Abstract
Tumor recurrence from residual local or micro-metastatic disease remains a problem in cancer therapy. In patients with soft-tissue sarcoma and patients with inoperable non-small cell lung cancer, local recurrence is common and significant mortality is caused by the subsequent emergence of metastatic disease. Thus, while the aim of the primary therapy is curative, the outcome may be improved by additional targeting of residual microscopic disease. We demonstrate in a murine model that surgical removal of a large primary sarcoma results in local recurrence in approximately 50% of animals. Depletion of CD8 T cells results in local recurrence in 100% of animals, indicating that these cells are involved in control of residual disease. We further demonstrate that systemic adjuvant administration of αOX40 at surgery eliminates local recurrences. In this model, αOX40 acts to directly enhance tumor antigen-specific CD8 T cell proliferation in the lymph node draining the surgical site, and results in increased tumor antigen-specific cytotoxicity in vivo. These results are also corroborated in a murine model of hypofractionated radiation therapy of lung cancer. Administration of αOX40 in combination with radiation significantly extended survival compared to either agent alone, and resulted in a significant proportion of long-term tumor free survivors. We conclude that αOX40 increases tumor antigen-specific CD8 T cell cytotoxic activity resulting in improved endogenous immune control of residual microscopic disease, and we propose that adjuvant αOX40 administration may be a valuable addition to surgical and radiation therapy for cancer.
Keywords: CD134, Costimulation, Surgery, Radiation, CD8
Introduction
It is becoming increasingly clear that suppression of host adaptive immune responses is a required element in the development of aggressively growing tumors to overcome their antigenicity 1–3. Surgery is an effective primary therapy in the majority of cancers, resulting in local control and increased duration of survival. In view of the immune suppressive effect of tumors on adaptive immune responses it has been proposed that surgical removal of the primary tumor has a net positive effect on anti-tumor immunity 4. Radiation therapy also provides local therapy to eliminate the primary tumor, though in this case the interaction between radiation and the endogenous immune response is likely mixed. In the case of both surgical therapy and radiation therapy, pre-existing microscopic disease beyond the primary tumor can develop into clinically relevant secondary tumors that represent a significant source of cancer-associated mortality. The ability to further enhance tumor-specific adaptive immune responses at the time of the initial procedure may be valuable for control of residual microscopic disease, allowing immune cells to additionally control local or distant tumor deposits 5.
In patients with soft-tissue sarcoma, despite significant efforts to remove all cancer cells at the time of the operation, microscopic disease has been demonstrated to infiltrate normal tissue at surprising distances from the primary tumor mass 6. Soft-tissue sarcomas have a 10–20% local recurrence rate when treated with surgical resection alone 7,8 and the presence of detectable cancer cells in the tumor margin is a prognostic factor for local recurrence 9. This residual microscopic disease is an excellent target for immunotherapy, since the relatively small number of cancer cells could allow effector-to-target ratios that are never achievable in large tumors. Enhancing the adaptive immune response at the time of the operation may permit clearance of the microscopic disease before it develops into more established disease.
Radiation therapy is a primary therapy for patients with inoperable localized non-small cell lung carcinoma. However, there is a high rate of local failure, and while it increases median survival, the therapy is often not curative 10. Standard radiation fractionation provides a daily dose on the order of 1.8-2Gy, to a final dose of 60–70Gy. By contrast, Stereotactic Body Radiation Therapy (SBRT) is a relatively novel technique in radiation therapy of lung carcinomas, delivering the total dose in 5 or fewer treatments of radiation (hypofractionation). Response rates in clinical trials suggest SBRT could be an important therapeutic advance 11. This approach may have significant relevance to the endogenous immune response, since lymphocytes are sensitive to even low radiation doses and are cleared rapidly from the radiation field 12. Standard fractionated radiation treatment may limit the effectiveness of the immune system by constantly removing tumor antigen-specific T cells at the target site. Thus, although standard fractionation has been shown to generate endogenous anti-tumor immune responses 13, SBRT hypofractionation may be a more optimal partner for immunotherapy.
Agonistic antibodies to OX40 (αOX40) are an effective adjuvant for both CD4 and CD8 activation. Provision of αOX40 immediately following antigen priming enhances T cell expansion and effector function, and the number of long-term memory CD4 and CD8 T cells 14–17. Therapy with αOX40 closely following tumor challenge significantly enhances survival in a wide variety of animal tumor models 18,19. In contrast, once the tumor has established beyond 9–10 days, we observe an inhibitory tumor environment, with significant numbers of T regulatory cells along with inhibitory macrophages that express arginase and TGFβ20. Treatment with αOX40 at this time provides tumor growth delay, enhanced infiltration of CD8 T cells and reduced suppression by tumor-infiltrating macrophages 20. Nevertheless, despite growth delay, αOX40 treatment at this stage results in only a few long-term tumor-free survivors due to the immune suppressive environment within the established tumor.
In this manuscript we present a model of sarcoma treatment where surgical removal of 10–14 day established tumors results in 50% local tumor recurrence. We demonstrate that the adaptive immune response is necessary for removal of residual disease. Critically, adjuvant αOX40 delivered at the time of the operation eliminates local recurrence in 100% of mice. To address the mechanism by which αOX40 therapy controls residual disease, we identify a temporal window of tumor antigen-specific T cell priming following surgery, and establish that administration of αOX40 in this window enhances the tumor antigen-specific response following the surgical procedure, and enhances tumor-specific cytotoxicity in vivo. We demonstrate the broad therapeutic applicability of adjuvant αOX40 using a model of SBRT for lung cancer. These data set the stage for the use of αOX40 as an adjuvant therapy with conventional treatment of primary tumors.
Materials and Methods
Animals, cell lines and in vivo antibodies
6–8 week old C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA) for use in these experiments. OT1 mice, with CD8 T cells specific for the SIINFEKL epitope of ovalbumin, have previously been described 21. OX40−/− C57BL/6 mice 22 were kindly provided by Dr. N Killeen, University of California San Francisco, CA. OX40−/− OT1 mice were kindly provided by Dr. M Croft, La Jolla Institute for Allergy and Immunology, CA. These experiments used the MCA205 (H12) sarcoma cell line and the MCA205ova cell line as previously described 20, and the Lewis Lung carcinoma (3LL). Control Ig antibody was purchased from Sigma (St Louis, MO) while the rat anti-OX40 antibody (OX86) and CD8-depleting antibody were produced in the laboratory from hybridomas and affinity purified over protein G columns. All animal protocols were approved by the Institution’s IACUC.
Surgical removal of tumors
Tumors were established subcutanteously (s.c.) on the right flank of 8–10 week old mice and removed at approximately day 10–14 when they reached an average size of 7–10mm in diameter. Mice were anesthetized by Isoflourane inhalation, and a 1cm incision was made immediately above the tumor. The encapsulated tumor was removed from the s.c. site by excising the surrounding fibrotic material with care taken to avoid cutting into the tumor mass. All animals were macroscopically tumor-free following surgery by visual inspection: however, we did not remove an additional margin beyond the primary tumor mass and associated fibrous material. Gel containing 20% bupicaine was administered to the surgical site, the wound closed with Reflex clips (Kent Scientific Corporation, Torrington, CT) and coated with antibiotic gel. Clips were removed 10 days following surgery.
Cytotoxic T Lymphocyte (CTL) assay
To measure the effect of αOX40 therapy on endogenous T cell cytotoxicity towards the MCA205 tumor, we established MCA205 tumors and treated with 250μg αOX40 or control Ig on day 3 and day 7 following tumor challenge. At day 12 following tumor challenge the tumor-draining lymph nodes were harvested and cells activated in vitro with 5μg/ml anti-CD3 for 2 days, followed by 60 U/ml IL-2 for a further 3 days. These cells were diluted to achieve a range of effector target ratios and combined in triplicate with [51]Cr-labeled MCA205 or 3LL tumor cells for 4 hours. Maximal release was calculated by adding 1% triton X100 and minimum release using media alone. Minimum release did not exceed 3% of maximal release. Free radiolabel in cell supernatants was measured using a MicroBeta Wallac scintillation counter (PerkinElmer, Waltham, MA) and percent specific cytotoxicity calculated using the formula: (Experimental − Minimum)/(Maximum − Minimum)×100. Experiments were performed with 3 mice per treatment group.
Adoptive transfer
For antigen-specific cell tracking studies using MCA205ova, mice were tolerized to ovalbumin by intravenous (i.v.) injection of 500μg ovalbumin (Sigma) 7 days and 2 days prior to MCA205ova challenge 20. For adoptive transfer of naïve OT1 cells, spleen and lymph nodes were harvested from Thy1.1+ OT1 mice and the percentage of CD8 T cells was calculated by FACS analysis. Where appropriate, OT1 splenocytes were washed into PBS, then labeled for 15 minutes in 1μl 5mM CFSE (Invitrogen)/5×107 cells, then washed 1× in media and 4× in PBS prior to adoptive transfer. 1×106 CD8+ (OT1) cells were transferred i.v. following surgical removal of the tumor. In some experiments, adoptive transfer was delayed for 3 days following the operation.
FACS antibodies and staining
Phenotyping of tumor and lymph node cells was performed using the following antibodies: CD8 PETxRD (Invitrogen); CD69 PE, H2Kb FITC, IFNγ APC, TNFα PECy5, CD62L APCCy7 and CD25 APC (each Ebiosciences, San Diego, CA); Unlabelled Thy1.1 was conjugated to PacificOrange in the laboratory using a PO-conjugation kit (Invitrogen). For intracellular cytokine staining, 1×106 cells were stimulated with 1μg/ml SIINFEKL for 6 hours at 37°C in the presence of Golgiplug (BD Biosciences). Cells were surface stained, then fixation and permeablization for intracellular staining was performed using a BD intracellular cytokine staining kit and anti-cytokine antibodies. Stained cells were analyzed on a BD LSRII.
In vivo cytotoxicity assay
For in vivo cytotoxicity assays, 1×106 unlabelled OT1 were adoptively transferred at the time of surgery, along with 250μg i.p. αOX40 or control Ig. Four or six days later naïve splenocytes were divided into two groups and pulsed with 1μM SIINFEKL peptide for 1 hour in vitro (target), or left untreated (internal control). These populations were then washed and labeled for 15 minutes in 1μl 5mM CFSE/5×107 cells (target CFSEhi) or 0.1μl 5mM CFSE/5×107 cells (internal control CFSElo) then washed 1× in media and 4× in PBS. The populations were counted and combined at a 1:1 ratio, then adoptively transferred i.v. to the 4 or 6 day post-operation mice, or naïve control mice. The draining lymph nodes of the surgery site were collected 4 hours later, and the proportion of CFSEhi/CFSElo cells used to calculate specific cytotoxicity using the formula: 100 – ((percentage of CFSEhi in treated mice/percentage of CFSElo in treated mice)/(percentage of CFSEhi in naive mice/percentage of CFSElo in naive mice) × 100).
Radiation therapy of tumors
Tumors were established s.c. in the right leg and allowed to established for 5–7 days before initiation of treatment. Three 20Gy treatment fractions were given over 10 days using Varian linear accelerator 6MV photons incorporating a half beam block to minimize dose to the torso. Tumor growth was determined by measurement of leg thickness, and animals were euthanized when leg thickness exceeded 15mm. Analysis of tumor infiltrating cells was performed as previously described 20. Briefly, the tumor was dissected into ~2 mm fragments followed by agitation in 1 mg/mL collagenase (Invitrogen, Carlsbad, CA), 100 μg/mL hyaluronidase (Sigma), and 20mg/mL DNase (Sigma) in PBS for 1 to 2 hr at room temperature. The digest was filtered through 100μm nylon mesh to remove macroscopic debris, and the final cell preparation was separated by layering over Ficoll. Viable cells were counted and stained for flow cytometry.
Results
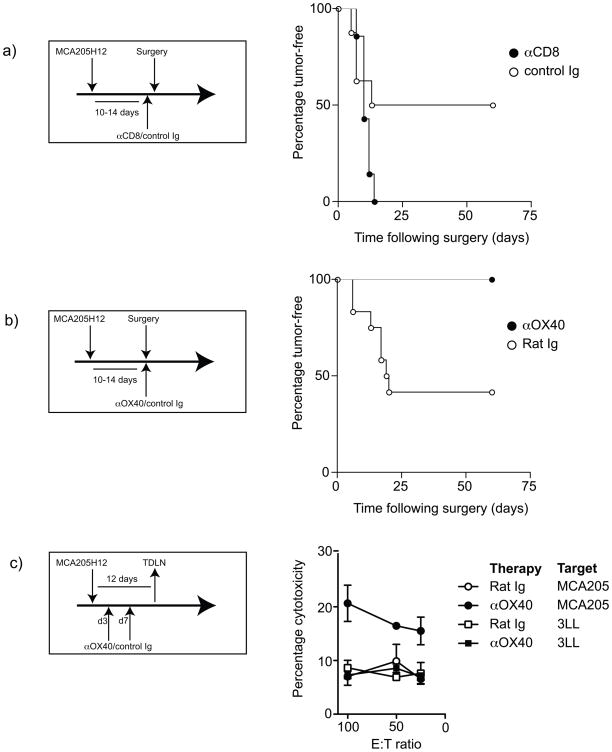
We developed a surgical model for treatment of large, established MCA205 sarcoma, such that surgical excision of the tumor resulted in local recurrence in approximately 50% of animals (Figure 1a). The recurrent tumors developed within the region of the primary tumor, and grew rapidly once detectable. Those mice remaining tumor-free following surgery did not develop tumors upon rechallenge with the parental tumor on the opposite flank (Table 1), indicating that they have developed immunity to the tumor. Thus, we hypothesized that the endogenous tumor antigen-specific immune response was a deciding factor in determining whether the tumor recurred. To test this hypothesis, we depleted CD8 T cells one day before surgery, and maintained depletion with weekly injections of the depleting antibody. Strikingly, all animals depleted of CD8 cells showed local recurrence following surgical removal of the primary tumor (Figure 1a). These data suggest that despite removal of macroscopic tumor all animals retain microscopic tumor deposits that have the potential to recur and are variably controlled by tumor antigen-specific CD8 T cells. Those animals that mount a sufficiently functional CD8 T cell response clear the residual tumor and maintain long-term tumor immunity.
Figure 1. Role of CD8 T cells in local recurrence following sarcoma surgery and influence of αOX40 therapy on local recurrence.
a) MCA205 tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed when they reached 7–10 mm in diameter. One day prior to the operation, mice began receiving weekly injections of 200μg of control (○) or CD8-depleting (●) antibody and followed for local tumor recurrence. b) MCA205 tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed when they reached 7–10 mm in diameter. At the time of the operation mice received a single injection of 250μg of control (○) or αOX40 (●) antibody and followed for local tumor recurrence. c) C57BL/6 mice bearing MCA205 tumors were treated with 250μg aOX40 or control Ig on day 3 and day 7 following tumor challenge. Draining lymph node cells were harvested on day 12 and activated in vitro. These cells were used in a [51]Cr release cytotoxicity assay to determine specific cytotoxicity against MCA205 (circles) or non-specific cytotoxicity against 3LL (squares). The graph shows the mean cytotoxicity at a range of effector: target ratios calculated from 3 individual mice per group treated with control Ig (empty shapes) or αOX40 (filled shapes).
Table 1. Protection against rechallenge in long-term survivors following surgery.
MCA205 tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed when they reached 7–10 mm in diameter. At the operation mice received a single injection of 250μg of control or αOX40 antibody and followed for local tumor recurrence. Mice remaining tumor-free at 60 days were following surgery were rechallenged with MCA205 on the opposite flank and monitored for recurrence.
| Treatment | Local recurrence | Tumors on rechallenge |
|---|---|---|
| Control Ig | 7/12 | 0/6 |
| αOX40 | 0/9 | 0/9 |
To test whether boosting the T cell response could improve the outcome of surgery, we surgically removed the MCA205 sarcoma when it reached 7–10mm, mice received a single dose of αOX40 or control antibody immediately following surgery, and were followed for local tumor recurrence. Again, in control treated groups, tumors recurred in approximately half of the animals. By contrast, anti-OX40 therapy completely eliminated local recurrence following surgery (Figure 1b), and these mice were protected from rechallenge with parental tumor (Table 1), demonstrating that long-term tumor-specific immune protection had been established. To determine whether OX40 therapy caused an increase in tumor antigen-specific cytotoxicity, we determined CTL activity in tumor draining lymph node cells at day 12 following tumor challenge. Our data shows that an endogenous tumor-specific response is not detectable by CTL assay. However, OX40 therapy generated a measureable cytotoxic T cell response that was tumor-specific (Figure 1c). These data demonstrate that boosting tumor antigen-specific immunity through adjuvant delivery of αOX40 reduces local recurrence.
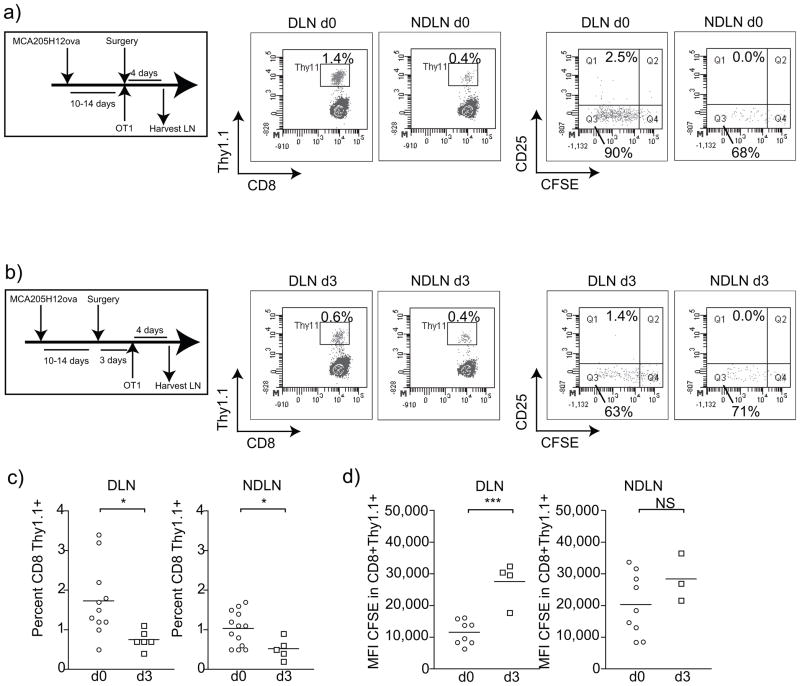
Tumor antigen has been shown to constantly drain to the tumor draining lymph nodes 23 implying that surgical resection would consequently remove the large majority of tumor antigen. Antigen is a required component in priming adaptive immune responses and OX40 is only expressed on T cells for a narrow temporal window following T cell activation. The majority of early studies focused on OX40 expression and function on CD4 T cells, 24–27: however, OX40 therapy also has powerful direct effects on the proliferation, effector function, and long-term survival of CD8 T cells 14,15,20,28–30. Therefore, we investigated the consequences of surgical removal of the tumor on antigen priming in vivo. We established MCA205 expressing the model antigen ovalbumin (MCA205ova) 20 in C57BL/6 mice and these tumors were surgically removed at 10–14 days. Mice were injected with CFSE-labeled naïve OT1 T cells at the time of surgery (Figure 2a) or 3 days following surgery (Figure 2b), and draining lymph nodes and non-draining lymph nodes were harvested 4 days following transfer. The majority of antigen-specific T cell expansion occurred in the draining lymph node (p<0.05 compared to non-draining lymph node), and significantly fewer CD8+Thy1.1+ OT1 T cells were present in the draining lymph node if adoptive transfer was delayed for 3 days following surgery (p<0.05) (Figure 2c). This difference is most likely a result of decreased proliferation of the tumor antigen-specific OT1 T cells as significantly less dilution of the CFSE signal was observed if cells were transferred 3 days post-surgery (Figure 2d). These data suggest that there may be a narrow temporal window following the operation for successful introduction of immune adjuvant therapies that target CD8 T cells. The dynamics of this temporal window may be different for endogenous antigens since these are recognized by T cells that likely have lower affinity for antigen than OT1 cells. It has been demonstrated that the activation threshold for T cells of lower affinity is more dependent on antigen load and the presence of co-stimulatory molecules compared to T cells with higher affinity. In this case the temporal window to expand endogenous immune responses following the operation may be shorter and more dependent on co-stimulation.
Figure 2. Loss of tumor antigen-specific priming following sarcoma surgery.
MCA205ova tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed when they reached 7–10mm in diameter. a) At the time of the operation or b) three days following the operation, mice received adoptive transfer of 1×106 naïve CFSE labeled OT1 T cells i.v.. Four days following adoptive transfer, the tumor-draining lymph node (DLN) and a non-draining lymph node (NDLN) were harvested and FACS analyzed for the proportion of OT1 T cells. b) Representative FACS plots identifying CD8+Thy1.1+ OT1 T cells or CFSE dilution and CD25 expression in gated CD8+Thy1.1+ OT1 T cells. c) Graphs showing percentage of CD8 T cells that are Thy1.1+ OT1 cells in DLN or NDLN 4 days following adoptive transfer. d) Graphs showing Mean Fluorescence Intensity (MFI) of CFSE Thy1.1+ OT1 cells in DLN or NDLN 4 days following adoptive transfer. Each symbol represents one animal. Key: NS - not significant; * - p<0.05; ** - p<0.01; *** - p<0.001.
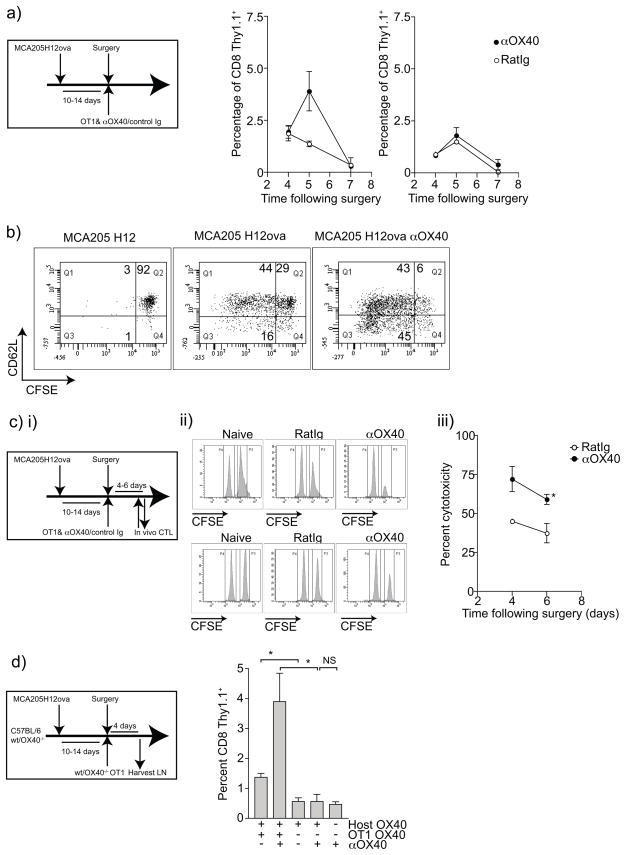
We hypothesized that adjuvant αOX40 therapy may reduce local recurrence by enhancing T cell activation and differentiation into tumor antigen-specific cytotoxic T cells. To test whether treatment with the T cell adjuvant αOX40 enhanced the tumor antigen-specific CD8 T cell response, we established subcutaneous MCA205ova tumors and on surgical removal of the primary tumor, mice received adoptive transfer of CFSE-labeled naïve OT1 cells along with αOX40 or control Ig. The tumor-draining and non-draining lymph nodes were harvested over a time course following surgery, adoptive transfer and antibody treatment. αOX40 therapy increased the proportion of OT1 cells in the tumor-draining lymph node, but not the non-draining lymph node 5 days following surgery (Figure 3a). However, 7 days following surgery this difference was not apparent. Based on previous studies 20 we hypothesize that these cells may have entered the peripheral tissues, perhaps including the site. Phenotypic analysis of the OT1 cells in draining lymph node of the negative control animals with surgically removed parental MCA205 tumor (no ovalbumin expression) showed no CFSE dilution in the CD8+Thy1.1+ OT1 population and a naïve CD62Lhi phenotype (Figure 3b). In contrast, we saw proliferation in the tumor antigen-specific T cells in the draining lymph node of surgically removed MCA205ova, and this proliferation was increased with αOX40 treatment (Figure 3b). Interestingly, we also saw evidence of increased differentiation of the OT1 cells, with decreased expression of CD62L in the CFSElo population after αOX40 administration, a phenotype that is associated with effector differentiation and improved peripheral trafficking 31. These data support the hypothesis that administration of αOX40 enhances a tumor-antigen specific immune response mediated by CD8-T cells to control tumor recurrence.
Figure 3. Influence of αOX40 therapy on tumor antigen-specific T cell proliferation and tumor antigen-specific cytotoxicity following sarcoma surgery.
a) MCA205ova tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed when they reached 7–10mm in diameter. At the time of the operation mice received adoptive transfer of 1×106 naïve CFSE-labeled Thy1.1+ OT1 T cells i.v. and 250μg control Ig or αOX40 i.p., and the tumor draining lymph node (DLN) and a non-draining lymph node (NDLN) were harvested at various times following surgery. b) Graphs show the time course of the percentage of CD8+Thy1.1+ OT1 cells in the draining and non-draining lymph nodes following surgery. c) Representative dot plots 5 days following surgery, gating on CD8+Thy1.1+ cells and showing CFSE dilution versus CD62L expression in the lymph node draining the surgical site of parental MCA205, MCA205ova treated with control Ig, or MCA205ova treated with αOX40. d)i) MCA205ova tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed when they reached 7–10mm in diameter. At the time of the operation, mice received adoptive transfer of 1×106 naïve OT1 T cells i.v. and 250μg control Ig or αOX40 i.p.. Four or six days later mice received adoptive transfer of 5×106 CFSEhi SIINFEKL-pulsed splenocytes and CFSElo control splenocytes in a 1:1 ratio. Naïve control mice also received adoptive transfer of target cells to determine background levels of cytotoxicity. Four hours later, draining lymph nodes were harvested and FACS analyzed for the proportion of CFSEhi to CFSElo cells. ii) Representative histograms showing the proportions of CFSElo and CFSEhi targets in draining lymph nodes day 4 following surgery, and day 6 following surgery. iii) Graph showing in vivo antigen-specific cytotoxicity calculated as described in the materials and methods. d) MCA205ova tumors were established s.c. in the flank of wild-type or OX40−/− C57BL/6 mice and were surgically removed when they reached 7–10mm in diameter. At the time of the operation mice received adoptive transfer of 1×106 naïve wild-type or OX40−/− Thy1.1+ OT1 T cells i.v.. Four days following adoptive transfer, the DLN was harvested and FACS analyzed for the proportion of OT1 T cells. Graphs showing percentage of CD8 T cells that are Thy1.1+ OT1 cells in DLN 4 days following adoptive transfer. Key: * - p<0.05
To determine whether αOX40 therapy enhanced tumor antigen-specific cytotoxicity, we performed cytotoxicity assays in mice following surgical removal of the tumor (Figure 3ci). MCA205ova tumors were surgically removed and mice received adoptive transfer of OT1 along with αOX40 or control Ig. Four or six days following surgery, mice were injected with CFSEhi SIINFEKL-loaded target splenocytes and CFSElo control splenocytes. Four hours following transfer of the target cell populations, the tumor-draining lymph node was harvested and analyzed for the proportions of CFSEhi and CFSElo cells. We saw tumor antigen-specific cytotoxicity following surgery (Figure 3cii), and this cytotoxicity was significantly increased in animals treated with αOX40 compared to those treated with control antibody (Figure 3ciii). These data support a model where αOX40 therapy delivered at the time of surgery enhances CD8 tumor antigen-specific cytotoxicity thereby enhancing the local control of tumor recurrence. To determine whether OX40 therapy acted directly on the tumor-specific CD8 T cells, or via host CD4 T helper or T regulatory cell populations, we established tumors in OX40−/− mice or used adoptive transfer of OX40−/− OT1 cells. The in vivo expansion of OT1 T cells was lost if the OT1 T cells were not able to express OX40 (Figure 3d). These data indicate that OX40 therapy directly targets the tumor antigen-specific T cells and enhances their expansion.
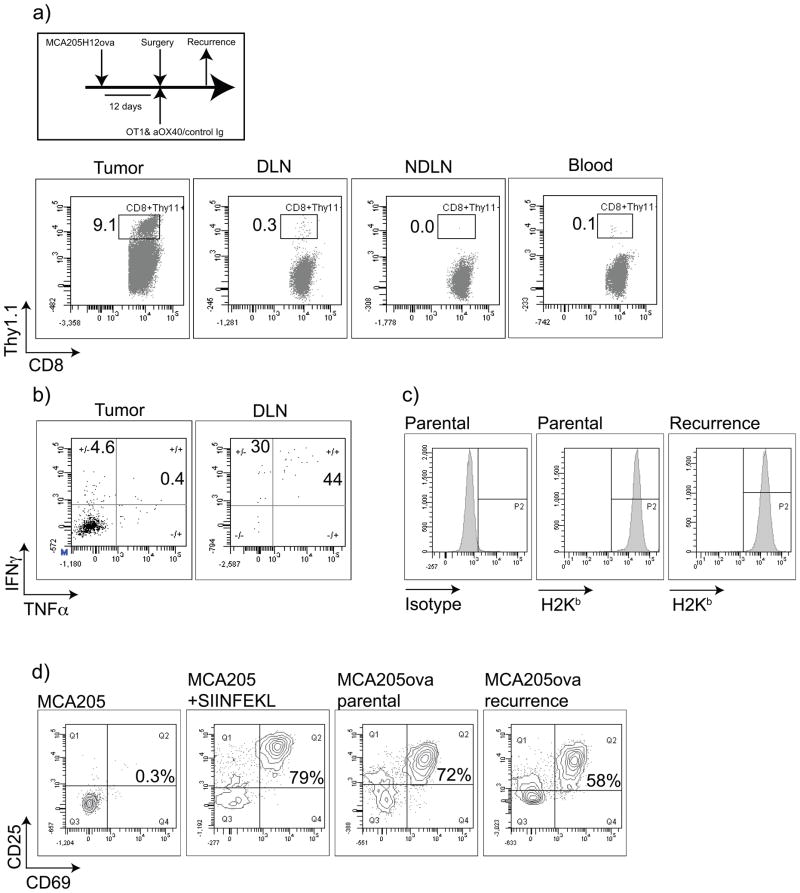
To understand the mechanisms by which tumors recur in the presence of tumor antigen-specific cells, we established MCA205ova for 12 days then surgically resected the tumor. At the time of the operation, mice received adoptive transfer of naïve Thy1.1+ OT1 T cells i.v. and 250μg control Ig or αOX40 i.p and were followed for local tumor recurrence. The OT1 transfer with tumor expression of ovalbumin resulted in tumor recurrence in only one of 5 mice receiving control treatment (and no recurrences in mice receiving OX40 therapy). We harvested this tumor and performed flow cytometry on the tumor-infiltrating cells. We demonstrated a small population of Thy1.1+ OT1 T cells in the blood and non-draining lymph node, with perhaps a slight enrichment in the tumor draining lymph node (Figure 4a). Importantly, we saw a large infiltrate of Thy1.1+ OT1 T cells in the tumor. In vitro restimulation of draining lymph node or tumor-infiltrating cells with SIINFEKL peptide demonstrated the majority of the small number of draining lymph node OT1 cells produced IFNγ in response to peptide stimulation, while the tumor infiltrating OT1 T cells we refractive to stimulation (Figure 4b). Based on these data we conclude that tumor recurrence occurred due to tumor-induced T cell suppression despite the heavy infiltration of tumor-specific T cells. To exclude the possibility that this tumor recurred due to loss of antigen presentation or ovalbumin expression, we generated primary cell cultures of tumor cells from the recurrent tumor. The recurrent tumors showed no loss of MHC class I expression compared to the parental MCA205ova culture (Figure 4c). In addition, the recurrent tumors retained their ability to activate OT1 cells (Figure 4d). Our experiments suggest that the failure to control tumor recurrence in the absence of OX40 therapy is not due to tumor antigen loss variants or loss of antigen presenting capacity, but due to tumor-induced suppression of T cell function.
Figure 4. Tumor recurrence involves loss of tumor-specific T cell function in the tumor.
MCA205H12ova tumors were established s.c. in the flank of C57BL/6 mice and were surgically removed at 12 days. At surgery, mice received adoptive transfer of 1×106 naïve CFSE-labeled Thy1.1+ OT1 T cells i.v. and 250μg control Ig or αOX40 i.p and followed for local tumor recurrence. In 1 (of 5) control mice the tumor recurred locally. The tumor, blood, draining lymph node (DLN) and non-draining lymph node (NDLN) were harvested and analyzed for the percentage of CD8+Thy1.1+ OT1 cells in each site. b) Tumor and DLN cells were stimulated with 1μg/ml SIINFEKL for 6 hours in the presence of secretion inhibitors, and analyzed for cytokine production by intracellular cytokine staining. c) Primary cell cultures of the recurrent tumor were established and tested for expression of H2Kb by flow cytometry. d) Naïve OT1 T cells were co-cultured for 48 hours with MCA205, MCA205 pulsed with SIINFEKL peptide, parental MCA205ova or the primary culture of recurrent MCA205ova. Antigen-specific recognition of presented ovalbumin by OT1 was determined by expression of the activation markers CD69 and CD25.
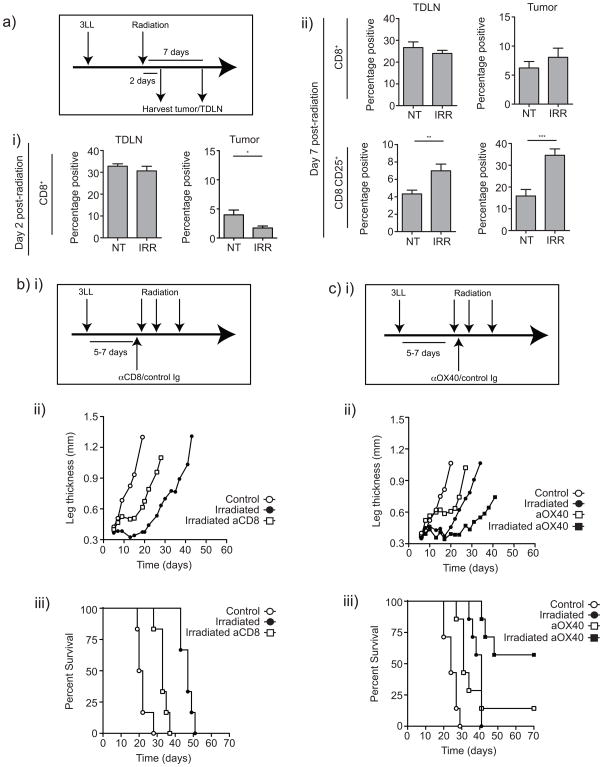
Like surgery, radiation is an effective local therapy that may benefit from the addition of adjuvant immunotherapy to target residual microscopic disease. We developed a mouse model of SBRT using the lung cancer cell line 3LL established in the leg to permit high dose focal radiation while avoiding radiosensitive organs. Tumors were irradiated 5–7 days following implantation, and analyzed for infiltrating T cells 2 or 7 days later (Figure 5a). As expected, 2 days following radiation CD8 T cells significantly decreased at the tumor site, which lies within the radiation field (Figure 5ai). However, CD8 T cells were not significantly altered in the tumor-draining lymph node that remains outside the radiation field. Seven days following radiation, CD8 T cells have returned to the irradiated tumor, and those T cells at the post-irradiation tumor site and tumor-draining lymph node significantly increased expression of CD25 compared to non-irradiated controls (Figure 5aii). These data suggest that while radiation initially locally depletes CD8 T cells, the consequence is increased proportions of activated T cells at the tumor site.
Figure 5. Role of CD8 T cells in local recurrence following radiation and influence of αOX40 therapy on local recurrence.
a) 3LL tumors were established s.c. in the leg of C57BL/6 mice and treated at 5–7 days with 20Gy focal radiation. Tumors and the tumor draining lymph node were harvested i) 2 days or ii) 7 days following treatment. Graphs show the proportion of lymph node or tumor-infiltrating cells that are CD8+, or the proportion of CD8+ cells that express CD25. b) 3LL tumors were established for 5–7 days and treated with 3×20Gy focal radiation over 10 days. One day prior to the first dose of radiation mice began weekly treatment with CD8-depleting or control antibody. Graph shows ii) mean leg thickness or iii) survival of tumor-bearing mice. c) 3LL tumors were established for 5–7 days and treated with 3x20Gy focal radiation over 10 days. One day after the first dose of radiation mice received a single dose of αOX40 or control antibody. Graph shows ii) mean leg thickness or iii) survival of tumor-bearing mice. Key: NS - not significant; * - p<0.05; ** - p<0.01; *** - p<0.001.
To determine the importance of activated CD8 T cells in the efficacy of radiation therapy, we depleted CD8 T cells prior to radiation therapy of 3LL tumor-bearing mice (Figure 5bi). Treatment with 3×20Gy caused a significant increase in median survival (21 days vs. 47 days p<0.001) (Figure 5bii and iii). CD8 depletion significantly decreased median survival caused by radiation (33 days p<0.001), though the survival benefit of radiation in the absence of CD8 T cells remained significantly better than no treatment (p<0.005). These data demonstrate that while radiation is an effective therapy, an adaptive T cell response mediated by CD8 T cells plays an important role in the success of radiation therapy. Nevertheless, it is relevant to note that in this model, radiation did not result in durable tumor cures. In view of these data, we investigated whether boosting the T cell response could improve the effectiveness of radiation therapy as demonstrated in the surgical model. Mice bearing 5–7 day 3LL tumors were treated with 3×20Gy focal radiation, and additionally treated with a single dose of αOX40 or control antibody one day following the first radiation dose (Figure 5ci). Consistent with the previous experiment, radiation significantly enhanced median survival compared to control-treated animals (24 vs. 41 days p<0.001) (Figure 5cii and iii). Treatment with αOX40 as a single agent also significantly enhanced median survival (31 days p<0.001), and the combination of radiation and αOX40 significantly increased survival compared to either agent alone (vs.αOX40 alone p<0.05, vs. radiation alone p<0.005). Importantly, the combination treatment resulted in a significant proportion of mice remaining tumor free, suggesting that tumor cells not killed by radiation were removed via immune mechanisms. In support of this hypothesis, mice tumor-free at 70 days were resistant to rechallenge with parental tumor on the opposite flank (data not shown). These data demonstrate that αOX40 therapy synergizes with radiation therapy to target residual disease and may represent a powerful strategy to augment existing primary therapies of cancer.
Discussion
The experiments reported here show that following resection of large, established MCA205 sarcoma we observed a high rate of local recurrence. Depletion of CD8 T cells substantially increased the local recurrence rate, indicating that the adaptive immune response played a role in the efficacy of the surgical therapy. Importantly, administration of αOX40 at surgery prevented local recurrence of the sarcoma. We characterized a temporal window following surgical removal of the tumor in which tumor antigen-specific CD8 T cells can be primed in the draining lymph node of the surgical site. Administration of agonistic antibodies to OX40 in this temporal window increased CD8 proliferation and effector differentiation in the draining lymph node of the surgical site and is dependent on direct ligation of OX40 on CD8 T cells. Moreover, we demonstrated increased tumor antigen-specific cytotoxicity in vivo with αOX40 therapy at surgery. We correlate tumor recurrence with suppression of tumor antigen-specific T cells in the tumor environment. To investigate the broad applicability of adjuvant αOX40 therapy, we developed a murine model of SBRT of lung cancer. While radiation initially depleted CD8 T cells, the returning CD8 T cell population was significantly more activated. We demonstrated that this CD8 T cell response was necessary for the full efficacy of radiation therapy, and that boosting the T cell response with adjuvant αOX40 therapy significantly enhanced tumor-free survival. These data strongly support the hypothesis that the adaptive immune system plays a role in the control of residual microscopic disease following surgical resection and radiation therapy of the primary tumor, and that adjuvant αOX40 antibodies are a powerful therapy to increase the local control rate following primary local therapies.
In the field of tumor immunotherapy, classical studies in chemically induced sarcomas were critical in identifying the role of tumor antigens, adaptive immune protection following surgery, and the emergence of immune suppression in established tumors 32–34. Though the wide variation in sarcomas will likely preclude development of antigen-directed immunotherapy approaches, it may be possible to enhance the endogenous immune response in patients to reduce the rate of recurrence. This relatively high local recurrence rate in sarcoma treated with surgery alone relates to the lack of distinct tumor margin, such that microscopic disease can be detected at large distances from the primary tumor mass 6. For this reason, multiple clinical trials have tested neoadjuvant or adjuvant chemotherapy and/or radiation therapy in patients with soft-tissue sarcoma 35. These additional cytotoxic therapies may have implications for tumor antigen specific immune responses, therefore careful consideration should be given to the combination of immunotherapy in these circumstances. Nevertheless, we demonstrate here that adjuvant αOX40 therapy shows significant synergy with both surgery and radiation, and adjuvant αOX40 therapy has also been shown to synergize with chemotherapy in a murine melanoma model 36. Thus, preclinical data demonstrates that with careful timing, adjuvant αOX40 therapy may be an important addition to conventional therapies for cancer.
While surgery creates a valuable temporal window where antigen is present in the draining lymph node and the suppressive effects of the tumor have been removed, there may be further confounding issues. TGFβ is a critical suppressive cytokine in the tumor environment 37, and the wound healing response that develops following surgery is directed by TGFβ secretion 38. This cytokine has been widely demonstrated to inhibit effector CD8 T cell activation, differentiation and effector function 37. Thus, the consequence of wound healing in the post-surgical region may significantly limit CD8 function. While TGFβ blockade may not be feasible since it may interfere with wound healing 38, strategies to diminish CD8 sensitivity to TGFβ significantly increase effector functions against tumors 39. In addition, residual microscopic disease may develop it’s own suppressive environment, potentially limiting the efficacy of adaptive immune responses. Future experiments will address the role of the immune environment of metastatic disease and the consequences of wound healing on the efficacy of adaptive immune responses following surgery.
Similar caveats should be considered when combining radiation with immunotherapy. Although CD8 T cell responses play an important role in the outcome of radiation therapy in immune competent animal models 40, fractionated radiation is locally immunosuppressive 41. Radiation of the lung is known to initiate a process that can result in radiation pneumonitis. While the initial phase may be caused by inflammatory cytokines 42, the later phases of pneumonitis leading to fibrosis and restricted lung function involve TGFβ 43–45. Accentuating the immune component of therapy may have unanticipated consequences on radiation toxicity, and the dominance of TGFβ in the repairing lung may limit effective long-term tumor immune surveillance. Future experiments will investigate the interaction between radiation therapy to the tumor site and the endogenous immune response to cancer.
The experiments described in this manuscript have focused on the use of agonistic antibodies to OX40 as an immune adjuvant. OX40 is an excellent target as an adjuvant for a number of reasons. Firstly, OX40 is inducibly expressed on recently antigen-activated T cells 14–17, therefore αOX40 therapy specifically targets only those T cells in the process of responding to antigen. Secondly, OX40 is constitutively expressed on T regulatory cells, and ligation of OX40 on T regulatory cells has been shown to decrease the suppressive function of these cells in vitro 46. This effect may be transitory, since in vivo suppression of T regulatory cells has been described within 6 hours of αOX40 treatment 47, but not 48 hours following αOX40 treatment 20. In these experiments we have shown a dependence on direct OX40 ligation on CD8 T cells to increase tumor specific cytotoxicity in vivo. This data is consistent with published data showing that CD8 T cells express OX40 and directly respond to OX40 ligation 14,15,20,28–30. Nevertheless, a transitory suppression of T regulatory cell function may be sufficient to increase the responsiveness of tumor-specific CD4 and CD8 T cells to antigen stimuli. Finally, the availability of OX40L appears to be limiting in vivo. OX40L is readily detectible in vivo in a number of highly inflammatory autoimmune scenarios 48–50, but deficient in tumors. The inflammatory environment of the tumor lacks the strong adjuvants that can induce OX40L expression 51, providing a rationale for αOX40 therapy in tumor-bearing individuals. While ligation of OX40 can transiently suppress T regulatory cells 52 and reduce their accumulation in the tumor 20, it can also drive proliferation of regulatory T cells if the appropriate inflammatory milieu is present 53. Recent data demonstrates that OX40 therapy is also an effective adjuvant immediately following systemic chemotherapy 36. We propose that there is a strong rationale for administration of OX40 therapy immediately post cytoreductive therapies to promote strong T helper and cytotoxic T cell differentiation while minimizing the suppressive differentiation pressures provided by the tumor.
The αOX40 reagent is one of a new class of immunotherapeutic adjuvant antibodies, which target receptors such as CD134 and CD137 to provide positive signals and CD152 (CTLA4) and CD279 (PD1) to block negative signals to activated T cells. We show here for the first time that αOX40 therapy is an effective adjuvant to surgical therapy and in agreement with Yokouchi et al demonstrate that αOX40 therapy is an effective adjuvant to radiation therapy 54. Radiation has also been shown to synergize with agonist antibodies specific for CD137 55, and with adenoviral delivery of the TNFR family member LIGHT (Homologous to lymphotoxin, exhibits inducible expression, competes with herpes virus glycoprotein D for HVEM on T cells) 40. αCTLA-4 synergized with surgical therapy in a prostate cancer model to enhance clearance of residual microscopic disease 56 and radiation therapy combined with αCTLA4 in a breast cancer model extended survival 57. Importantly, in these models αCTLA4 treatment reduced the number of metastases. These data suggest that antibodies targeting T cell regulatory receptors have significant potential as adjuvant therapies in combination with existing primary therapies such as surgery and radiation and may have a particular role in the control of residual microscopic disease.
In summary, antibodies to OX40 are known to specifically target T cells that have recently been triggered by antigen 14–17, may cause a transitory reduction in immune suppression 20,46, and recapitulate an element of an inflammatory state that is more akin to tissue autoimmunity than the standard suppressive tumor environment 48–50. The consequence is a synergy between αOX40 and primary therapy that can eliminate local tumor recurrence, with a potential to improve overall outcome. Residual microscopic disease following primary local therapy remains a problem in delivering curative treatment. We hypothesize that primary treatment of the tumor provides an excellent opportunity to exploit the immune system to target residual microscopic disease. For this reason it is critical to study the immunological consequences of primary therapies, and to harness the immune response in combination with surgery and radiation to enhance their therapeutic efficacy. Agonistic antibodies to OX40 are in a Phase I Clinical Trial at our institution, and the preclinical experiments described in this manuscript suggest translation of αOX40 as an adjuvant therapy to reduce local recurrence rates.
Acknowledgments
These experiments would not have been possible without the assistance of the animal core facility at Providence Portland Medical Center and the staff of Providence Radiation Oncology. We appreciate the efforts of Dr Walter Urba and Dr Charles Thomas in their review of the manuscript.
This work was funded by NIH grants CA122701 and CA102577, and by a 2007 Philips Medical Systems/RSNAResearch Resident Grant (MRC).
Footnotes
Financial Disclosure
Dr. Weinberg has patents covering the use of OX40 agonists in cancer. All other authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Klein G, Klein E. Immune surveillance against virus-induced tumors and nonrejectability of spontaneous tumors: contrasting consequences of host versus tumor evolution. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2121–2125. doi: 10.1073/pnas.74.5.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan DH, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton DL. Changing concepts of cancer surgery: surgery as immunotherapy. Am J Surg. 1978;135:367–371. doi: 10.1016/0002-9610(78)90067-3. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh EC, et al. Does endogenous immune response determine the outcome of surgical therapy for metastatic melanoma? Annals of surgical oncology. 2000;7:232–238. doi: 10.1007/BF02523659. [DOI] [PubMed] [Google Scholar]
- 6.White LM, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. International journal of radiation oncology, biology, physics. 2005;61:1439–1445. doi: 10.1016/j.ijrobp.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Fabrizio PL, Stafford SL, Pritchard DJ. Extremity soft-tissue sarcomas selectively treated with surgery alone. International journal of radiation oncology, biology, physics. 2000;48:227–232. doi: 10.1016/s0360-3016(00)00601-5. [DOI] [PubMed] [Google Scholar]
- 8.Geer RJ, Woodruff J, Casper ES, Brennan MF. Management of small soft-tissue sarcoma of the extremity in adults. Arch Surg. 1992;127:1285–1289. doi: 10.1001/archsurg.1992.01420110027007. [DOI] [PubMed] [Google Scholar]
- 9.Fleming JB, et al. Long-term outcome of patients with American Joint Committee on Cancer stage IIB extremity soft tissue sarcomas. J Clin Oncol. 1999;17:2772–2780. doi: 10.1200/JCO.1999.17.9.2772. [DOI] [PubMed] [Google Scholar]
- 10.Wisnivesky JP, Bonomi M, Henschke C, Iannuzzi M, McGinn T. Radiation therapy for the treatment of unresected stage I–II non-small cell lung cancer. Chest. 2005;128:1461–1467. doi: 10.1378/chest.128.3.1461. [DOI] [PubMed] [Google Scholar]
- 11.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 12.Rosen EM, Fan S, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer investigation. 1999;17:56–72. [PubMed] [Google Scholar]
- 13.Nesslinger NJ, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 14.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 15.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 16.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 17.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL Promote the Persistence of CD8 T Cells to Recall Tumor-Associated Antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 18.Morris A, et al. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg AD, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 20.Gough MJ, et al. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 21.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 22.Pippig SD, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–6529. [PubMed] [Google Scholar]
- 23.Marzo AL, et al. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–5845. [PubMed] [Google Scholar]
- 24.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 26.Gramaglia I, et al. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 27.Weatherill AR, Maxwell JR, Takahashi C, Weinberg AD, Vella AT. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell Immunol. 2001;209:63–75. doi: 10.1006/cimm.2001.1783. [DOI] [PubMed] [Google Scholar]
- 28.Lee SW, et al. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 29.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 30.Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009 doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer SL, et al. Reduced L-selectin (CD62LLow) expression identifies tumor-specific type 1 T cells from lymph nodes draining an autologous tumor cell vaccine. Cell Immunol. 2004;227:93–102. doi: 10.1016/j.cellimm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 33.Delorme EJ, Alexander P. Treatment of Primary Fibrosarcoma in the Rat with Immune Lymphocytes. Lancet. 1964;2:117–120. doi: 10.1016/s0140-6736(64)90126-6. [DOI] [PubMed] [Google Scholar]
- 34.Bursuker I, North RJ. Generation and decay of the immune response to a progressive fibrosarcoma. II. Failure to demonstrate postexcision immunity after the onset of T cell-mediated suppression of immunity. J Exp Med. 1984;159:1312–1321. doi: 10.1084/jem.159.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlieman M, Smith R, Kraybill WG. Adjuvant therapy for extremity sarcomas. Current treatment options in oncology. 2006;7:456–463. doi: 10.1007/s11864-006-0021-x. [DOI] [PubMed] [Google Scholar]
- 36.Hirschhorn-Cymerman D, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 38.Faler BJ, Macsata RA, Plummer D, Mishra L, Sidawy AN. Transforming growth factor-beta and wound healing. Perspectives in vascular surgery and endovascular therapy. 2006;18:55–62. doi: 10.1177/153100350601800123. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, et al. Adoptive transfer of tumor-reactive transforming growth factor-beta-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer. Cancer Res. 2005;65:1761–1769. doi: 10.1158/0008-5472.CAN-04-3169. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009 doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart CC, Perez CA. Effect of irradiation on immune responses. Radiology. 1976;118:201–210. doi: 10.1148/118.1.201. [DOI] [PubMed] [Google Scholar]
- 42.Weichselbaum RR, Hallahan D, Fuks Z, Kufe D. Radiation induction of immediate early genes: effectors of the radiation-stress response. Int J Radiat Oncol Biol Phys. 1994;30:229–234. doi: 10.1016/0360-3016(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 43.Haiping Z, et al. Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene. Cancer Gene Ther. 2006;13:864–872. doi: 10.1038/sj.cgt.7700959. [DOI] [PubMed] [Google Scholar]
- 44.Nishioka A, et al. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys. 2004;58:1235–1241. doi: 10.1016/j.ijrobp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Anscher MS, et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 46.Takeda I, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 47.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsumura Y, et al. Expression of CD134 and CD134 ligand in lesional and nonlesional psoriatic skin. Archives of dermatological research. 2003;294:563–566. doi: 10.1007/s00403-002-0363-6. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg AD, et al. Target organ-specific up-regulation of the MRC OX-40 marker and selective production of Th1 lymphokine mRNA by encephalitogenic T helper cells isolated from the spinal cord of rats with experimental autoimmune encephalomyelitis. J Immunol. 1994;152:4712–4721. [PubMed] [Google Scholar]
- 50.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–863. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohshima Y, et al. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 52.Valzasina B, et al. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 53.Ruby CE, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokouchi H, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99:361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res. 2006;26:3445–3453. [PubMed] [Google Scholar]
- 56.Kwon ED, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci U S A. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]