Abstract
The metabolic syndrome epidemic, including a marked increase in the prevalence of obesity and gestational diabetes mellitus (GDM) among pregnant women, represents a significant public health problem. There is increasing recognition that the risk of adult obesity is clearly influenced by prenatal and infant environmental exposures, particularly nutrition. This tenet is the fundamental basis of developmental programming. Low birth weight, together with infant catch-up growth, is associated with a significant risk of adult obesity. Exposure to maternal obesity, with or without GDM, or having a high birth weight also represents an increased risk for childhood and adult obesity. Animal models have replicated human epidemiologic findings and elucidated potential programming mechanisms that include altered organ development, cellular signaling responses, and epigenetic modifications. Prenatal care has made great strides in optimizing maternal, fetal, and neonatal health, and now has the opportunity to begin interventions which prevent or reduce childhood/adult obesity. Guidelines that integrate optimal pregnancy nutrition and weight gain, management of GDM, and newborn feeding strategies with long-term consequences on adult obesity, remain to be elucidated.
Keywords: Maternal obesity, gestation diabetes mellitus, birth weight, catch-up growth, developmental origins of obesity, programmed adipogenesis
Introduction
It is difficult to overestimate the significance of the steadily developing epidemic of global obesity, the resultant pathologies that develop, and their collective impact on health, well-being, and quality of life. Obesity and its related diseases are the leading cause of death in western society. Currently, 65% of adults in the United States are overweight and more than one third are obese [1], representing a modern health crisis. Worse, epidemiologic data indicate that obesity continues to increase relentlessly, particularly among blacks and Hispanics. In parallel the rates of type 2 diabetes mellitus (DM) is increasing in the United States and worldwide [2]. Of concern to obstetricians, there is a marked and continuing increase in the prevalence of obesity and gestational DM (GDM) among pregnant women (~30%) [3,4], a factor associated with both high birth weight newborns and a known risk factor for childhood obesity [5,6]. As childhood obesity is a major risk factor for adult obesity [7], the 20% incidence of childhood obesity [8] portends a further increase in the prevalence of adult obesity and DM.
Obesity is often attributed to a Western style, high-fat diet combined with decreased activity levels. While there is little doubt that these factors are strong determinants of obesity, the long-term sustainability of dieting combined with exercise have largely proved unsuccessful. In recent years, there is compelling data from our laboratory and others which support the concept that origins of obesity begin in utero. As the developing fetus is dependent upon the maternal nutritional, hormonal and metabolic environments, any perturbation which “programs” organ structure, cellular composition, gene expression and/or the epigenome may ultimately alter metabolism and function. Importantly, interactions with the postnatal environment and neonatal growth further modulate susceptibility to obesity. This review focuses on the influence of prenatal/neonatal growth and adipogenesis in developmental origins of obesity.
Nutrition and Growth
Growth of tissues and organs during development involves proliferation, differentiation and migration of cells into organized structures. In humans, as in other mammalian species, the major part of the developmental process pertaining to cell division occurs during intrauterine life, emphasizing the need for optimal in utero environment. Unquestionably, therefore, nutrition is one of the cornerstones of growth, development and health. The merit of nutritional supplementation especially during pregnancy is obvious, as demonstrated with iodine and folate supplementation in preventing iodine deficiency-induced cretinism and spina bifida, respectively. The field of developmental origins of adult disease has incorporated this phenomenon and portends that sub-optimal maternal nutrition impacts fetal growth leading to adult diseases. In addition to nutritional influences, factors including GDM, maternal stress, preterm delivery and maternal glucocorticoid therapy, among others, may significantly impact adult health and disease. Evidence for the concept of programming health and disease is provided by both human studies and animal models that have used birth weight as a proximate measure for in utero growth and development.
Maternal Influence on Birth Weight
Beyond fetal genetic potential, maternal nutrition, oxygenation and placental perfusion have predominant effects on birth weight. Animal models using maternal nutrient restriction, placental uterine artery ligation or glucocorticoid exposure, have effectively replicated findings associated with low birth weight (LBW) [9-12]. Conversely, maternal overnutrition, resulting from obesity, high fat diet, or excess weight gain during pregnancy, has reported variable effects on birth weight. However, the adult offspring consistently exhibit obesity and metabolic abnormalities [13-15], evidence of in utero programming. Offspring of women with GDM are consistently larger than normal controls, with birth weight proportional to the mean glucose levels [16].
Whether the programming effects of GDM-associated macrosomia differ from that of maternal obesity alone, is unknown at present.
Association between Birth Weight and Obesity
Epidemiological studies and animal models link birth weight to risk of adult obesity and metabolic syndrome, including insulin resistance. Notably in humans, both low and high birth weights lead to increased risk for childhood and adult obesity, suggesting increased risk of obesity at both ends of the birth weight spectrum [17,18].
High Birth Weight
Obesity in pregnancy has not only adverse effects on maternal health and pregnancy outcome but also on the developing fetus. Specifically, maternal obesity before and during pregnancy, including increased weight gain in pregnancy, has been associated with higher birth weight [16,19] as well as lower birth weight newborns, the later a result in part of the increased risk of preterm delivery [20]. The 25–36% increase in maternal BMI over the last decade has translated to an approximately 25% increase in the incidence of high birth weight babies [21]. This is of particular importance, as high birth weight newborns show increased adipose tissue mass and an increased risk of obesity and diabetes risk in later life (review [22]). However, both human and animal studies indicate that increased maternal prepregnancy BMI and excessive maternal weight gain during pregnancy are greater predictors of offspring obesity than high newborn birthweight [5,23,24]. As the majority of GDM women have accompanying obesity, the independent programming effects of GDM are uncertain.
Low Birth weight
Early epidemiologic studies initially demonstrated that the LBW infants with rapid catch-up growth have higher risk of obesity and metabolic syndrome. The prevalence of metabolic syndrome increased progressively in both men and women, from those who had the highest to those who had the lowest birth weights. Of 64-year-old men whose birth weights were 6.5 pounds or less, 22% had metabolic syndrome. Those with the lowest birth weight were 10 times more likely to have metabolic syndrome as compared to those who were heaviest at birth [25,26]. The reduced rate of obesity amongst the heavier infants (born from 1935-1943), further suggests that maternal obesity and pregnancy diet/weight gain have greater effect on programming of offspring obesity than birthweight alone. Numerous epidemiologic studies from diverse populations confirm this relationship [17].
U-Shaped Curve
Epidemiologic studies confirm that the relationship between human birth weight and adult obesity, hypertension, and/or insulin resistance is a “U-shaped curve” [27-30]. Perhaps most importantly, the relation of fetal growth to offspring obesity and metabolic syndrome is a continuum [25], rather than a threshold response. There may well be an optimal newborn weight (potentially specific to an individual mother) at which the programming of obesity potential is minimized. However, within ranges of lower or higher birth weights in comparison to mean values, studies indicate a gradation of propensity to programming sequelae. Thus, deviations from “optimal” in utero growth, be it from limited or excess nutrition, increase the relative risk of adult metabolic syndrome (Figure 1).
Figure 1. Developmental Programming of Obesity.
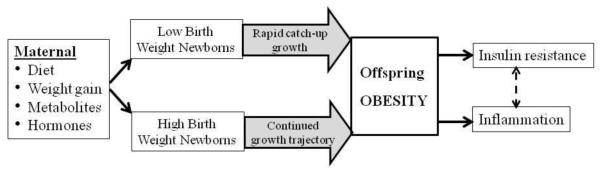
Altered maternal nutrition, hormonal or metabolite milieu impacts fetal growth resulting in low or high birth weight newborns. As a result of this growth deviation in utero combined with accelerated/similar postnatal growth causes enhanced adipogenesis, resulting in childhood and adult obesity. Obesity in turn leads to insulin resistance and inflammation.
Additive Risk of Postnatal Catch-up Growth
Although the long term effects of LBW are linked to adult obesity, several studies have demonstrated detrimental effects of newborn or childhood catch-up growth among the LBW infants (Figure 1). Those infants that are born small, and remain small exhibit a lower risk of obesity and metabolic syndrome, then those born small who catch up and exceed normal weights through infancy or early adolescence [31,32]. Importantly, LBW or preterm infants with catch-up growth during early life have less lean body mass and higher body fat that shows predominant abdominal distribution [33,34]. A similar phenomenon is seen in normal birth weight newborns that exhibit accelerated weight gain in first two years of life [35].
These findings have been successfully replicated in animal models using prenatal nutrient restriction to produce LBW newborns, followed by normal nursing to promote catch-up growth. As adults, the LBW offspring not only have higher body weights and body fat [36-39] but show greater susceptibility to high fat diets [40]. Conversely, prevention of catch-up growth in LBW newborns prevents an obese adult phenotype [37]. These results suggest that the degree of newborn nutrient enhancement and timing of newborn catch-up growth may determine the programming of offspring obesity [37,41]. A fundamental question that arises is what mechanism regulates preferential catch-up of fat [42] in these offspring. Again, animal models have provided initial insight that prenatal factors result in programming of hyperphagia, reduced energy expenditure and/or enhanced adipogenesis, which result in a propensity for fat accrual in the offspring [43-46].
Catch-up Growth and Fat Accrual
Adipocytes are highly specialized cells that maintain whole body energy homeostasis by regulating glucose and lipid metabolism [47]. More recently, adipocytes are recognized for their role in inflammation and immune response [48]. Adipose tissue contains functionally distinct cellular subtypes with white adipocytes serving as energy storage depots whereas brown adipocytes dissipate energy through thermogenesis. Fat storage is facilitated by insulin which stimulates adipocyte glucose uptake and lipogenesis. Alteration in either adipose tissue mass, increased circulating free fatty acids, and/or fuel partitioning into adipocytes may result in dyslipidemia, obesity, insulin resistance and DM.
Increase in fat mass or adipogenesis occurs primarily during the prenatal and postnatal development, though some adipogenesis continues throughout adulthood [49]. The process of adipogenesis involves differentiation of preadipocytes to mature adipocytes that can store fat. The differentiation pathway is tightly regulated by a cascade of transcription factors that are salient within the preadipocytes and are sequentially expressed in response to stimuli (nutrient, hormones) probably under the influence of epigenetic mechanism (Figure 2). Obesogens, including environmental factors, have the potential to alter key adipogenic pathways, including adipogenic transcription factors, via epigenetic modifications of promoters or histones [50].
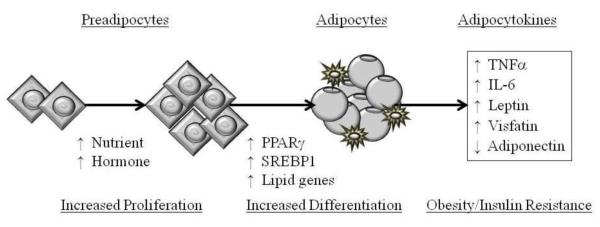
Figure 2. Increased Adipogenesis Mediated Diabetes Mellitus.
Adipogenesis is a process of cell differentiation by which preadipocytes become adipocytes. Increased nutrient supply or elevated hormonal levels (e.g., insulin, corticosterone, IGF1) stimulate cell proliferation and differentiation. Induction of adipocyte differentiation is facilitated by adipogenic transcription factor (PPARg) and fat storage by lipogenic transcription factor (SREBP1). Increased adipogenesis is associated with increased macrophage infiltration and increased secretion of pro-diabetic (TNFα, IL-6, leptin, visfatin) with decreased secretion of anti-diabetic (adiponectin) adipocytokines.
Regulation of Adipogenesis and Lipogenesis
The induction of adipocyte differentiation is driven by transcription factors PPAR (peroxisome proliferator-activated receptor) and C/EBP (CCAAT-enhancer-binding proteins) [51-53]. Of these, the principal adipogenic transcription factor, PPAR 2γ induces lipogenic transcription factor SREBP1 (sterol regulatory element binding protein) thereby initiating both adipocyte differentiation and lipogenesis [54-56]. SREBP1 can also activate PPARγ, by both stimulating the production of an endogenous ligand [57], as well as by inducing PPAR promoter activity [54,57]. These data are suggestive of a feed-forward mechanism, in which PPAR activates SREBP1 and vice-versa, and which is aimed at promoting adipogenesis and lipogenesis [55]. SREBP1 facilitates lipogenesis by induction of extracellular lipolytic enzyme (lipoprotein lipase) and lipogenic enzyme (fatty acid synthase) that in turn, lead to an increase in fatty acid uptake and synthesis, promoting lipid accumulation within the adipocyte [58,59]. The release of free fatty acid from adipocytes is facilitated by an intracellular lipolytic enzyme, hormone-sensitive lipase [60].
Synthesis of fatty acids (via de novo lipogenesis) and triglycerides are important factors in fat accumulation. Triglycerides destined for fat storage in adipose tissue are composed of fatty acids from dietary sources and from de novo synthesis. De novo synthesized fatty acids can undergo modification through creation of double bonds via desaturation, and/or further lengthening via chain elongation. While de novo synthesis and chain elongation promote energy storage, breakdown of fatty acids by chain shortening and β-oxidation promote energy release. Since triglycerides become incorporated into adipose tissue for storage, an increase in the monounsaturated to saturated fatty acid ratio, therefore, increases propensity for fat storage [61].
Perturbation of the metabolic network may shift the energy balance toward increased energy release, or, as in obesity, increased energy storage. Animal studies provide some insight into underlying mechanistic basis for programmed enhanced adipogenesis/lipogenesis or alteration in function/response of adipocytes.
Effects of Increased Adipogenesis on DM in offspring
Increased fat accumulation, especially visceral fat, has been shown to cause impaired glucose and lipid metabolism, leading to insulin resistance and DM [62]. The underlying mechanistic basis involves perturbation in the production of adipose-derived ‘adipocytokines’ that modulate insulin sensitivity. In the obese state, adipose tissue secretes proportionally more adipokines that cause insulin resistance (e.g., TNFα, IL-6, leptin) and fewer that promote insulin sensitivity( e.g., adiponectin) [63-65]. Indeed, numerous human studies have confirmed that increased plasma TNFα, IL-6 and leptin, and decreased plasma adiponectin levels are associated with obesity/insulin resistance [66-69] This relationship has recently been demonstrated in childhood obesity, suggesting that adipocytokines may serve as early markers of development of DM [68,70].
LBW Offspring
LBW rat newborns, either as a result of maternal nutrient restriction in pregnancy or uteroplacental insufficiency, which demonstrate subsequent postnatal catch-up growth exhibit an altered adipocyte phenotype and function. Early studies of offspring exposed to maternal protein restriction during pregnancy show that obese adult offspring have increased expression of insulin receptor, hypertrophic adipocytes and upregulation of genes involved in adipocyte differentiation [71-73]. Recent studies, including those from our laboratory, have specifically investigated proximate mechanisms that predispose LBW offspring to fat accrual. These include demonstration of altered adipocyte gene expression, morphologic variations, and differential response to modulators at birth and at end of nursing period (prior to onset of obesity). For example, growth restricted LBW newborns have an upregulated adipogenic signaling cascade, specifically increased adipose tissue expression of PPAR [44,74] with increased de novo fatty acid synthesis [75]. Ex-vivo cultures indicate that newborns have higher rates of preadipocyte proliferation at birth [76], and early induction of adipocyte differentiation together with increased PPAR expression [77]. At the end of the nursing period, LBW rats exhibit elevated plasma leptin levels, hypertrophic adipocytes and increased expression of PPARγ, SREBP1 and lipid enzymes that influence adipocyte lipid synthesis, storage and release. Cell culture studies indicate a continued higher preadipocyte proliferation rate [78] with higher de novo fatty acid synthesis, greater glucose utilization for fatty acid synthesis and lipid accumulation in adipocytes of LBW offspring [37,38,44,74,79-81]. Collectively, these findings indicate increased susceptibility to retain fat in adipocytes of LBW offspring, and thus an increased propensity for adiposity. Furthermore, increased lipid accumulation is likely to alter adipocyte endocrine function with resultant impact on insulin sensitivity and inflammation.
As these changes are evident early in life, it suggests a programmed pathway of increased adipocyte differentiation and lipogenesis which likely promotes the development of obesity and DM in LBW offspring (Figure 2).
Maternal Obesity/High Fat Diet Offspring
Programming of adipose tissue as a result of in utero overnutrition likely involves an interplay of effects: preexisting maternal obesity, maternal weight gain during pregnancy, high fat Western diet, and varying degrees of maternal glucose intolerance. Adipogenesis programming may occur in the presence of absence of increased newborn birth weight. Limited mechanistic studies on programmed adipogenesis due to maternal obesity or high fat diet show remarkably similar phenotype as LBW offspring. This includes increased expression of PPAR in fetal and newborn adipose tissue [82,83] as well as increased expression of enzymes mediating fatty acid biosynthesis [84].
Clinical Implications and Conclusions
A major public health challenge in the 21st century is to devise an effective policy and practice to combat the epidemic of obesity across all spectrums of age groups. Prevention of childhood obesity remains a high priority for many health professionals. There is irrefutable evidence that departures from optimal growth in utero, whether from limited or excess nutrition, increase the relative risk of adult obesity and metabolic syndrome. This predisposition is especially paramount within a postnatal environment that facilitates neonatal catch-up growth as well as access to energy-intense childhood and adult diets. Collectively, these findings have great significance for neonatal and childhood care. For example, a major goal of treatment for premature, LBW newborn infants is the achievement of a weight satisfactory for hospital discharge. Contrary to existing practice, it may be advisable to limit the rapid weight gain in the neonatal period. Fortunately, the recent enthusiasm for exclusive breastfeeding may provide one approach to prevention of offspring obesity [85] and the accompanying insulin resistance, perhaps due to favorable nutrient and hormone composition and the natural limitation which avoids excessive feeding. Although macro- and micronutrient guidelines for nutrition in pregnancy continue to evolve, there is critical need for additional research as to how these guidelines may influence offspring long-term sequelae, particularly among obese or gestationally diabetic pregnant women.
Acknowledgments
Our work reported is supported by the National Institutes of Health Grants R01DK081756 and R01HD054751.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012 Jan;82:1–8. [PubMed] [Google Scholar]
- 2.Shamseddeen H, Getty JZ, Hamdallah IN, Ali MR. Epidemiology and economic impact of obesity and type 2 diabetes. Surg Clin North Am. 2011 Dec;916:1163–72. vii. doi: 10.1016/j.suc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Nodine PM, Hastings-Tolsma M. Maternal obesity: improving pregnancy outcomes. MCN Am J Matern Child Nurs. 2012 Mar;372:110–115. doi: 10.1097/NMC.0b013e3182430296. [DOI] [PubMed] [Google Scholar]
- 4.Evensen AE. Update on gestational diabetes mellitus. Prim Care. 2012 Mar;391:83–94. doi: 10.1016/j.pop.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 5*.Laitinen J, Jaaskelainen A, Hartikainen AL, Sovio U, et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG. 2012 May;1196:716–723. doi: 10.1111/j.1471-0528.2012.03319.x. [DOI] [PubMed] [Google Scholar]
- 6.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983 Feb;3085:242–245. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- 7.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002 Sep;763:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 8.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998 Mar;1013(Pt 2):518–525. [PubMed] [Google Scholar]
- 9.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005 Apr;852:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 10.Hales CN, Desai M, Ozanne SE. The Thrifty Phenotype hypothesis: how does it look after 5 years? Diabet Med. 1997 Mar;143:189–195. doi: 10.1002/(SICI)1096-9136(199703)14:3<189::AID-DIA325>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biol Rev Camb Philos Soc. 1997 May;722:329–348. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- 12.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004 Dec;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 13.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009 Feb;587(Pt 4):905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferezou-Viala J, Roy AF, Serougne C, Gripois D, et al. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007 Sep;2933:R1056–R1062. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- 15.Samuelsson AM, Matthews PA, Argenton M, Christie MR, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008 Feb;512:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 16.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005 Mar;1153:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman PD, Hanson MA, Morton SM, Pinal CS. Life-long echoes--a critical analysis of the developmental origins of adult disease model. Biol Neonate. 2005;872:127–139. doi: 10.1159/000082311. [DOI] [PubMed] [Google Scholar]
- 18.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001 Feb;301:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 19.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008 Nov;1125:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djelantik AA, Kunst AE, van der Wal MF, Smit HA, Vrijkotte TG. Contribution of overweight and obesity to the occurrence of adverse pregnancy outcomes in a multi-ethnic cohort: population attributive fractions for Amsterdam. BJOG. 2012 Feb;1193:283–290. doi: 10.1111/j.1471-0528.2011.03205.x. [DOI] [PubMed] [Google Scholar]
- 21.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004 Oct;1044:720–726. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- 22.Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- 23.Hull HR, Thornton JC, Ji Y, Paley C, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011 Sep;2053:211–217. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 2010 Jan;341:67–74. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ, Hales CN, Fall CH, Osmond C, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993 Jan;361:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 26.Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci. 2006 Dec;1092:138–147. doi: 10.1196/annals.1365.012. [DOI] [PubMed] [Google Scholar]
- 27.Pettitt DJ, Jovanovic L. Birth weight as a predictor of type 2 diabetes mellitus: the U-shaped curve. Curr Diab Rep. 2001 Aug;11:78–81. doi: 10.1007/s11892-001-0014-x. [DOI] [PubMed] [Google Scholar]
- 28.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65(Suppl 3):65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 29.Pettitt DJ, Jovanovic L. Low birth weight as a risk factor for gestational diabetes, diabetes, and impaired glucose tolerance during pregnancy. Diabetes Care. 2007 Jul;30(Suppl 2):S147–S149. doi: 10.2337/dc07-s207. [DOI] [PubMed] [Google Scholar]
- 30.Launer LJ, Hofman A, Grobbee DE. Relation between birth weight and blood pressure: longitudinal study of infants and children. BMJ. 1993 Dec;3076917:1451–1454. doi: 10.1136/bmj.307.6917.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005 May;62:143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 32.Baird J, Fisher D, Lucas P, Kleijnen J, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005 Oct;3317522:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, et al. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr. 2005 Feb;812:480–487. doi: 10.1093/ajcn.81.2.480. [DOI] [PubMed] [Google Scholar]
- 34.Finken MJ, Keijzer-Veen MG, Dekker FW, Frolich M, et al. Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia. 2006 Mar;493:478–485. doi: 10.1007/s00125-005-0118-y. [DOI] [PubMed] [Google Scholar]
- 35.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000 Apr;3207240:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996 Oct;764:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- 37.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005 Jan;2881:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 38.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinol. 2007 Mar;1483:1350–1358. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- 39.Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J Anim Sci. 1998 Sep;769:2354–2367. doi: 10.2527/1998.7692354x. [DOI] [PubMed] [Google Scholar]
- 40.Desai M, Babu J, Ross MG. Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007 Dec;2936:R2306–R2314. doi: 10.1152/ajpregu.00783.2006. [DOI] [PubMed] [Google Scholar]
- 41.Desai M, Gayle D, Babu J, Ross MG. Permanent reduction in heart and kidney organ growth in offspring of undernourished rat dams. Am J Obstet Gynecol. 2005 Sep;1933(Suppl):1224–1232. doi: 10.1016/j.ajog.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 42.Summermatter S, Marcelino H, Arsenijevic D, Buchala A, et al. Adipose tissue plasticity during catch-up fat driven by thrifty metabolism: relevance for muscle-adipose glucose redistribution during catch-up growth. Diabetes. 2009 Oct;5810:2228–2237. doi: 10.2337/db08-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007 May;144:329–337. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- 44.Desai M, Guang H, Ferelli M, Kallichanda N, Lane RH. Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod Sci. 2008 Oct;158:785–796. doi: 10.1177/1933719108318597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orozco-Solis R, Matos RJ, Lopes de SS, Grit I, et al. Perinatal nutrient restriction induces long-lasting alterations in the circadian expression pattern of genes regulating food intake and energy metabolism. Int J Obes (Lond) 2011 Jul;357:990–1000. doi: 10.1038/ijo.2010.223. [DOI] [PubMed] [Google Scholar]
- 46.Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (Lond) 2006 Dec;30(Suppl 4):S23–S35. doi: 10.1038/sj.ijo.0803516. [DOI] [PubMed] [Google Scholar]
- 47.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001 Feb;1044:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 48.Saltiel AR. You are what you secrete. Nat Med. 2001 Aug;78:887–888. doi: 10.1038/90911. [DOI] [PubMed] [Google Scholar]
- 49.Wabitsch M. The acquisition of obesity: insights from cellular and genetic research. Proc Nutr Soc. 2000 May;592:325–330. doi: 10.1017/s0029665100000367. [DOI] [PubMed] [Google Scholar]
- 50.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int J Androl. 2012 Jun;353:437–448. doi: 10.1111/j.1365-2605.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001 Oct;27641:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 52.Morrison RF, Farmer SR. Insights into the transcriptional control of adipocyte differentiation. J Cell Biochem. 1999;(Suppl 32-33):59–67. doi: 10.1002/(sici)1097-4644(1999)75:32+<59::aid-jcb8>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005 Mar;29(Suppl 1):S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 54.Fajas L, Schoonjans K, Gelman L, Kim JB, et al. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999 Aug;198:5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes dipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996 May;109:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 56.Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARgamma in the control of lipid metabolism. J Lipid Res. 2002 Feb;432:177–186. [PubMed] [Google Scholar]
- 57.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998 Apr;958:4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JB, Sarraf P, Wright M, Yao KM, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998 Jan;1011:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boizard M, Le L,X, Lemarchand P, Foufelle F, et al. Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem. 1998 Oct;27344:29164–29171. doi: 10.1074/jbc.273.44.29164. [DOI] [PubMed] [Google Scholar]
- 60.Samra JS. Sir David Cuthbertson Medal Lecture. Regulation of lipid metabolism in adipose tissue. Proc Nutr Soc. 2000 Aug;593:441–446. doi: 10.1017/s0029665100000604. [DOI] [PubMed] [Google Scholar]
- 61.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005 Oct;24:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes (Lond) 2008 Dec;32(Suppl 7):S83–S92. doi: 10.1038/ijo.2008.243. [DOI] [PubMed] [Google Scholar]
- 63.Matsuzawa Y. Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006 Jan;31:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 64**.Desai M, Ross MG. Fetal programming of adipose tissue: effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin Reprod Med. 2011 May;293:237–245. doi: 10.1055/s-0031-1275517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;1518:1851–1862. doi: 10.2174/092986708785133004. [DOI] [PubMed] [Google Scholar]
- 66.Widjaja A, Stratton IM, Horn R, Holman RR, et al. UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J Clin Endocrinol Metab. 1997 Feb;822:654–657. doi: 10.1210/jcem.82.2.3744. [DOI] [PubMed] [Google Scholar]
- 67.Hauner H, Bender M, Haastert B, Hube F. Plasma concentrations of soluble TNF-alpha receptors in obese subjects. Int J Obes Relat Metab Disord. 1998 Dec;2212:1239–1243. doi: 10.1038/sj.ijo.0800773. [DOI] [PubMed] [Google Scholar]
- 68.Yan WJ, Wu J, Mo J, Huang CW, et al. [Plasma levels of adiponectin and tumor necrosis factor-alpha in children with obesity] Zhongguo Dang Dai Er Ke Za Zhi. 2009 Jan;111:47–50. [PubMed] [Google Scholar]
- 69.Haluzik M, Parizkova J, Haluzik MM. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res. 2004;532:123–129. [PubMed] [Google Scholar]
- 70.Weiss R, Dziura J, Burgert TS, Tamborlane WV, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004 Jun;35023:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 71.Shepherd PR, Crowther NJ, Desai M, Hales CN, Ozanne SE. Altered adipocyte properties in the offspring of protein malnourished rats. Br J Nutr. 1997 Jul;781:121–129. doi: 10.1079/bjn19970124. [DOI] [PubMed] [Google Scholar]
- 72.Bieswal F, Ahn MT, Reusens B, Holvoet P, et al. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 2006 Aug;148:1330–1343. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- 73.Guan H, Arany E, van Beek JP, Chamson-Reig A, et al. Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab. 2005 Apr;2884:E663–E673. doi: 10.1152/ajpendo.00461.2004. [DOI] [PubMed] [Google Scholar]
- 74.Joss-Moore LA, Wang Y, Campbell MS, Moore B, et al. Uteroplacental insufficiency increases visceral adiposity and visceral adipose PPARgamma2 expression in male rat offspring prior to the onset of obesity. Early Hum Dev. 2010 Mar;863:179–185. doi: 10.1016/j.earlhumdev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yee JK, Lee WN, Han G, Ross MG, Desai M. Organ-specific alterations in Fatty Acid de novo synthesis and desaturation in a rat model of programmed obesity. Lipids Health Dis. 2011;10:72. doi: 10.1186/1476-511X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desai M, Magee TR, Han G, Ross MG. Insulin resistant adipocyte mediated mechanism for programmed obesity in intrauterine growth restricted newborns. Reprod Sci. 2009;16(Suppl):87A. [Google Scholar]
- 77.Desai M, Lane RH, Han G, Magee TR, Ross MG. Epigenetic mediated early induction of adipcyte differentiation contributes to programmed obesity in intrauterine growth restricted newborns. Reprod Sci. 2012;19(Suppl):157A. [Google Scholar]
- 78.Bol VV, Reusens BM, Remacle CA. Postnatal catch-up growth after fetal protein restriction programs proliferation of rat preadipocytes. Obesity (Silver Spring) 2008 Dec;1612:2760–2763. doi: 10.1038/oby.2008.417. [DOI] [PubMed] [Google Scholar]
- 79*.Yee JK, Lee PW, Ross MG, Desai M. Enhancement of de novo fatty acid synthesis in IUGR adipose tissue prior to onset of programmed obesity. Reprod Sci Suppl. 2010;17:104A–137. [Google Scholar]
- 80.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007 Jun;1966:555–557. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yee JK, Lee WN, Ross MG, Lane RH, et al. Peroxisome proliferator-activated receptor gamma modulation and lipogenic response in adipocytes of small-for-gestational age offspring. Nutr Metab (Lond) 2012 Jun;91:62. doi: 10.1186/1743-7075-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, Rattanatray L, McMillen IC, Suter CM, Morrison JL. Periconceptional nutrition and the early programming of a life of obesity or adversity. Prog Biophys Mol Biol. 2011 Jul;1061:307–314. doi: 10.1016/j.pbiomolbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Desai M, Han G, Li T, Ross MG. Transcriptional regulation of adipogenesis in newborns exposed to maternal obesity. Reprod Sci Suppl. 2010;17:217A–532. [Google Scholar]
- 84.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci. 2012 Jan; doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]
- 85.Dewey KG. Is breastfeeding protective against child obesity? J Hum Lact. 2003 Feb;191:9–18. doi: 10.1177/0890334402239730. [DOI] [PubMed] [Google Scholar]