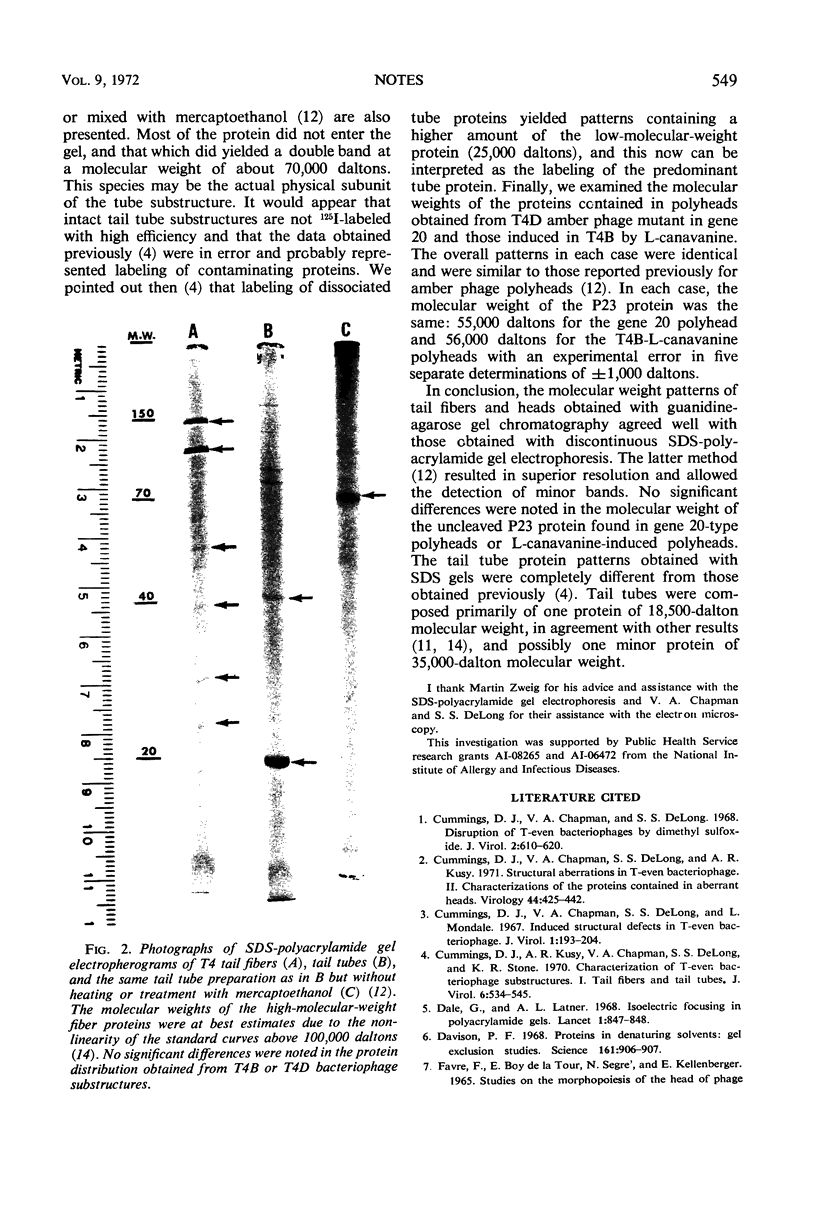
Abstract
T-even bacteriophage substructural proteins were studied by using discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis. It was found that tail fibers are composed of two major proteins of 155,000 and 120,000 daltons molecular weight and four minor proteins of 51,000, 38,000, 27,000, and 23,000 daltons. Tail tubes were composed of one predominant protein of 18,500 daltons and one minor protein of 35,000 daltons molecular weight. Tubular polyheads obtained from a T4D amber mutant and by treatment of T4B-infected cells with L-canavanine were also examined, and no significant differences were noted in the molecular weight of the P23 protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cummings D. J., Chapman V. A., De Long S. S., Mondale L. Induced structural defects in T-even bacteriophage. J Virol. 1967 Feb;1(1):193–204. doi: 10.1128/jvi.1.1.193-204.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., Kusy A. R., DeLong S. S. Structural aberrations in T-even bacteriophage. II. Characterization of the proteins contained in aberrant heads. Virology. 1971 May;44(2):425–442. doi: 10.1016/0042-6822(71)90273-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Kusy A. R., Chapman V. A., DeLong S. S., Stone K. R. Characterization of T-even bacteriophage substructures. I. Tail fibers and tail tubes. J Virol. 1970 Oct;6(4):534–544. doi: 10.1128/jvi.6.4.534-544.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale G., Latner A. L. Isoelectric focusing in polyacrylamide gels. Lancet. 1968 Apr 20;1(7547):847–848. doi: 10.1016/s0140-6736(68)90303-6. [DOI] [PubMed] [Google Scholar]
- Davison P. F. Proteins in denaturing solvents: gel exclusion studies. Science. 1968 Aug 30;161(3844):906–907. doi: 10.1126/science.161.3844.906. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]





