Abstract
Background. Septic arthritis is a severe and rapidly debilitating disease mainly caused by Staphylococcus aureus. Here, we assess the antiarthritic efficiency of glutaminyl cyclase (QC) inhibitors.
Methods. Mice were inoculated with an arthritogenic amount of S. aureus intravenously or by local administration into the knee joint. Animals were treated with QC inhibitors (PBD155 and PQ529) via chow during the experiment. QC and isoQC knockout mice were also analyzed for arthritis symptoms after local administration of bacteria.
Results. Both QC inhibitors significantly delayed the onset of clinical signs of arthritis, and inhibitors significantly decreased weight loss in treated animals. Following intraarticular injection of S. aureus, PBD155-treated mice had lower levels of synovitis and bone erosion, as well as less myeloperoxidase in synovial tissue. Fluorescence-activated cell sorter analysis revealed that PBD155 treatment affected the expression pattern of adhesion molecules, preventing the upregulation of cells expressing CD11b/CD18.
Conclusion. The compounds investigated here represent a novel class of small molecular antiarthritic inhibitors. In our studies, they exerted strong antiinflammatory actions, and therefore they might be suited for disease-modifying treatment of infectious arthritis.
Keywords: Glutaminyl cyclases, septic arthritis, posttranslational modifications, neutrophils
Staphylococcus aureus–induced septic arthritis is a medical emergency because of its potential for causing mortality and rapid joint destruction, particularly in patients with rheumatoid arthritis or immunocompromised status [1]. To study the pathogenesis of this disease, we used a mouse model of S. aureus–induced arthritis in which intravenous administration of S. aureus leads to clinical and histological signs of arthritis within 48 hours [2]: neutrophils and activated macrophages infiltrate the synovium within 1 day, and T cells follow within a few days.
The extravasation of circulating neutrophils into inflamed tissues, with the subsequent release of proteases and production of reactive oxygen species, is an essential component of the innate immune response [3]. Neutrophils are also heavily involved in the recruitment of monocytes by releasing chemokines [4]. Although neutrophils are important effector cells for the elimination of invading pathogens, they can also cause collateral damage to surrounding tissue [5]. Indeed, cartilage destruction caused by neutrophil accumulation is a prominent feature of septic arthritis. Neutrophil-mediated tissue damage has been attributed to a variety of proteolytic enzymes released by these cells, particularly elastase and cathepsin G [6, 7]. In addition, neutrophils produce numerous inflammatory cytokines, including interleukin 8 and tumor necrosis factor α, that significantly contribute to the destructive cycle [8]. Therefore, the influx of neutrophils into joints represents an attractive target for the development of new therapeutic strategies for septic arthritis.
The migration of circulating neutrophils and monocytes to the site of inflammation is tightly controlled by their interaction with the vascular endothelium [9]. Two β2 integrins expressed on the cell surface of leukocytes, lymphocyte function-associated antigen 1 (CD11a/CD18) and Mac-1 (CD11b/CD18), and their counterpart expressed on endothelial cells, intercellular adhesion molecule 1, are crucial for neutrophil adhesion and migration [10, 11]. Furthermore, E-selectin and P-selectin, expressed mainly on the endothelium, together with L-selectin, expressed on the surface of neutrophils, are abundant at sites of inflammation and are also important in controlling migration across the endothelium [12].
We recently reported that the isoenzyme of glutaminyl cyclase (isoQC) has an important role in monocyte infiltration under inflammatory conditions by mediating the posttranslational modification of monocyte chemoattractant protein 1 (MCP-1/CCL2) [13]. This might be relevant to septic arthritis because the switch from neutrophil to monocyte recruitment is regulated by CCL2 [14].
CCL2 is one of the major chemokines produced by activated neutrophils and is crucial for monocyte migration to the site of inflammation and for the switch from acute to chronic inflammation [15]. CCL2 can exert a direct effect on neutrophil recruitment in vitro [16], and administration of CCL2 in the presence of endotoxins induces strong migration of neutrophils to the site of inflammation [17]. Similarly, neutralization of CCL2 causes a decrease in neutrophil influx in a septic peritonitis model through effects on the neutrophil chemoattractant leukotriene B4 [18].
The activity and stability of CCL2 are dependent on posttranslational conversion of its N-terminal glutamine into pyroglutamate [13, 19]. This conversion is catalyzed by QCs and is required for the function of several proteins [20, 21]. QC is involved in the pathology of diseases such as Alzheimer's disease [22], melanoma [23], osteoporosis [24], and rheumatoid arthritis [25]. Recently, an isoenzyme of QC was discovered in both humans and mice. This isoenzyme possesses nearly identical substrate specificity but differs in subcellular localization, with QC being secreted from cells and isoQC being retained in the Golgi apparatus [13, 26, 27].
To balance the inflammatory response and protect the host from pathogens while avoiding extensive tissue damage from excessive activation of the immune system, all cells, chemokines, and other components of the immune system have to work in concert. In the present study, we evaluated the effect of 2 inhibitors of QC/isoQC, PBD155 and PQ529, on inflammatory disease, using an animal model of S. aureus–induced septic arthritis.
METHODS
Mice
NMRI mice were obtained from Charles River Laboratories. Ten QC knockout mice [28] with the genetic background of 99.2% C57Bl/6 and 0.8% 129Sv, 10 isoQC knockout mice [13] produced in the C3J strain, and 20 wild-type littermates (10 C3J mice and 10 C57Bl/6/129Sv mice) were used.
Treatment With QC/isoQC Inhibitors
Isoform-nonspecific QC/isoQC inhibitors PQ529 [13] and PBD155 (Probiodrug) were used. Standard laboratory chow supplemented with PQ529 or PBD155 was generated by Ssniff at a concentration of 6.8 g/kg. Mice were pretreated with supplemented chow 3 days before induction of septic arthritis. Ethics approval was obtained from the Animal Research Committee of University of Gothenburg.
Bacterial Strain and Induction of Septic Arthritis
Staphylococcus aureus strain LS-1, originating from a joint of a spontaneously arthritic NZB/W mouse, was used [29]. The bacteria were diluted in phosphate-buffered saline (PBS), and mice received intravenous inoculation of 107 colony-forming units (CFU) on day 0.
Determination of Bacterial Growth
Growth of staphylococci in the kidneys was determined 3 and 14 days after intravenous injection of S. aureus. Kidneys were removed aseptically, homogenized in PBS, and plated on blood agar plates. After incubation for 24 hours at 37°C, the number of CFU per animal was calculated.
Induction and Registration of Olive Oil–Induced Inflammation
Inflammation was induced by intradermal injection of 30 μL of olive oil (Apoteksbolaget) in the hind footpad. Footpad swelling was measured 26 hours after injection, using an Oditest spring caliper. The intensity of the olive oil–induced inflammation was expressed as [(footpad thickness at 26 hours) – (footpad thickness at 0 hours)] × 10–3 cm.
Clinical Evaluation of Arthritis
Clinical scoring of arthritis of the paws was assessed every second day through the following scoring system: 0, no sign of inflammation; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; and 3, marked swelling and erythema. Swelling and erythema of a toe or finger was scored as 0.5. Clinical score was calculated by adding scores for all 4 paws, resulting in a maximum score of 12 per mouse. In addition, mice weights were monitored.
Histological Evaluation
Mice were sacrificed on day 3 and 14, and paws with elbows and knees were removed for histological assessment. Following decalcification with Parengy (Histolab), samples were embedded in paraffin, cut, and stained with hematoxylin-eosin, and sections were evaluated by a blinded examiner for synovitis and erosion of bone and/or cartilage. Synovitis was defined as a membrane thickness of >2 cell layers. A mean score of 12 joints for all inspected animals was calculated, giving a maximum arthritis index of 3 per animal. The following histological scoring system of synovitis and bone erosion was used: 1, mild; 2, moderate; and 3, severe [30].
Intraarticular Challenge of S. aureus
Treated mice and knockout mice were intraarticularly inoculated with 104 CFU of S. aureus and sacrificed 3 days later. Injected knees were obtained for histological evaluation, as previously described.
Determination of Synovial Myeloperoxidase Content
The synovial membrane was dispersed into a single-cell suspension, and cells were lysed for 1 hour at room temperature in 20 µL of lysis buffer containing 0.2% cetrimonium bromide (Sigma-Aldrich) and 0.2% bovine serum albumin (Sigma-Aldrich) in PBS. The peroxidase substrate 1,2-phenylenediamine dihydrochloride (Dako) was dissolved according to the manufacturer's instructions and mixed with H2O2 immediately before use. A total of 40 μL of peroxidase substrate was added to the samples, and the samples were incubated for 1 hour at room temperature. The absorbance was measured at 450 nm on a Spectra Max 340PC (Molecular Devices).
Flow Cytometric Analysis
Blood was obtained from 14 untreated or PBD155-treated mice 3 days after intraarticular inoculation of 104 CFU of S. aureus and compared with blood from 12 noninfected mice. Whole blood was subjected to isotonic lysis for elimination of erythrocytes. Cells were pelleted in a 96-well plate and blocked with Fc-block (BD Pharmingen). Cells were stained with eFluor 450–conjugated anti-CD11b (clone M1/70, BD Biosciences), fluorescein isothiocyanate (FITC)–conjugated CD18 (clone M18/2, eBioscience), and peridinin-chlorophyll-protein complex–conjugated anti-CD62L (clone MEL-14, BioLegend) for 30 minutes at 4°C. Cells were collected using FACSCantoII (BD-Bioscience) equipped with FACSDiva software. Analysis was performed using FlowJo software (Tree Star), and Fluorochrome Minus One was used for determination of positive populations and gating when needed.
Phagocytosis Assay
Samples of whole blood from 5 healthy NMRI mice treated for 3 days with inhibitor and samples from 4 untreated controls were evaluated by flow cytometry for phagocytosis of FITC-labeled E. coli, using Phagotest (Orpegen Pharma). Measurement was performed according to the manufacturer's instructions. Cells were collected using FACSCantoII with FACSDiva software. Analysis was performed using FlowJo software.
Statistical Analysis
Differences between 2 groups were tested for statistical significance by means of the Mann–Whitney U test. Kaplan-Meier plots were prepared, and the log-rank test was used for comparing the curves of arthritis frequency. Weight curves were analyzed using 2-way analysis of variance with the Bonferroni posttest. All statistical analyses were performed using GraphPad Prism, version 5.0d for Mac (GraphPad), and a P value of <.05 was considered statistically significant.
RESULTS
QC/isoQC Inhibitors Delay the Onset and Progression of Septic Arthritis
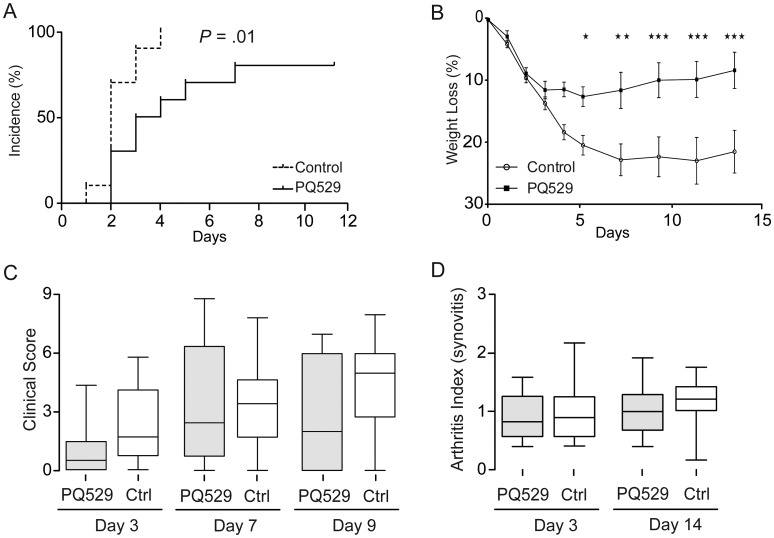
To investigate the role of QC/isoQC inhibition on the development of septic arthritis, mice were intravenously inoculated with S. aureus LS-1 and provided with chow impregnated with the inhibitors PQ529 (for 10 mice) and PBD155 (for 10 mice) for the duration of the experiments. Treatment with PQ529 resulted in a significant delay in the development of arthritis; at day 3, 90% of control mice displayed arthritis as compared to 50% of treated mice (P = .01; Figure 1A). Considerable weight loss occurs during septic arthritis because of inflammation. Mice treated with PQ529 showed a significantly lower rate of weight loss and recovered more rapidly (Figure 1B). Despite the effect of PQ529 on arthritis development and weight loss, it had no effect on the clinical scoring of joints (Figure 1C). Histological scoring of sectioned joints and paws at end point confirmed the clinical observations, with no differences between PQ529-treated mice and controls in both short-term and long-term experiments (Figure 1D).
Figure 1.
Glutaminyl cyclase inhibitor PQ529 delays development of arthritis. A, Kaplan-Meier plots displaying clinical onset of arthritis in 10 mice treated with PQ529 and 10 controls (P = .01, by the log-rank test). B, Weight curve during experiment. Initial weight was taken as 100% (*P < .05, **P < .01, and ***P < .005, by 2-way analysis of variance with the Bonferroni posttest). C, Clinical score of arthritis in mice. Analysis was performed by the Mann–Whitney U test. D, Articular inflammation at days 3 and 14. Analysis was performed by the Mann–Whitney U test. The horizontal line in the box-and-whiskers plot indicates the median, and the whiskers indicate the minimum and maximum. Abbreviation: Ctrl, control.
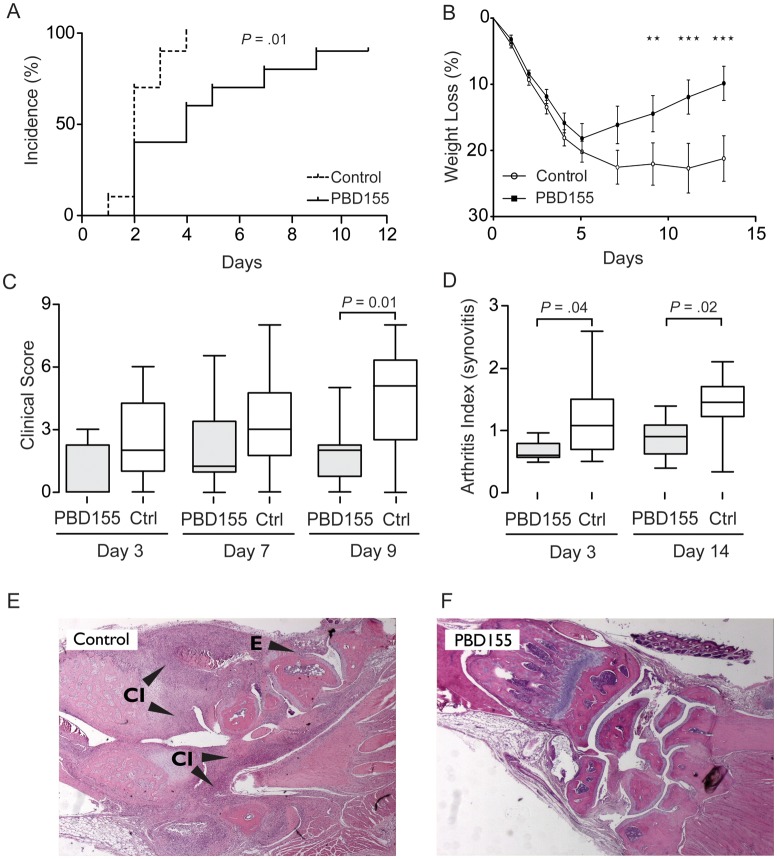
PBD155 was more effective than PQ529 in delaying the progression of septic arthritis. At day 3 after inoculation, only 40% of treated mice displayed clinical signs of arthritis, whereas 90% of control mice had arthritis (P = .01; Figure. 2A). Mice treated with PBD155 also had significantly less severe arthritis as compared to control mice. The clinical score of the control group increased rapidly until day 9, when it reached a mean of 5.1 (combined score, 40.5), compared with a mean of 1.9 (combined score, 19.0) in the treated group (P = .01; Figure 2C). All mice experienced a decrease in body weight during the development of septic arthritis. However, unlike the control group, mice treated with PBD155 started to gain weight after 5 days (Figure 2B).
Figure 2.
Glutaminyl cyclase inhibitor (PBD155) delays the onset and ameliorates the progression of septic arthritis. A, Kaplan–Meyer plot of onset of arthritis in 10 PBD155-treated mice and 10 controls (P = .01, by the log-rank test). B, Weight curve during the experiment with PBD155 (**P < .01 and ***P < .005, by 2-way analysis of variance with the Bonferroni posttest). C, Clinical scoring during the experiment (P = .01, by the Mann–Whitney U test). D, Articular inflammation at days 3 and 14 (P = .04 and P = .02, by the Mann–Whitney U test, on days 3 and 14, respectively). E and F, Representative histological changes in the front paw joints of PBD155-treated animals and nontreated controls (original magnification ×50). The horizontal line in the box-and-whiskers plot indicates the median, and the whiskers indicate the minimum and maximum. Abbreviations: CI, cell infiltration; Ctrl, control; E, erosions.
Histological evaluation for synovitis and erosions was performed at the early phase (day 3, for 20 mice) and late phase (day 14, for 20 mice) of inflammation. Morphological examination confirmed that the inhibition of QC/isoQC with PBD155 alleviated arthritis. Cell infiltration at both time points was significantly reduced by PBD155 treatment at day 3 (mean arthritis index [±SEM], 0.55 ± 0.04) and at day 14 (mean arthritis index [±SEM], 0.72 ± 0.08) as compared to the control group (mean arthritis index [±SEM], 1.01 ± 0.17 and 1.18 ± 0.15 at days 3 and 14, respectively; Figure 2E and 2F). The extent of erosion did not differ between the groups at any time point. Because PBD155 was more effective than PQ529 in our model, we focused on PBD155 in subsequent experiments.
PBD155 Treatment Does Not Affect Bacterial Survival and Phagocytic Clearance In Vivo
Bacterial load in the kidneys was examined by counting the number of CFU per kidney. Kidneys from mice treated with PBD155 contained a similar number of bacteria as kidneys from control mice after 3 days (mean CFU/kidney [±SEM], 1.6 ± 0.7 × 107 in the PBD155 group vs 0.98 ± 0.8 × 107 in the control group; P = .15) and 14 days (mean CFU/kidney [±SEM], 7.8 ± 0.3 × 107 in the PBD155 group vs 4.3 ± 0.3 × 107 in the control group; P = .15), suggesting that QC/isoQC inhibition had no impact on bacterial clearance. Treatment with PQ529 also did not affect bacterial count in kidneys (data not shown). We also found that QC/isoQC inhibition had no effect on bacterial phagocytosis by neutrophils derived from control and inhibitor-treated mice (Supplementary Figure 1; P = .11).
PBD155 Significantly Reduces Joint Damage After Local Administration of Bacteria
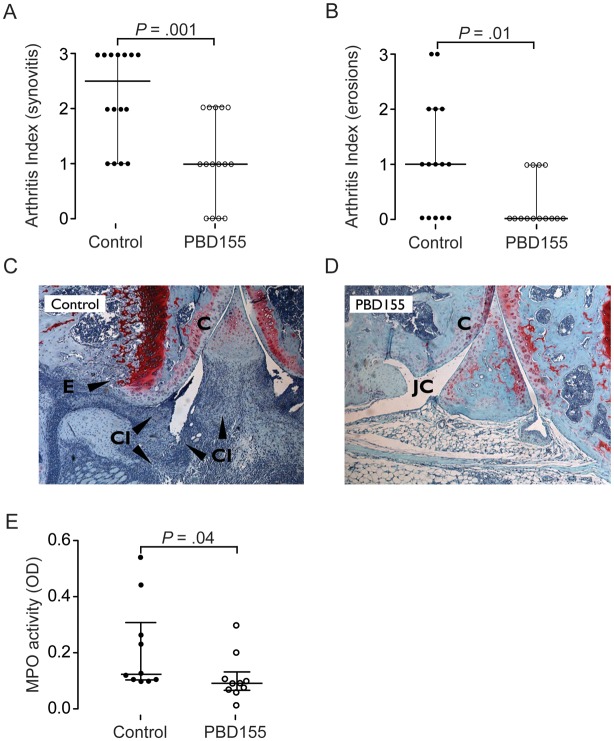
To further investigate the effects of PBD155 on inflammatory processes within the joint cavity, PBD155-treated mice and control mice received an intraarticular injection of 104 CFU of S. aureus. Histological evaluation of the knees after 3 days revealed that the treatment had a significant effect on synovial inflammation and bone/cartilage destruction. Synovitis in the control group was 2.25-fold higher (mean arthritis index [±SEM], 2.25 ± 0.21) than that in the treated group (mean arthritis index [±SEM], 1.0 ± 0.20) (P = .001; Figure 3A and 3C). Furthermore, bone and cartilage destruction was reduced in the treatment group (P = .01; Figure 3B and 3D).
Figure 3.
Glutaminyl cyclase inhibition reduces arthritis after local Staphylococcus aureus infection. A and B, Synovitis (A) and erosion (B) following intraarticular injection of S. aureus in 15 treated mice and 15 control mice (P = .001 and P = .01, by the Mann–Whitney U test). The bars represent the median. C and D, Representative histopathological (safranin O) stainings of specimens from the knee joint of a control mouse (C) and treated mouse (D). The black arrowheads indicate areas with cartilage and bone destruction. E, The myeloperoxidase activity of neutrophils in the joint cavity of 10 treated mice and 10 nontreated littermates (P = .04, by the Mann–Whitney U test). Abbreviations: C, cartilage; CI, cell infiltration; E, erosions; JC, joint cavity; MPO, myeloperoxidase; OD, optical density.
QC/isoQC Inhibition Affects Leukocyte Migration to the Site of Inflammation
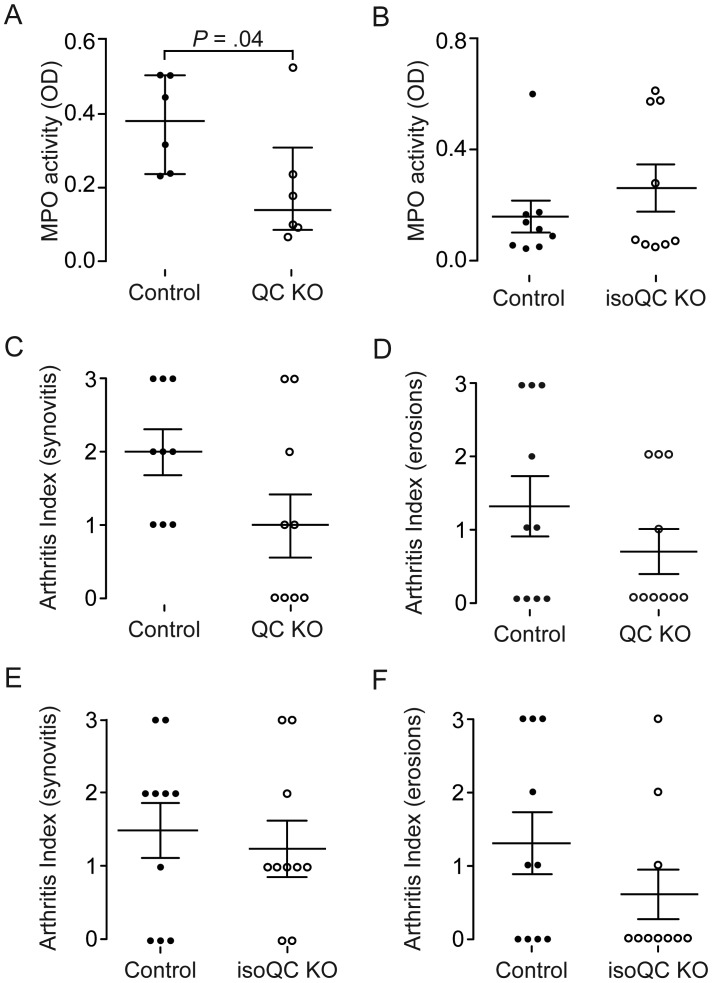
Three days after intraarticular inoculation with S. aureus, synovial membranes were excised to determine myeloperoxidase (MPO) activity as a means of enumerating infiltrating neutrophils. Treated mice showed lower MPO levels within the synovial membrane as compared to control mice (P = .04; Figure 3E). To delineate the role of QC and isoQC in neutrophil influx, we repeated the experiment, using QC knockout mice and isoQC knockout mice. Interestingly, QC knockout mice had much lower levels of MPO within their synovial membranes (P = .04; Figure 4A), whereas isoQC knockout mice showed no differences in the number of infiltrating neutrophils (Figure 4B). Additionally, we tested the capacity of inflammatory cells to migrate to the site of inflammation, using a paw edema assay. This assay revealed that PBD155 decreased cell influx at 26 hours after injection, compared with control mice (P = .02; Supplementary Figure 2).
Figure 4.
Knockout (KO) of a single glutaminyl cyclase (QC) isoform does not protect against septic arthritis. Ten homozygous QC KO mice and 10 isoform QC (isoQC) KO mice were intraarticulary inoculated with 1 × 104 Staphylococcus aureus. Knee joints and synovial membranes were excised on day 3 for histological evaluation and assessment of myeloperoxidase (MPO) activity in the joints. A, Levels of MPO activity in 6 QC KO mice and 7 wild-type mice (P = .04, by the Mann–Whitney U test). B, MPO activity in synovia of 9 isoQC KO mice and 9 wild-type littermates. C and D, Histological evaluation of lymphocyte infiltration (C) and erosions (D) in the joints of 10 QC KO mice and 10 wild-type controls. E and F, Evaluation of synovitis (E) and erosions (F) in 10 isoQC KO mice and 10 wild-type mice. Abbreviation: OD, optical density.
Inhibition of Both Isoforms Is Required to Prevent Joint Damage
To explore the importance of both isoenzymes in protection against inflammatory joint damage after bacterial inoculation, QC knockout mice, isoQC knockout mice, and wild-type mice received intraarticular inoculations of 1 × 104 CFU of S. aureus. Three days after S. aureus inoculation, there was a histological trend toward decreased synovitis and bone/cartilage destruction in the QC knockout mice and isoQC knockout mice, but the difference was not significant. This outcome contrasted with the significant difference in synovitis and bone/cartilage destruction obtained with QC/isoQC inhibition. However, in the QC knockout group, synovitis was present in 60% of mice (mean arthritis index [±SEM], 1.1 ± 0.38), compared with 100% of wild-type controls (mean arthritis index [±SEM], 1.9 ± 0.28). In addition, 3 of 10 wild-type mice had high bone/cartilage erosion scores (mean erosion index [±SEM], 1.3 ± 0.42), whereas QC knockout mice had a considerably reduced degree of damage (mean erosion index [±SEM], 0.7 ± 0.3; Figure 4C and 4D). In isoQC knockout mice, synovitis was similar level to that in controls but erosion was decreased, although the difference in erosion was not statistically significant (Figure 4E and 4F).
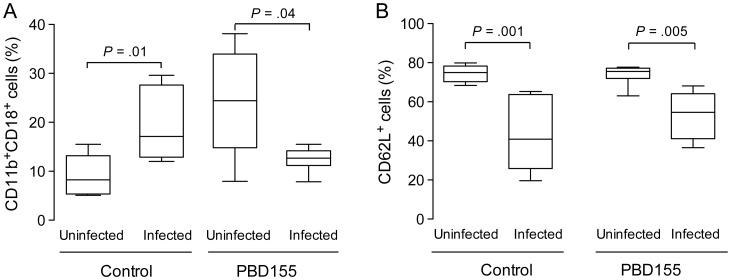
QC/isoQC Inhibition Changes the Pattern of CD11b/CD18 and CD62L Expression in Blood
Changes in integrin expression in the blood of mice were observed by flow cytometry 72 hours after intraarticular inoculation with S. aureus. Upregulation of the CD11b/CD18 complex in infected mice could be clearly seen (P = .01; Figure 5A). In contrast, mice treated with PBD155 and inoculated with S. aureus showed a significantly decreased number of cells positive for CD11b/CD18 after 72 hours (P = .04; Figure 5A). This suggests that PBD155 treatment may affect the ability of neutrophils to migrate to the site of inflammation. On the basis of these results, we explored the activation level of immune cells by measuring cell surface expression of CD62L (L-selectin). The percentage of cells expressing CD62L in the control group decreased significantly, by 34.1%, 72 hours after infection (P = .001; Figure 5B). CD62L expression also decreased in the PDB155-treated group as compared to the uninfected group, but by 20.9% (P = .005; Figure 5B).
Figure 5.
Treatment with glutaminyl cyclase inhibitor (PBD155) changes the pattern of integrin expression. NMRI mice were inoculated with 1 × 104 Staphylococcus aureus in the joint cavity, and on day 3 the expression of integrins was evaluated through flow cytometric analysis. A, CD11b/CD18 expression on cells circulating in the blood of control mice (P = .01), and treated mice (P = .04). B, Expression of CD62L in the blood of the control group (P = .001) and in infected mice treated with PBD155 (P = .005). The horizontal line in the box-and-whiskers plot indicates the median, and the whiskers indicate the minimum and maximum. Differences between groups were analyzed using the Mann–Whitney U test.
DISCUSSION
Posttranslational modification of amino acids with various functional groups is used as a cellular strategy to fine-tune the function of numerous proteins [31]. We previously showed that the activity of CCL2, a key mediator in several inflammatory diseases, relies on the enzymatic conversion of the N-terminal glutamine into pyroglutamate in a reaction catalyzed by iso-glutaminyl cyclase [13]. The formation of N-terminal pyroglutamate modulates bioactivity but also protects proteins and peptides against degradation by aminopeptidases [32]. Here, we used oral inhibitors (PQ529 and PBD155) to show the importance of QC/isoQC for disease development, neutrophil migration to the inflammation site, and synovitis and bone/cartilage erosion in hematogenously induced S. aureus arthritis.
In S. aureus arthritis, mice treated with PQ529 developed clinical signs of arthritis significantly later than control mice. PQ529-treated mice also had less weight loss during the acute phase of the disease and gained weight faster during recovery. PBD155, a more optimized compound than PQ529, was also effective, leading to a significant delay in arthritis development, reduced weight loss, and a lower clinical score. Histological assessments were consistent with clinical observations: treatment with PBD155 led to a significant reduction in synovitis at days 3 and 14.
The peak bacterial burden after intravenous S. aureus inoculation typically occurs in blood within the first 3 days and in kidneys within 1 week [33]. Bacterial clearance is dependent on phagocytosis by neutrophils [34], which is the primary leukocyte population in the joint lesions [2]. However, activated neutrophils also serve as the primary cells responsible for tissue damage by releasing damaging proteinases and proinflammatory cytokines [35]. NADPH oxidase–derived superoxide discharged from infiltrating neutrophils also contributes to the degradation of the joint surface [36].
We detected lower MPO activity, a measure of neutrophil numbers, in QC inhibitor–treated mice, which suggests reduced infiltration of neutrophils. This contrasts with previous results showing that granulocyte infiltration in thioglycollate-induced peritonitis and lipopolysaccharide-induced lung inflammation was not affected in QC and isoQC knockout mice [13]. QC and isoQC exhibit nearly identical substrate specificity and are differentiated only by their subcellular localization: QC is secreted to the extracellular milieu, and isoQC remains in the Golgi apparatus [26]. Because the compounds used in this study inhibit both isoenzymes, we used QC and isoQC knockout mice to determine whether the beneficial effects of treatment depend on the inhibition of a certain isoform or whether both isoforms need to be blocked for the therapeutic effect.
In QC-deficient mice and isoQC-deficient mice, there was a trend toward a lower arthritis score following local inoculation of bacteria, compared with the score for wild-type mice. However, only in the QC knockout mice were MPO levels significantly lower than those in the other infected groups. Under septic conditions, CCL2 regulates neutrophil recruitment via direct chemotactic activity and via modulatory effects on CCL8 and leukotriene B4 [37]. In addition, bone marrow neutrophils express fully functional CCR2, which suggests a role for CC chemokines in the differentiation and regulation of neutrophil functions [16].
The data presented here provide evidence that QC/isoQC might play a role in leukocyte infiltration under certain experimental settings, such as septic arthritis. Clearly, CCL2 is not the sole inflammatory effector molecule affected by QC/isoQC inhibition. Apart from known targets such as CCL-2, -7, -8, and -13, there might be other unknown targets for QC/isoQC that may affect the inflammatory response in this experimental setting. These studies of QC/isoQC knockout mice provide clear evidence that the inhibition of both isoforms is required to alleviate septic arthritis in the intraarticular injection model.
FACS analysis suggests that QC inhibition affected integrin expression. Activation of circulating neutrophils results in rapid upregulation of CD11b/CD18 expression, which allows neutrophil immobilization and subsequent transmigration to the site of inflammation [38]. Upregulation of CD11b/CD18 was observed in the control group after S. aureus administration into the joint but not in the group treated with PBD155. Treatment with PBD155 by itself caused a higher extent of CD11b/CD18 expression before infection, followed by a reduction during infection. Although the cause of this increased expression in healthy mice is unknown, it is clear that the infiltration of leukocytes at site of inflammation is reduced. Downregulation of CD11b/CD18 could result in decreased affinity between neutrophils and the endothelium, which could explain the reduced neutrophil transmigration to the site of inflammation in PBD155-treated mice. The observed downregulation of CD62L ligand may also have contributed to the impaired migration to the inflamed tissue in PBD155-treated mice. Leukocytes constitutively express CD62L, which plays a pivotal role in attachment to the endothelium at the site of inflammation [39]. In response to cell activation by various stimuli, including lipoteichoic acid, a major component of S. aureus cell wall [40], and lipopolysaccharide [41], the expression of CD62L is downregulated [42]. We observed downregulation of CD62L in the treated group, indicating that L-selectin is not responsible for the reduced infiltration in joint.
To summarize, we have shown that treatment of mice with specific QC/isoQC inhibitors alleviates the development and progression of experimental septic arthritis. Collectively, we provide compelling evidence that the treatment decreases cell infiltration into the synovium and reduces synovitis and bone/cartilage destruction. Although not all substrates targeted by QC inhibitors are known, we suggest that the therapeutic effect is at least partially based on the impaired ability of neutrophils to massively migrate to the site of inflammation and inflict major damage. However, even though we see a decreased capacity of neutrophils to migrate into the inflamed synovial tissue, our phagocytosis experiments proved polymorphonuclear cells to be fully capable of clearing bacteria and preventing dissemination of the infection. Because QC/isoQC inhibitors are approaching the regulatory stage for the treatment of various inflammatory disorders, this study provides novel insights into their mechanism of action and opens doors to the development of new combination therapies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the Medical Society of Göteborg; the Swedish Society of Medicine, Tore Nilsson Foundation; the Swedish Association Against Rheumatism; the Nanna Swartz Foundation; the Rune and Ulla Amlövs Trust; and the European Commission FP7 (Gums & Joints no. 261460) and Marie Curie ITN (RAPID no. 290246). K. M. and J. P. are supported by a Marie Curie Reintegration Grant (PIRG03-GA-2008-230850JP), the National Institutes of Health (grant DE 09761), the National Science Center (2011/01/B/NZ6/00268, Kraków, Poland), and the Foundation for Polish Science (TEAM project DPS/424-329/10). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (POIG.02.01.00-12-064/08).
Potential conflicts of interest. S. S., U. H., and H. C. are former or present employees of Probiodrug. S. G. is employee of Ingenium Pharmaceuticals. H. U. D. is chief science officer of Probiodrug and managing director of Ingenium Pharmaceuticals, a daughter company of Probiodrug, and holds stock in the Probiodrug group. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Koopman WJ, Moreland LW. Arthritis and allied conditions: a textbook of rheumatology. 15th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 2577–92. [Google Scholar]
- 2.Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–85. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Soehnlein O, Zernecke A, Eriksson EE, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–71. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–4. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 6.Velvart M, Fehr K. Degradation in vivo of articular cartilage in rheumatoid arthritis and juvenile chronic arthritis by cathepsin G and elastase from polymorphonuclear leukocytes. Rheumatol Int. 1987;7:195–202. doi: 10.1007/BF00541377. [DOI] [PubMed] [Google Scholar]
- 7.Hilbert N, Schiller J, Arnhold J, Arnold K. Cartilage degradation by stimulated human neutrophils: elastase is mainly responsible for cartilage damage. Bioorg Chem. 2002;30:119–32. doi: 10.1006/bioo.2002.1242. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 9.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–35. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 10.Henderson RB, Lim LH, Tessier PA, et al. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med. 2001;194:219–26. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol. 2010;185:7057–66. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–12. [PubMed] [Google Scholar]
- 13.Cynis H, Hoffmann T, Friedrich D, et al. The isoenzyme of glutaminyl cyclase is an important regulator of monocyte infiltration under inflammatory conditions. EMBO Mol Med. 2011;9:545–58. doi: 10.1002/emmm.201100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 15.Yamashiro S, Kamohara H, Yoshimura T. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-alpha plays a role in maximal MCP-1 mRNA expression. J Leukoc Biol. 1999;65:671–9. doi: 10.1002/jlb.65.5.671. [DOI] [PubMed] [Google Scholar]
- 16.Iida S, Kohro T, Kodama T, Nagata S, Fukunaga R. Identification of CCR2, flotillin, and gp49B genes as new G-CSF targets during neutrophilic differentiation. J Leukoc Biol. 2005;78:481–90. doi: 10.1189/jlb.0904515. [DOI] [PubMed] [Google Scholar]
- 17.Maus U, Huwe J, Maus R, Seeger W, Lohmeyer J. Alveolar JE/MCP-1 and endotoxin synergize to provoke lung cytokine upregulation, sequential neutrophil and monocyte influx, and vascular leakage in mice. Am J Respir Crit Care Med. 2001;164:406–11. doi: 10.1164/ajrccm.164.3.2009055. [DOI] [PubMed] [Google Scholar]
- 18.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–54. [PubMed] [Google Scholar]
- 19.Van Coillie E, Proost P, Van Aelst I, et al. Functional comparison of two human monocyte chemotactic protein-2 isoforms, role of the amino-terminal pyroglutamic acid and processing by CD26/dipeptidyl peptidase IV. Biochemistry. 1998;37:12672–80. doi: 10.1021/bi980497d. [DOI] [PubMed] [Google Scholar]
- 20.Fischer WH, Spiess J. Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. Proc Natl Acad Sci U S A. 1987;84:3628–32. doi: 10.1073/pnas.84.11.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busby WH, Jr, Quackenbush GE, Humm J, Youngblood WW, Kizer JS. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem. 1987;262:8532–6. [PubMed] [Google Scholar]
- 22.Schilling S, Zeitschel U, Hoffmann T, et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat Med. 2008;14:1106–11. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- 23.Muthusamy V, Duraisamy S, Bradbury CM, et al. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 2006;66:11187–93. doi: 10.1158/0008-5472.CAN-06-1274. [DOI] [PubMed] [Google Scholar]
- 24.Ezura Y, Kajita M, Ishida R, et al. Association of multiple nucleotide variations in the pituitary glutaminyl cyclase gene (QPCT) with low radial BMD in adult women. J Bone Miner Res. 2004;19:1296–301. doi: 10.1359/JBMR.040324. [DOI] [PubMed] [Google Scholar]
- 25.Batliwalla FM, Baechler EC, Xiao X, et al. Peripheral blood gene expression profiling in rheumatoid arthritis. Genes Immun. 2005;6:388–97. doi: 10.1038/sj.gene.6364209. [DOI] [PubMed] [Google Scholar]
- 26.Cynis H, Rahfeld JU, Stephan A, et al. Isolation of an isoenzyme of human glutaminyl cyclase: retention in the Golgi complex suggests involvement in the protein maturation machinery. J Mol Biol. 2008;379:966–80. doi: 10.1016/j.jmb.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 27.Stephan A, Wermann M, von Bohlen A, et al. Mammalian glutaminyl cyclases and their isoenzymes have identical enzymatic characteristics. FEBS J. 2009;276:6522–36. doi: 10.1111/j.1742-4658.2009.07337.x. [DOI] [PubMed] [Google Scholar]
- 28.Schilling S, Kohlmann S, Bauscher C, et al. Glutaminyl cyclase knock-out mice exhibit slight hypothyroidism but no hypogonadism: implications for enzyme function and drug development. J Biol Chem. 2011;286:14199–208. doi: 10.1074/jbc.M111.229385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremell T, Lange S, Svensson L, et al. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–44. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- 30.Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Genistein as an anti-inflammatory agent. Inflamm Res. 2003;52:341–6. doi: 10.1007/s00011-003-1182-8. [DOI] [PubMed] [Google Scholar]
- 31.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol. 2004;16:753–8. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Rink R, Arkema-Meter A, Baudoin I, et al. To protect peptide pharmaceuticals against peptidases. J Pharmacol Toxicol Methods. 2010;61:210–8. doi: 10.1016/j.vascn.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Verba V, Tarkowski A. Participation of V beta 4(+)-, V beta 7(+)-, and V beta 11(+)-T lymphocytes in haematogenously acquired Staphylococcus aureus nephritis. Scand J Immunol. 1996;44:261–6. doi: 10.1046/j.1365-3083.1996.d01-309.x. [DOI] [PubMed] [Google Scholar]
- 34.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–21. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–50. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 36.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275:20069–76. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 37.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun. 2011;79:2567–77. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheikh S, Nash GB. Continuous activation and deactivation of integrin CD11b/CD18 during de novo expression enables rolling neutrophils to immobilize on platelets. Blood. 1996;87:5040–50. [PubMed] [Google Scholar]
- 39.Griffin JD, Spertini O, Ernst TJ, et al. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990;145:576–84. [PubMed] [Google Scholar]
- 40.Lotz S, Aga E, Wilde I, et al. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J Leukoc Biol. 2004;75:467–77. doi: 10.1189/jlb.0803360. [DOI] [PubMed] [Google Scholar]
- 41.Wilson ME. Effects of bacterial endotoxins on neutrophil function. Rev Infect Dis. 1985;7:404–18. doi: 10.1093/clinids/7.3.404. [DOI] [PubMed] [Google Scholar]
- 42.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.