Abstract
Aims
Atypical (WHO grade II) meningiomas have moderately high recurrence rates; even for completely resected tumours, approximately one-third will recur. Postoperative radiotherapy (RT) may aid local control and improve survival, but carries the risk of side effects. More accurate prediction of recurrence risk is therefore needed for patients with atypical meningioma. Previously, we used high-resolution array CGH to identify genetic variations in 47 primary atypical meningiomas and found that approximately 60% of tumors show gain of 1q at 1q25.1 and 1q25.3 to 1q32.1 and that 1q gain appeared to correlate with shorter progression-free survival. This study aimed to validate and extend these findings in an independent sample.
Methods
86 completely resected atypical meningiomas (with 25 recurrences) from two neurosurgical centres in Ireland were identified and clinical follow up was obtained. Utilizing a dual-colour interphase FISH assay, 1q gain was assessed using BAC probes directed against 1q25.1 and 1q32.1.
Results
The results confirm the high prevalence of 1q gain at these loci in atypical meningiomas. We further show that gain at 1q32.1 and age each correlate with progression-free survival in patients who have undergone complete surgical resection of atypical meningiomas.
Conclusions
These independent findings suggest that assessment of 1q copy number status can add clinically useful information for the management of patients with atypical meningiomas.
Keywords: meningioma, atypical, pathology, classification, genetics
Introduction
Meningiomas collectively constitute nearly 34% of all central nervous system tumours in the United States, with annual incidence between four and six per 10, 000[1, 2]. Although a spectrum of behaviour exists for these tumours, the major factor governing prognosis is completeness of resection. While the majority of meningiomas are indolent tumours (World Health Organization grade I) and can be cured by complete resection, more aggressive behaviour is noted in a smaller proportion comprising grade II (atypical) and grade III (anaplastic) meningiomas[3–5]. Such higher grade tumours are associated with increased local recurrence and subsequent excess risk of death, even when completely resected[6]. Approximately 40% of atypical meningiomas recur within 5 years of gross total resection[7]. The relatively high likelihood of recurrence has led to the use of radiation therapy for patients with atypical meningioma. In most centres, radiation is given to patients who have residual tumour after initial surgery[8, 9], but there is debate as to the best treatment for patients who have undergone complete resection of their atypical meningiomas[10]. Given the latter debate, prognostic biomarkers that can estimate the risk of recurrence for patients would help in making treatment decisions, particularly for those patients who have undergone complete resection.
Much is now known about the genetic aberrations common in meningiomas, although few thus far have proven to be of clear prognostic value. Increasing numbers of chromosomal aberrations, in particular loss of 1p, 6q, 9p, 10, 14q and 18q and gain of 1q, 9q, 12q, 15q, 17q and 20q are associated with meningiomas of progressively higher histological grade[11–13]. Progressive chromosomal aberrations occur in a non-random pattern, and 1p and 14q loss have been proposed as potential markers of tumour recurrence[14, 15]. We recently performed an aCGH study on a series of clinically annotated atypical meningiomas and demonstrated that gain at 1q occurred at a higher rate than previously shown. Gains occurred at two common regions: 1q25.1 and 1q25.1-q32.1[16]. In addition, analysis of the frequently identified copy number aberrations showed a significant association between 1q gain and shorter progression-free survival[16].
In the present study, we sought to evaluate the potentially important association between 1q gain and time to recurrence in an independent series of atypical meningiomas managed in the two neurosurgical centres in Ireland. This series provided a larger set of patients who were all followed carefully and long-term in two centres. Importantly, all tumours included in this series had been reportedly completely resected, thus allowing us to determine if 1q gain could provide useful information in this challenging clinical management situation.
Materials and Methods
Tumours
One hundred cases of atypical meningioma with at least five years of follow up from initial resection were retrospectively identified from tumour databases and histology reports from the two national Irish neurosurgical centres in Cork (Cork University Hospital) and Dublin (Beaumont Hospital). For those cases in Cork and Dublin that were pathologically graded prior to the implementation of the WHO Classification 2000 criteria for atypical meningioma, records were hand searched, and cases in which the terms “malignant”, “high grade”, “necrosis” and “mitotic activity” were used as descriptors in the report were selected, reviewed and regraded by a neuropathologist (MJ). None of the cases retrieved were known to have neurofibromatosis or multiple meningiomas. Approval for the use of these archival tumours for this study was obtained from the Cork Research Ethics Committee.
For each case, medical charts were reviewed to determine extent of resection, further therapy and recurrence. In most cases, a formal Simpson grade was not assigned or not recorded in the operative note, instead the note contained a comment on completeness of resection, which was the parameter recorded for the study. Neurosurgical teams initially followed patients for a variable number of years post surgery with interval scans as deemed appropriate. After discharge to primary care; patients who re-presented with symptoms were investigated and returned to the same centre for further management.
Overall survival was ascertained from patient records, by obtaining the date of death from a publicly accessible death notification service website (www.rip.ie) or through telephonic contact with the patient’s primary care practitioner. If follow-up could not be obtained, the case was excluded from further analysis.
Fluorescence in situ hybridization (FISH)
All studies were performed on formalin-fixed, paraffin-embedded tissue. Representative slides and corresponding tissue blocks were selected by one neuropathologist (MJ) and a region suitable for hybridization was marked. FISH was performed to ascertain copy number at 1q25.1 and 1q32.1. Dual colour FISH assays were done as follows on 5-μm sections: the first slide used Bacterial Artificial Chromosome (BAC) probes RP11-203F10 (chromosome 1q32.1, labeled red) and RP11-496O2 (chromosome 13q12, labeled green); the second assay used probes RP-11-299P5 (chromosome 1q25.1, labeled red) and RP11-845I20 (chromosome 1p21.1, labeled green). (Prior to assays being performed, the chromosomal location of each probe was confirmed using metaphase FISH). Control probes at 13q12 and 1p21.1 were selected based on their known lack of aberration in meningioma. Slides were viewed under oil using an Olympus BX40 microscope with fluorescent light source under red, green and blue filters. Hybridization was considered successful if at least 80% of the selected area showed a relatively clean signal. Signal quantitation of 100 non-overlapping nuclei was used to derive a 1q32.1/13q12 ratio, a 1q25.2/13q12 ratio and a 1p21.1/13q12 ratio, via manual counting of the total number of signals. A 1q32.1/13q12 ratio (or 1q25.2/13q12 ratio) of >1.2 was designated as gain.
Statistical analysis
Univariate Cox proportional hazards models were fit for time to progression or death; we considered age (continuous and dichotomized at 55 years), gender and copy number ratios at the three loci of interest. We considered both the continuous ratios, as well as the dichotomized gain/loss assessments. We estimated the distributions of time to progression or death and time to death using the Kaplan-Meier method. To assess the added discriminatory value of copy number changes at the two loci of interest, we calculated the C-index, with adjustment for censoring[17]. Comparisons of C-indices among models capture the net improvement in discrimination by one model versus the other. The C-index can be interpreted as the probability that the rank ordering of marker values of two subjects is consistent with the ordering of their times to progression or death.
Results
Clinicopathological characteristics and correlations with outcome
Of the originally identified 100 cases, follow-up was not available for two patients, nine cases had subtotal resection and three cases were subsequently found to have a history of whole brain radiotherapy administered for acute lymphoblastic leukemia and were excluded. Complete follow up was available for 82 patients and partial follow up for a further four. The study therefore comprised 86 patients (38 males and 48 females) with atypical meningioma. The cases originated from all over Ireland, reflecting the concentration of neurosurgical services in the two centres. The average age at first resection was 57 years and the median age at first resection was 58.5 years (range 11.1 to 83). Twenty-four patients (28%) had brain invasion and two tumours invaded bone. Three patients died within the first month following surgery, presumably of complications related to resection. Among the 83 subjects who were followed for progression, twenty-five patients (30%) had recurrent tumours, of whom eight were still alive at the time of the study. Among those patients who had recurrences, the mean time to first tumour recurrence was 54.5 months and the median time to recurrence was 42 months (range 11 to 244 months). The median progression-free survival time was 103 months (95% CI: 56, 245) and the median overall survival time was 215 months (95% CI: 116, 262). Of the 25 patients who had recurrent tumours, 11 were re-operated. The remaining patients were referred for stereotactic radiotherapy or external beam radiation therapy.
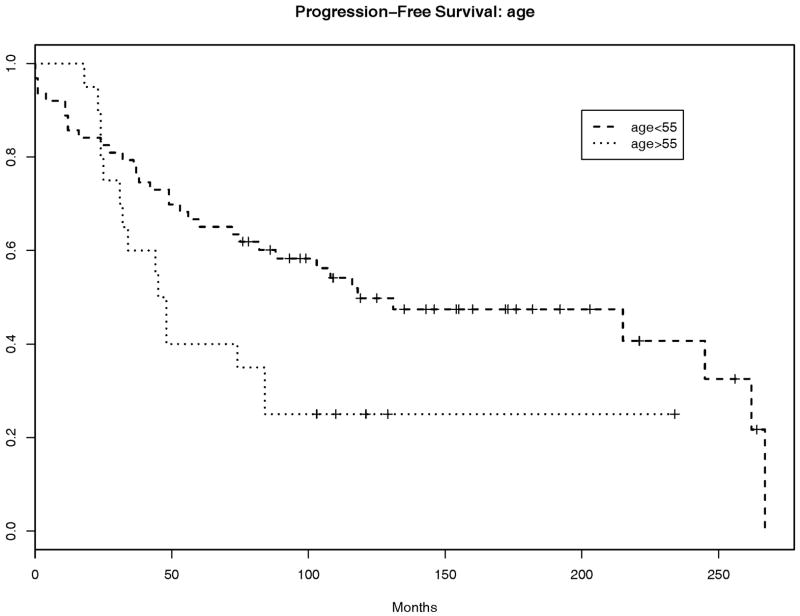
Thirty-nine patients (45%) remained alive at the time of the study. Patients older than 55 years had a higher hazard for progression or death than those younger than 55 (hazard ratio = 2.4, p=0.005, 95% CI: 1.31, 4.37). Gender was not significantly associated with progression-free survival (hazard ratio for males versus females=1.42, p=0.23, 95% CI: 0.81, 2.49). Bone invasion was documented in only two cases, and therefore could not be evaluated as a clinicopathological factor relating to progression-free survival. Brain invasion was not significantly associated with progression-free survival (p=0.62, hazard ratio=1.17).
FISH analysis and correlations with outcome
FISH analysis of the 86 atypical meningiomas revealed 1q32.1 gain in 21 tumours (24.4%) and 1q25.1 gain in 49 tumours (56.9%). Both 1q32.1 gain and 1q25.1 gain were present in 15 tumours (17.4%). 1q gain was thus present in a total of 55 of 86 (63.9%) of tumours in this series. Fourteen tumours showed intratumoral variability in the number of 1q25.1 signals ranging from three to five signals per nucleus, with a higher 1q25.1/13q12 ratio ranging from 1.6 to 2. This phenomenon was also noted when quantitating the 1q32.1 signals and 1q32.1/13q12 ratios in four atypical meningiomas, including three of the 14 tumours showing gain at both 1q loci. A representative image is shown in Figure 1.
Figure 1.
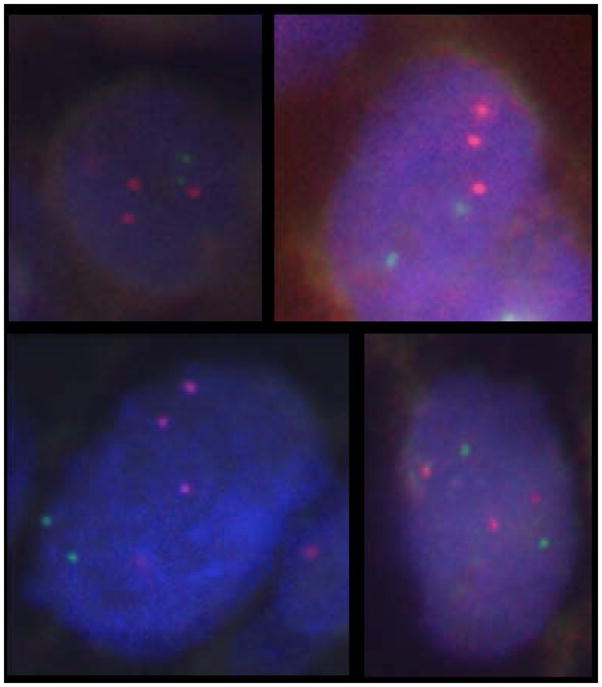
Representative examples of 1q32.1 gain by FISH on interphase nuclei. Note two green signals from probes to chromosome 13q12, but three red signals from probes to 1q32.1 [red signal = BAC probe RP11-203F10 (chromosome 1q32.1); green signal = BAC probe RP11-496o2 (chromosome 13q12)].
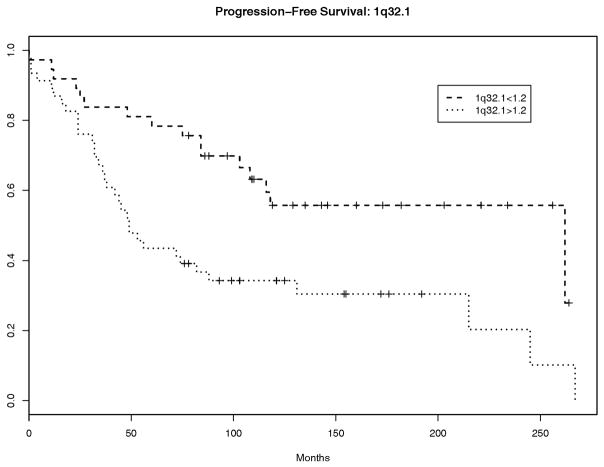
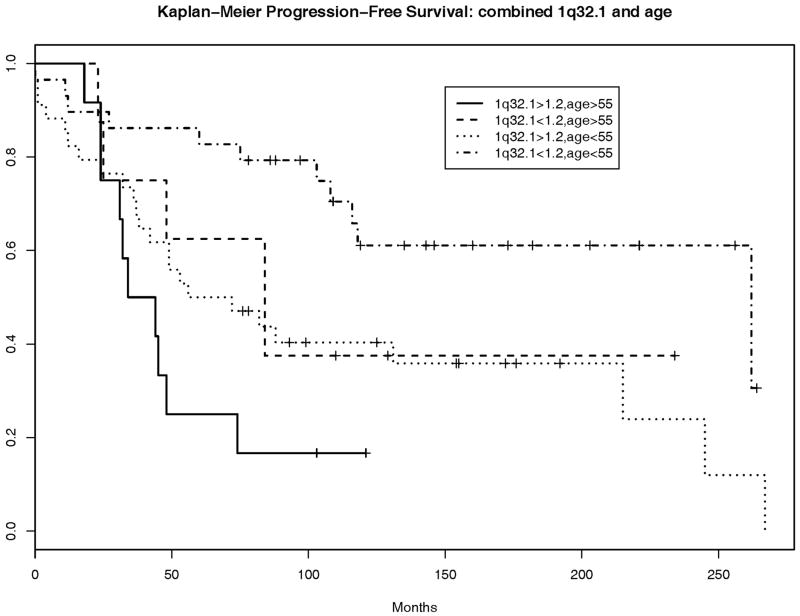
Neither gain at 1q32.1 nor gain at 1q25.1 provided improved discrimination for progression-free survival, e.g., addition of gain at 1q32.1 to age provides a net improvement in discrimination of only 0.03 and yields a C-index of 0.63 (95% CI: 0.5, 0.76) versus a C-index of 0.6 (95% CI: 0.45, 0.75) for the model including age alone However, discrimination does not reflect the capacity for risk prediction as it is based on relative orderings of event times and not on the actual times. In fact, gain of 1.2 or greater at 1q32.1 was associated with an increased hazard for progression or death (hazard ratio=1.94, p=0.037, 95% CI: 1.04, 3.60). In a multivariate model with age and 1q32.1, both variables remained significantly associated with progression-free survival: adjusted hazard ratios are 1.93 (p=0.039, 95% CI: 1.03, 3.61) for 1q32.1 ratio>1.2 and 2.39 (p=0.005, 95% CI: 1.31, 4.37) for age>55. The non-parametric Kaplan-Meier survival curves for patients according to their combined 1q32.1 and age status (not based on the Cox proportional hazards model) and those based on age alone are depicted in Figure 2. The additional information about 1q32.1 separates the survival curves beyond their separation due to age alone, though the sample sizes are small. Interestingly, 1q32.1 gain<1.2 and age<55 appear to be associated with improved predicted survival of 10–20% at each time point relative to that associated with age<55 alone. We note that the separation in these curves may be slightly optimistic, as we selected 1q32.1 and age because of their significant univariate associations with PFS
Figure 2.
Atypical meningiomas occurring at age > 55years (A) and atypical meningiomas showing gain at 1q32.1 (B) have worse progression-free survival than those occurring in younger patients or those that do not show gain at 1q32.1 respectively. On multivariate analysis, additional information about 1q32.1 status separates the survival curves beyond separation due to age alone (C). The combination of 1q32.1 gain<1.2 and age<55 appears to be associated with improved predicted survival of 10–20% at each time point relative to that associated with age<55 alone.
Three subjects died within the first month following diagnosis. For sensitivity analysis, we re-analyzed the data after excluding these subjects, who likely died of operative complications. The results remained similar to the original analysis.
Discussion
Using high-resolution oligonucleotide aCGH, we recently demonstrated that chromosome 1q gain occurs in 59% of the atypical meningiomas in a series of 47 atypical meningiomas. 1q gain occurred at two regions: 1q25.1 with a peak region spanning 727kb including two genes; and 1q25.3 to q32.1 spanning 1.48Mb and including 42 genes. Although 1q gain had been noted in previous series[11, 18], it had not been noted to occur at such a high frequency. Moreover, in our prior study, of all chromosome arms lost or gained, 1q gain was the only one associated with reduced progression-free survival[16]. In the present study, therefore, we sought to verify the prevalence of 1q gain in an independent, clinically annotated series of meningiomas and to examine the utility of 1q gain, as assessed by FISH, as a predictive biomarker for progression; such findings would validate 1q gain not only as a genetic marker of atypical meningioma but also as a marker of adverse prognosis, thus indicating a subset of tumours possibly warranting more aggressive initial management.
The present results confirm the high incidence of 1q gain in atypical meningioma. Of 86 tumours, 55 (63.9%) showed gain at 1q: 21 (24.4%) had gain at 1q32.1 and 49 (56.9%) at 1q25.1. The current findings also clarify the association of 1q gain with reduced progression-free survival: this correlation only holds true for gain at 1q32.1 and not for gain at 1q25.1. The association would further suggest that a significant oncogenic gene may reside in this region.
Gain of 1q has been associated with a poor prognosis in other tumours. In particular, 1q gain has been associated with greater likelihood of recurrence in Wilms tumour, neuroblastoma and ependymoma[19–21] and as a marker of reduced survival in primary neuroepithelial tumours such as ependymoma, medulloblastoma and low grade paediatric glioma, as well as in myeloma and Ewing sarcoma[20, 22–25]. Whole-arm single copy 1q gain has also been associated with reduced progression-free and overall survival in paediatric nonependymal, nonpilocytic gliomas[25]. Our findings add to this list of tumours for which 1q copy number assessment could provide clinically useful information.
Our demonstration that 1q gain can be readily detected using FISH in formalin-fixed, paraffin-embedded atypical meningiomas shows that the assay could be set up readily for clinical purposes in many laboratories (as opposed to high-resolution aCGH, which still remains largely a research tool). The presence of 1q gain in a completely resected atypical meningioma should therefore at least prompt the patient’s oncologist or surgeon to pursue follow-up imaging sooner than standard for atypical meningioma, and should raise the estimation that the patient might benefit from radiation therapy.
The average age of this sample of patients with atypical meningioma was younger than that cited for meningiomas overall (mean age = 57 years vs 64 years)[26]. In our series, age greater older than 55 was independently associated with shorter progression-free survival. Few studies have specifically acquired follow up data on higher-grade meningioma; of these, older age has been noted to influence time to recurrence and/or overall survival[10, 27, 28]. Two of these studies, however, evaluated irradiated patients with both atypical and malignant meningioma[27, 28]. Pasquier et al reported 119 patients (82 with atypical meningiomas and 37 with malignant meningioma) and found that age > 60 years was found to impact overall survival negatively on multivariate analysis[28]. Similarly, Milosevic et al reported 59 patients (17 with atypical meningiomas and 42 with malignant meningiomas) finding that age < 58 years was independently associated with cause specific survival[27]. Aghi et al evaluated only patients with completely resected atypical meningiomas, of whom 8 were irradiated, noting that older age appeared to confer a higher risk of recurrence[10]. Conversely, Jo et al recently followed up 35 patients with atypical meningioma, finding that age had no significant effect on overall survival or progression free survival[29]. Our data support the suggestion that older age may confer worse prognosis in patients with atypical meningioma, including in those that have undergone complete resection.
While 1q32.1 gain does not appear to improve the discrimination capacity (i.e., the C-index) of age alone for progression-free survival, its addition to age may lead to clinically meaningful changes in progression-free survival predictions. This finding should be validated in independent and larger studies; moreover, the region of gain is relatively large and improved genetic determinants of aggressive behaviour may be localized within this area. The confirmation of this molecular prognostic determinant should prompt further examination of this region to identify genes with oncogenic potential, to clarify their role in tumorigenesis and to enable future targeted therapy.
Acknowledgments
The authors wish to thank Dr. N. Bermingham, Dr. M. Farrell and Dr. F. Brett for their assistance retrieving cases in Cork and Dublin and Ms. M. Nitta for technical assistance with FISH experiments.
Abbreviations used
- CGH
comparative genomic hybridization
- aCGH
array comparative genomic hybridization
- FISH
fluorescence in situ hybridization
Footnotes
Conflicts of Interest
We declare that we have no conflicts of interest.
References
- 1.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006 Dec;5(12):1045–54. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 2.CBTRUS. CBTRUS statistical report: primary brain and central nevous system tumours diagnosed in the United States in 2004–2006. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999 May 1;85(9):2046–56. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Budka H. Meningiomas. In: Kleihues P, editor. World Health Organization classification of tumours; pathology and genetics of tumours of the central nervous system. Lyon: IARC Press; 2000. pp. 176–84. [Google Scholar]
- 5.Perry A, Scheithauer BW, Budka H, von Deimling A. Meningeal tumours. In: Louis DN, Wiestler OD, Cavanee WK, editors. WHO classification of tumours of the central nervous system. Lyon: IARC; 2007. pp. 164–72. [Google Scholar]
- 6.Kallio M, Sankila R, Hakulinen T, Jaaskelainen J. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992 Jul;31(1):2–12. doi: 10.1227/00006123-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Perry A. Meningiomas. In: McLendon R, Bigner DD, editors. Russell and Rubinstein’s pathology of tumours of the nervous system. London: Hodder Arnold; 2006. pp. 427–74. [Google Scholar]
- 8.Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, et al. Meningioma. Crit Rev Oncol Hematol. 2008 Aug;67(2):153–71. doi: 10.1016/j.critrevonc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Rogers L, Vogelbaum MA. Intracranial meningiomas of atypical (WHO Grade II) histology. J Neurooncol. 2010;99:393–405. doi: 10.1007/s11060-010-0343-1. [DOI] [PubMed] [Google Scholar]
- 10.Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009 Jan;64(1):56–60. doi: 10.1227/01.NEU.0000330399.55586.63. discussion. [DOI] [PubMed] [Google Scholar]
- 11.Weber RG, Bostrom J, Wolter M, Baudis M, Collins VP, Reifenberger G, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14719–24. doi: 10.1073/pnas.94.26.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Finkelstein S, Hamilton RL, Rekha R, King JT, Jr, Omalu B. Loss of heterozygosity analysis of benign, atypical, and anaplastic meningiomas. Neurosurgery. 2004 Nov;55(5):1163–73. doi: 10.1227/01.neu.0000141081.07086.a0. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Liu J, Patel S, Cloughesy T, Lai A, Farooqi H, et al. Genomic landscape of meningiomas. Brain Pathol. 2010 Jul;20(4):751–62. doi: 10.1111/j.1750-3639.2009.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillo A, Orfao A, Sayagues JM, Diaz P, Gomez-Moreta JA, Caballero M, et al. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J Clin Oncol. 2003 Sep 1;21(17):3285–95. doi: 10.1200/JCO.2003.07.156. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz J, Martinez A, Hernandez S, Zimman H, Ferrer M, Fernandez C, et al. Clinicopathological variables, immunophenotype, chromosome 1p36 loss and tumour recurrence of 247 meningiomas grade I and II. Histol Histopathol. 2010 Mar;25(3):341–9. doi: 10.14670/HH-25.341. [DOI] [PubMed] [Google Scholar]
- 16.Gabeau-Lacet D, Engler D, Gupta S, Scangas GA, Betensky RA, Barker FG, 2nd, et al. Genomic profiling of atypical meningiomas associates gain of 1q with poor clinical outcome. J Neuropathol Exp Neurol. 2009 Oct;68(10):1155–65. doi: 10.1097/NEN.0b013e3181ba3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uno H, Cai T, Tian L, Wei LJ. Graphical Procedures for Evaluating Overall and Subject-Specific Incremental Values from New Predictors with Censored Event Time Data. Biometrics. 2011 Apr 19; doi: 10.1111/j.1541-0420.2011.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki S, Nishizaki T, Ito H, Sasaki K. Comparative genomic hybridization analysis of genetic alterations associated with malignant progression of meningioma. J Neurooncol. 1999 Jan;41(2):167–74. doi: 10.1023/a:1006086723607. [DOI] [PubMed] [Google Scholar]
- 19.Hing S, Lu YJ, Summersgill B, King-Underwood L, Nicholson J, Grundy P, et al. Gain of 1q is associated with adverse outcome in favorable histology Wilms’ tumors. Am J Pathol. 2001 Feb;158(2):393–8. doi: 10.1016/S0002-9440(10)63982-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendrzyk F, Korshunov A, Benner A, Toedt G, Pfister S, Radlwimmer B, et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2070–9. doi: 10.1158/1078-0432.CCR-05-2363. [DOI] [PubMed] [Google Scholar]
- 21.Pezzolo A, Rossi E, Gimelli S, Parodi F, Negri F, Conte M, et al. Presence of 1q gain and absence of 7p gain are new predictors of local or metastatic relapse in localized resectable neuroblastoma. Neuro Oncol. 2009 Apr;11(2):192–200. doi: 10.1215/15228517-2008-086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattinger CM, Potschger U, Tarkkanen M, Squire J, Zielenska M, Kiuru-Kuhlefelt S, et al. Prognostic impact of chromosomal aberrations in Ewing tumours. Br J Cancer. 2002 Jun 5;86(11):1763–9. doi: 10.1038/sj.bjc.6600332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca R, Van Wier SA, Chng WJ, Ketterling R, Lacy MQ, Dispenzieri A, et al. Prognostic value of chromosome 1q21 gain by fluorescent in situ hybridization and increase CKS1B expression in myeloma. Leukemia. 2006 Nov;20(11):2034–40. doi: 10.1038/sj.leu.2404403. [DOI] [PubMed] [Google Scholar]
- 24.Lo KC, Ma C, Bundy BN, Pomeroy SL, Eberhart CG, Cowell JK. Gain of 1q is a potential univariate negative prognostic marker for survival in medulloblastoma. Clin Cancer Res. 2007 Dec 1;13(23):7022–8. doi: 10.1158/1078-0432.CCR-07-1420. [DOI] [PubMed] [Google Scholar]
- 25.Miwa T, Hirose Y, Sasaki H, Ezaki T, Yoshida K, Kawase T. Single-copy gain of chromosome 1q is a negative prognostic marker in pediatric nonependymal, nonpilocytic gliomas. Neurosurgery. 2011 Jan;68(1):206–12. doi: 10.1227/NEU.0b013e3181fd2c2e. [DOI] [PubMed] [Google Scholar]
- 26.Asthagiri AR, Helm GA, Sheehan JP. Current concepts in management of meningiomas and schwannomas. Neurol Clin. 2007 Nov 25;4:1209–30. xi. doi: 10.1016/j.ncl.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Milosevic MF, Frost PJ, Laperriere NJ, Wong CS, Simpson WJ. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996 Mar 1;34(4):817–22. doi: 10.1016/0360-3016(95)02166-3. [DOI] [PubMed] [Google Scholar]
- 28.Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008 Aug 1;71(5):1388–93. doi: 10.1016/j.ijrobp.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Jo K, Park HJ, Nam DH, Lee JI, Kong DS, Park K, et al. Treatment of atypical meningioma. J Clin Neurosci. 2010 Nov;17(11):1362–6. doi: 10.1016/j.jocn.2010.03.036. [DOI] [PubMed] [Google Scholar]