholar]
- 69.Le Jossic-Corcos C, Gonthier C, Zaghini I, Logette E, Shechter I, Bournot P. Hepatic farnesyl diphosphate synthase expression is suppressed by polyunsaturated fatty acids. Biochem J. 2005;385:787–794. doi: 10.1042/BJ20040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka N, Zhang X, Sugiyama E, Kono H, Horiuchi A, Nakajima T, Kanbe H, Tanaka E, Gonzalez FJ, Aoyama T. Eicosapentaenoic acid improves hepatic steatosis independent of PPARalpha activation through inhibition of SREBP-1 maturation in mice. Biochem Pharmacol. 2010;80:1601–1612. doi: 10.1016/j.bcp.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caputo M, Zirpoli H, Torino G, Tecce MF. Selective regulation of UGT1A1 and SREBP-1c mRNA expression by docosahexaenoic, eicosapentaenoic, and arachidonic acids. J Cell Physiol. 2011;226:187–193. doi: 10.1002/jcp.22323. [DOI] [PubMed] [Google Scholar]
- 72.Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K, Gonzalez FJ, Willson TM, Edwards PA. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 73.Zhao A, Yu J, Lew JL, Huang L, Wright SD, Cui J. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell Biol. 2004;23:519–526. doi: 10.1089/1044549041562267. [DOI] [PubMed] [Google Scholar]
- 74.Minihane AM. Nutrient gene interactions in lipid metabolism. Curr Opin Clin Nutr Metab Care. 2009;12:357–363. doi: 10.1097/MCO.0b013e32832c94a5. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Soldado I, Avella M, Botham KM. Suppression of VLDL secretion by cultured hepatocytes incubated with chylomicron remnants enriched in n-3 polyunsaturated fatty acids is regulated by hepatic nuclear factor-4alpha. Biochim Biophys Acta. 2009;1791:1181–1189. doi: 10.1016/j.bbalip.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–393. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- 77.Yessoufou A, Wahli W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Med Wkly. 2010;140:w13071. doi: 10.4414/smw.2010.13071. [DOI] [PubMed] [Google Scholar]
- 78.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 79.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 80.Altmann R, Hausmann M, Spottl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Scholmerich J, Falk W, Rogler G. 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochem Pharmacol. 2007;74:612–622. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 81.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, Liu Y, Sangras B, Falck JR, Weintraub NL, Spector AA. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H55–63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 82.Wray JA, Sugden MC, Zeldin DC, Greenwood GK, Samsuddin S, Miller-Degraff L, Bradbury JA, Holness MJ, Warner TD, Bishop-Bailey D. The epoxygenases CYP2J2 activates the nuclear receptor PPARalpha in vitro and in vivo. PLoS One. 2009;4:e7421. doi: 10.1371/journal.pone.0007421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry. 1999;38:185–190. doi: 10.1021/bi9816094. [DOI] [PubMed] [Google Scholar]
- 84.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids. 2008;79:215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci U S A. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- 89.Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem Cell Biol. 2010;88:251–267. doi: 10.1139/o09-168. [DOI] [PubMed] [Google Scholar]
- 90.Wang H, Chen X, Fisher EA. N-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J Clin Invest. 1993;91:1380–1389. doi: 10.1172/JCI116340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lang CA, Davis RA. Fish oil fatty acids impair VLDL assembly and/or secretion by cultured rat hepatocytes. J Lipid Res. 1990;31:2079–2086. [PubMed] [Google Scholar]
- 92.Berge RK, Madsen L, Vaagenes H, Tronstad KJ, Gottlicher M, Rustan AC. In contrast with docosahexaenoic acid, eicosapentaenoic acid and hypolipidaemic derivatives decrease hepatic synthesis and secretion of triacylglycerol by decreased diacylglycerol acyltransferase activity and stimulation of fatty acid oxidation. Biochem J. 1999;343(Pt 1):191–197. [PMC free article] [PubMed] [Google Scholar]
- 93.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 94.Clarke SD, Jump D. Polyunsaturated fatty acids regulate lipogenic and peroxisomal gene expression by independent mechanisms. Prostaglandins Leukot Essent Fatty Acids. 1997;57:65–69. doi: 10.1016/s0952-3278(97)90494-4. [DOI] [PubMed] [Google Scholar]
- 95.Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray-McGuire C, Schauer PR, Gupta M, Feldstein AE, Hazen SL, Stein CM. Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring) 2009;17:1696–1701. doi: 10.1038/oby.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, Salles GF. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011;31:700–706. doi: 10.1111/j.1478-3231.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- 97.Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 98.Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 99.Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alwayn IP, Gura K, Nose V, Zausche B, Javid P, Garza J, Verbesey J, Voss S, Ollero M, Andersson C, Bistrian B, Folkman J, Puder M. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005;57:445–452. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 101.Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol. 1993;264:R1111–1118. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- 102.Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48:2365–2375. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- 103.Okamoto T, Takamizawa S, Arai H, Bitoh Y, Nakao M, Yokoi A, Nishijima E. Esophageal atresia: prognostic classification revisited. Surgery. 2009;145:675–681. doi: 10.1016/j.surg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 104.Arai T, Kim HJ, Chiba H, Matsumoto A. Anti-obesity effect of fish oil and fish oil-fenofibrate combination in female KK mice. J Atheroscler Thromb. 2009;16:674–683. doi: 10.5551/jat.1313. [DOI] [PubMed] [Google Scholar]
- 105.Rokling-Andersen MH, Rustan AC, Wensaas AJ, Kaalhus O, Wergedahl H, Rost TH, Jensen J, Graff BA, Caesar R, Drevon CA. Marine n-3 fatty acids promote size reduction of visceral adipose depots, without altering body weight and composition, in male Wistar rats fed a high-fat diet. Br J Nutr. 2009;102:995–1006. doi: 10.1017/S0007114509353210. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda I, Kumamaru J, Nakatani N, Sakono M, Murota I, Imaizumi K. Reduced hepatic triglyceride secretion in rats fed docosahexaenoic acid-rich fish oil suppresses postprandial hypertriglyceridemia. J Nutr. 2001;131:1159–1164. doi: 10.1093/jn/131.4.1159. [DOI] [PubMed] [Google Scholar]
- 107.Alexander Aguilera A, Hernandez Diaz G, Lara Barcelata M, Angulo Guerrero O, Oliart Ros RM. Induction of Cd36 expression elicited by fish oil PUFA in spontaneously hypertensive rats. J Nutr Biochem. 2006;17:760–765. doi: 10.1016/j.jnutbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Murphy MC, Brooks CN, Rockett JC, Chapman C, Lovegrove JA, Gould BJ, Wright JW, Williams CM. The quantitation of lipoprotein lipase mRNA in biopsies of human adipose tissue, using the polymerase chain reaction, and the effect of increased consumption of n-3 polyunsaturated fatty acids. Eur J Clin Nutr. 1999;53:441–447. doi: 10.1038/sj.ejcn.1600774. [DOI] [PubMed] [Google Scholar]
- 109.Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord. 1997;21:637–643. doi: 10.1038/sj.ijo.0800451. [DOI] [PubMed] [Google Scholar]
- 110.Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr. 1999;70:817–825. doi: 10.1093/ajcn/70.5.817. [DOI] [PubMed] [Google Scholar]
- 111.Kunesova M, Braunerova R, Hlavaty P, Tvrzicka E, Stankova B, Skrha J, Hilgertova J, Hill M, Kopecky J, Wagenknecht M, Hainer V, Matoulek M, Parizkova J, Zak A, Svacina S. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol Res. 2006;55:63–72. doi: 10.33549/physiolres.930770. [DOI] [PubMed] [Google Scholar]
- 112.Rustan AC, Hustvedt BE, Drevon CA. Dietary supplementation of very long-chain n-3 fatty acids decreases whole body lipid utilization in the rat. J Lipid Res. 1993;34:1299–1309. [PubMed] [Google Scholar]
- 113.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 114.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rahn Landstrom T, Mei J, Karlsson M, Manganiello V, Degerman E. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3-L1 adipocytes induced by tumour necrosis factor alpha and cAMP. Biochem J. 2000;346(Pt 2):337–343. doi: 10.1042/bj3460337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kawamata Y, Imamura T, Babendure JL, Lu JC, Yoshizaki T, Olefsky JM. Tumor necrosis factor receptor-1 can function through a G alpha q/11-beta-arrestin-1 signaling complex. J Biol Chem. 2007;282:28549–28556. doi: 10.1074/jbc.M705869200. [DOI] [PubMed] [Google Scholar]
- 118.Laurencikiene J, van Harmelen V, Arvidsson Nordstrom E, Dicker A, Blomqvist L, Naslund E, Langin D, Arner P, Ryden M. NF-kappaB is important for TNF-alpha-induced lipolysis in human adipocytes. J Lipid Res. 2007;48:1069–1077. doi: 10.1194/jlr.M600471-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg AP, Shuldiner AR, Fried SK, Gong DW. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morisset AS, Huot C, Legare D, Tchernof A. Circulating IL-6 concentrations and abdominal adipocyte isoproterenol-stimulated lipolysis in women. Obesity (Silver Spring) 2008;16:1487–1492. doi: 10.1038/oby.2008.242. [DOI] [PubMed] [Google Scholar]
- 121.Kawakami M, Murase T, Ogawa H, Ishibashi S, Mori N, Takaku F, Shibata S. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J Biochem. 1987;101:331–338. doi: 10.1093/oxfordjournals.jbchem.a121917. [DOI] [PubMed] [Google Scholar]
- 122.Halle M, Berg A, Northoff H, Keul J. Importance of TNF-alpha and leptin in obesity and insulin resistance: a hypothesis on the impact of physical exercise. Exerc Immunol Rev. 1998;4:77–94. [PubMed] [Google Scholar]
- 123.Lorente-Cebrian S, Bustos M, Marti A, Fernandez-Galilea M, Martinez JA, Moreno-Aliaga MJ. Eicosapentaenoic acid inhibits tumour necrosis factor-alpha-induced lipolysis in murine cultured adipocytes. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 124.Oster RT, Tishinsky JM, Yuan Z, Robinson LE. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARgamma mRNA, in 3T3-L1 adipocytes. Appl Physiol Nutr Metab. 2010;35:783–789. doi: 10.1139/H10-076. [DOI] [PubMed] [Google Scholar]
- 125.Perez-Matute P, Perez-Echarri N, Martinez JA, Marti A, Moreno-Aliaga MJ. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2007;97:389–398. doi: 10.1017/S0007114507207627. [DOI] [PubMed] [Google Scholar]
- 126.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 128.Herzberg GR, Rogerson M. The effect of dietary fish oil on muscle and adipose tissue lipoprotein lipase. Lipids. 1989;24:351–353. doi: 10.1007/BF02535176. [DOI] [PubMed] [Google Scholar]
- 129.Lam YY, Hatzinikolas G, Weir JM, Janovska A, McAinch AJ, Game P, Meikle PJ, Wittert GA. Insulin-stimulated glucose uptake and pathways regulating energy metabolism in skeletal muscle cells: The effects of subcutaneous and visceral fat, and long-chain saturated, n-3 and n-6 polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1811:468–475. doi: 10.1016/j.bbalip.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 130.Wanders D, Plaisance EP, Judd RL. Pharmacological effects of lipid-lowering drugs on circulating adipokines. World J Diabetes. 2010;1:116–128. doi: 10.4239/wjd.v1.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]


