Abstract
New therapies to control tuberculosis are urgently required because of the inability of the only available vaccine, BCG, to adequately protect against tuberculosis. Here we demonstrate that proteins of the Mycobacterium tuberculosis sulfate-assimilation pathway (SAP) represent major immunogenic targets of the bacillus, as defined by strong T-cell recognition by both mice and humans infected with M. tuberculosis. SAP proteins displayed increased expression when M. tuberculosis was resident within host cells, which may account in part for their ability to stimulate anti-M. tuberculosis host immunity. Vaccination with the first enzyme in this pathway, adenosine-5′-triphosphate sulfurylase, conferred significant protection against murine tuberculosis and boosted BCG-induced protective immunity in the lung. Therefore, we have identified SAP components as a new family of M. tuberculosis antigens, and we have demonstrated that these components are promising candidate for inclusion in new vaccines to control tuberculosis in humans.
Keywords: tuberculosis, vaccine, sulfate metabolism, human immune response, ATP sulfurylase
Mycobacterium tuberculosis, the causative agent of tuberculosis, claims almost 2 million lives annually [1] and globally is the leading cause of death due to a single bacterial agent. The situation has become more critical in the past decade because of coinfection with human immunodeficiency virus (HIV) and the inefficiency of the current vaccine, BCG, to protect adults against tuberculosis [1]. The identification of novel and protective antigens recognized by infected individuals would represent a major advance in the control of tuberculosis and may form the basis of new therapeutics to limit disease spread. Current tuberculosis vaccines in clinical trials include viral-vectored or adjuvant-based subunit vaccines, as well as whole mycobacterial vaccines, that express 1 or more immunogenic M. tuberculosis antigens [2]. Most of these strategies are based on secreted antigens of M. tuberculosis that are presumably more readily “seen” by the host immune response [2]. The efficacy of these new candidate vaccines in protecting humans against M. tuberculosis infection is yet to be determined.
M. tuberculosis can survive in a broad spectrum of environmental conditions, including high levels of oxidative stress, low pH, and nutrient deprivation [3]. Exposure and adaption of M. tuberculosis to these conditions during infection requires the coordinated regulation of gene expression [4]. Genes involved in the metabolism of sulfur have consistently been identified as upregulated in conditions that mimic the macrophage environment [5–9] and during macrophage infection [10]. These genes encode enzymes of the sulfate-assimilation pathway (SAP) of M. tuberculosis, required for the reduction of sulfur. Indeed, sulfur-containing compounds are fundamental in a wide range of biological activities. In its reduced form, sulfur is used in the biosynthesis of the amino acid cysteine, one of the prime targets for reactive nitrogen intermediates encountered by M. tuberculosis in the intracellular environment [11]. Cysteine is subsequently incorporated into mycothiol, which functions analogously to glutathione [12] and is crucial to M. tuberculosis within the granuloma for regulating the redox balance on encountering free radicals released by host cells. Mutants of Mycobacterium smegmatis in which mycothiol biosynthesis has been abrogated exhibit high-level resistance to isoniazid and are more susceptible than wild-type strains to oxidative stress and antibiotics [13, 14]. This first line of defense by M. tuberculosis therefore is linked to the availability of cysteine, and as a result the SAP pathway is required for the organism's survival [15–17]. Reinforcing this increased need for cysteine in the macrophage environment is the upregulation of adenosine-5′-triphosphate (ATP) sulfurylase, the first enzyme in the SAP, on exposure to oxidative stress [9, 10]. Disabling the biosynthesis of cysteine attenuates bacterial virulence and persistence during the chronic phase of infection in mice [18].
Although members of the SAP are highly expressed under conditions encountered within the host, it is not known whether these proteins constitute immunogenic components of M. tuberculosis. The search for M. tuberculosis antigens has focused on secreted proteins of mycobacteria, as these are predicted to be recognized by early host immune responses [19, 20]. Sulfate reduction takes place within the cell, and for this reason SAP enzymes are either intracellular or membrane-bound components [21–23] and would not be detected in screens for immunogenic secreted antigens of M. tuberculosis. In this report, we demonstrate that SAP components are highly immunogenic components of M. tuberculosis, as they are recognized by T cells of M. tuberculosis-infected individuals and confer protective immunity in a murine model of tuberculosis. Our results suggest that SAP components are potential candidates for inclusion in new tuberculosis vaccines.
METHODS
Bacterial Strains and Growth Conditions
Escherichia coli K-12 and BL21 (DE3) was grown in Luria-Bertani broth or agar (Sigma-Aldrich). M. tuberculosis H37Rv was grown in Middlebrook 7H9 medium (Difco Laboratories) supplemented with 0.5% glycerol, 0.05% Tween 80%, and 10% albumin-dextrose-catalase (ADC) or on solid Middlebrook 7H11 medium (Difco Laboratories) supplemented with oleic acid–ADC. All cultures were grown at 37°C, with or without shaking. Antibiotics were added to media when required, at 25 µg/mL for kanamycin and 100 µg/mL for ampicillin.
Protein Antigens and DNA Vaccines
Culture filtrate protein (CFP) was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository (NR-14825). Concanavalin A (ConA) was purchased from Sigma-Aldrich. Purification of SAP protein antigens and construction of DNA vectors encoding SAP genes are described in Supplementary Table 1.
Macrophage Infection and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
The RAW264.7 mouse macrophage cell line was grown at 37°C in 5% CO2 in Roswell Park Memorial Institute (RPMI) medium (Gibco-BRL) supplemented with 10% fetal calf serum (Gibco-BRL) and 2 mM l-glutamine (Invitrogen; Complete RPMI). Adherent RAW264.7 cells were infected with M. tuberculosis at a multiplicity of infection of 1:1. Four hours after infection, macrophage monolayers were washed with phosphate-buffered saline, cells were incubated for an additional 48 hours in fresh medium, and total RNA was extracted for quantitative real-time RT-PCR analysis.
M. tuberculosis pellets from broth culture or M. tuberculosis–infected macrophages were resuspended in TRI reagent (Invitrogen) and disrupted with 0.1-mm zirconia/silica beads in a BioSpec Products Bead Beater. RNA was extracted, treated with TURBO DNase (Ambion), and resuspended in DPEC-treated water (Invitrogen) as described previously [8]. cDNA was synthesized from 1 μg of total RNA by using Superscript III reverse transcriptase (Invitrogen). Quantitative real-time RT-PCR was performed using 4 μL of complementary DNA, SYBR green I PCR Master Mix (Qiagen), and 5 μM of the gene-specific primer pair (Supplementary Table 1) in a reaction volume of 25 μL. Quantitative real-time RT-PCR reactions were run on a Rotogene 6000-series sequence detector (Corbett Research, Mortlake, Australia) in triplicate per primer pair. Relative expression levels were determined using the comparative threshold cycle method of Livak and Schmittgen [24], with noninduced M. tuberculosis 16S ribosomal RNA (encoded by rrs) as the control [25].
Human Studies
Subjects
Fifteen M. tuberculosis–infected, HIV-negative patients were recruited from the tuberculosis clinic at Royal Prince Alfred Hospital (New South Wales, Australia). Peripheral blood mononuclear cells (PBMCs) were obtained from patients with biopsy- or culture-proven tuberculosis who had recently started anti-tuberculosis treatment and from 11 healthy, tuberculin skin test (TST)–negative individuals. Ethical approval for this study was provided by the Sydney South West Area Health Service (protocol X06–0248).
T-Cell Proliferation Assay
PBMCs from whole blood were isolated on a Ficoll gradient (Histopaque-1077, Sigma Aldrich). A total of 2.5 × 105 cells/well of PBMCs were incubated at 37°C in 5% CO2 for 5 days in the presence of 10 µg/mL of the SAP proteins, 10 µg/mL of CFP, M. tuberculosis antigen (Ag) 85B, or 3 µg/mL of ConA. T-cell proliferation was assayed by 3H-thymidine incorporation (MP Biomedicals; 1 µCi/well) at day 5, using liquid scintillation spectroscopy (Microbeta Luminescence Counter, Wallace). The lymphocyte stimulation index was calculated using the following formula: (mean counts per minute in the presence of antigen)/(mean counts per minute in the absence of antigen). A lymphocyte stimulation index of ≥3 was considered a positive response to antigen.
Murine Studies
Female C57BL/6 mice aged 6–8 weeks were obtained from Animal Resources Centre (Perth, Australia) and maintained in specific pathogen-free conditions. For determination of immunogenicity, mice (4 per group) were infected via the intranasal route with 5 × 104 colony-forming units (CFU) of M. tuberculosis H37Rv. Three and 8 weeks after infection, single-cell suspensions were prepared from the mediastinal lymph node (MLN) of immunized mice in complete RPMI medium, and the number of interferon γ (IFN-γ)–producing cells was determined by enzyme-linked immunosorbent spot analysis, as described previously [26] using SAP enzymes, CFP, and Ag85B at a concentration of 10 µg/mL, with ConA used at a concentration of 3 µg/mL. For analysis of protective efficacy, mice (5 per group) were immunized intramuscularly 3 times at 2-week intervals with (1) 10 µg of CysDNC protein, coadministered with dimethyl dioctadecyl ammonium bromide (1.25 mg/mL) and monophosphoryl lipid A (125 μg/mL), or (2) 100 µg of a mix of DNA vaccines. At the time of the first injection of DNA vaccines, mice were immunized subcutaneously once with 5 × 105 CFU of BCG. Eight weeks after the final vaccination, mice were challenged with aerosolized M. tuberculosis H37Rv, using an inhalation exposure apparatus (Glas-Col) with an infective dose of <100 viable bacilli per lung [27]. Bacterial load was determined 4 weeks after challenge by plating homogenates of lung and spleen.
Statistical Analysis
For assessment of protective efficacy, the significance of differences was evaluated by 1-way analysis of variance, with pairwise comparison of multigrouped data sets achieved using the Bonferroni post hoc test. The Mann–Whitney U test was used to compare induction of host immune responses by SAP enzymes from infected mice or M. tuberculosis–infected macrophages from humans to induction in uninfected mice and TST-negative individuals, respectively. Differences with a P value of <.05 were considered statistically significant.
RESULTS
Induction of M. tuberculosis ATP Sulfurylase Messenger RNA (mRNA) in the Intracellular Environment Correlates With Potent Antigen-Specific Immunity
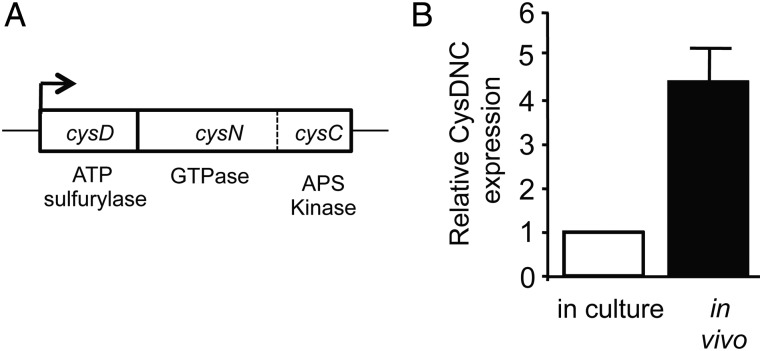
The sulfate-activating complex (SAC) of M. tuberculosis is the first step in the SAP and constitutes 3 catalytic activities, ATP sulfurylase, GTPase, and APS kinase activity, which are all encoded by the cysDNC operon (Figure 1A). The ability of M. tuberculosis SAC to upregulate its expression in culture conditions that mimic intracellular stress [9] suggests that its expression may also be induced in the intracellular environment. To test this, we examined the changes in cysDNC mRNA levels within RAW264.7 cells during M. tuberculosis infection. We found that expression of cysDNC was significantly enhanced, displaying an approximately 4.4-fold increase over the level found in broth-cultured bacilli during the first 48 hours of infection (Figure 1B). This implies that CysDNC may be involved in the ability of M. tuberculosis to adapt to conditions encountered in the intracellular environment.
Figure 1.
Upregulation of the Mycobacterium tuberculosis sulfate-activation complex within host cells. A, Genetic organization of the cysDNC locus encoding the sulfate-activation complex. In M. tuberculosis, the cysN (GTPase) and cysC (kinase) activities are fused together in 1 polypeptide, and cysDNC constitute an operon. B, Relative expression level of cysDNC, measured by quantitative real-time reverse transcription polymerase chain reaction, of bacilli grown for 48 hours in culture (clear bar) or 48 hours after infection of RAW cells (black bar). Data are the mean relative expression ± standard error of the mean, measured in triplicate, and are representative of 2 independent experiments. Abbreviation: ATP, adenosine triphosphate.
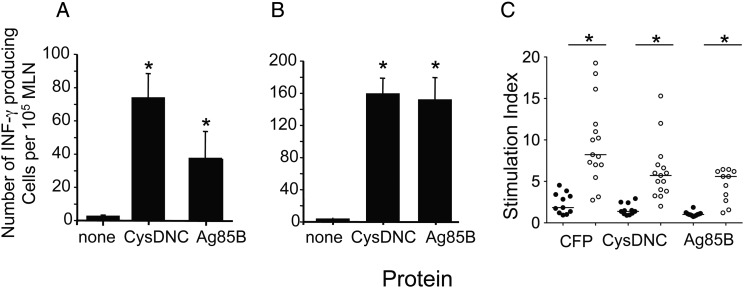
Because CysDNC was induced at high levels within the intracellular environment, we hypothesized that the enzyme may be recognized by the immune response during M. tuberculosis infection. To test this, we infected mice with M. tuberculosis by the intranasal route and examined the frequency of IFN-γ–secreting cells in the MLNs. At 3 weeks after infection, stimulation of MLN cells with CysDNC ex vivo resulted in a strong induction of IFN-γ–secreting T cells, which was similar to the levels induced by the immunodominant secreted Ag85B protein of M. tuberculosis (Figure 2A). This strong T-cell response was maintained up to 8 weeks after infection (Figure 2B). Similar patterns of antigen-specific IFN-γ–secreting cellular responses in response to CysDNC and Ag85B were observed in the lung (data not shown). In addition, lymphocyte proliferation assays of human PBMCs revealed that CysDNC was recognized during human M. tuberculosis infection (Figure 2C). CysDNC responses were similar to those following recall to Ag85B yet lower than those induced by CFP. Furthermore, CysDNC induced significantly greatly proliferation of PBMCs from tuberculosis patients as compared to TST-negative individuals (Figure 2C). These results indicated that M. tuberculosis CysDNC encoding ATP sulfurylase was a potent immunostimulatory antigen of M. tuberculosis.
Figure 2.
Host immune recognition of the Mycobacterium tuberculosis sulfate-activation complex. Antigen-specific T-cell responses in the mediastinal lymph node (MLN) of mice were measured 3 (A) and 8 (B) weeks following intranasal M. tuberculosis challenge. Interferon γ (IFN-γ)–secreting cells were enumerated by enzyme-linked immunosorbent spot following stimulation with CysDNC or 85B proteins (10 μg/mL). Data are the means ± standard error of the mean for 4 mice and are representative of duplicate experiments. The significance of differences between protein-stimulated and unstimulated cells was determined by analysis of variance; *P <.0001. C, Antigen-specific T-cell responses were measured in the peripheral blood of M. tuberculosis–infected patients (open circles; n = 15) and tuberculin skin test–negative individuals (filled circles; n = 11). T-cell proliferation in response to M. tuberculosis CFP, CysDNC, and Ag85B proteins at 10 μg/mL was measured via the incorporation of 3H-thymidine, and a stimulation index was calculated. Horizontal lines represent the median for each group. Significance of differences between M. tuberculosis–infected patients and TST-negative individuals was determined by the Mann–Whitney U test; *P < .001.
Protective Immunity Against Virulent M. tuberculosis Challenge Following Vaccination With DNA Encoding ATP Sulfurylase
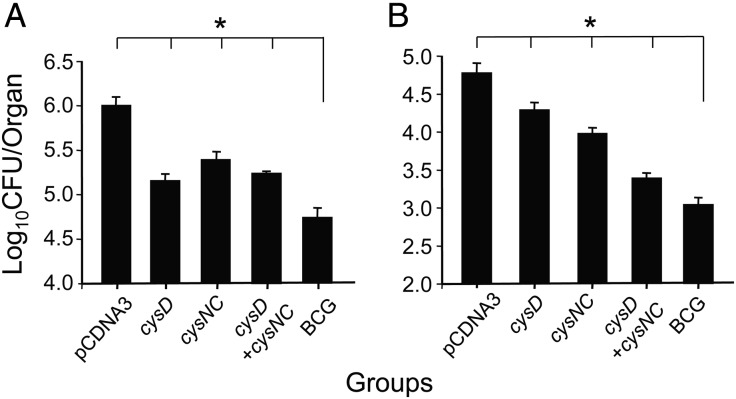
The enhanced expression of the genes encoding ATP sulfurylase in the intracellular environment (Figure 1B) and the ability of this protein complex to induce a robust, antigen-specific T-helper 1 type cytokine response (Figure 2) may render the encoded products effective targets for antimycobacterial protective immunity. To determine this, mice were immunized with DNA vectors expressing cysD and/or cysNC and challenged with M. tuberculosis by aerosol. Immunization with vectors expressing either cysD or cysNC resulted in a significantly reduced bacterial load as compared to mice vaccinated with the control vector, in both the lung (Figure 3A) and spleen (Figure 3B; P < .01). In all experiments, there was an increasing trend for DNA-cysD to afford better protective efficacy than DNA-cysNC in the lung, while use of a combination of DNA-cysD and DNA-cysNC resulted in equivalent protection to that observed with DNA-cysD alone. The protective efficacy was significantly greater in the spleen when mice were immunized with a combination of these 2 plasmids, and this protection approached the level achieved with BCG (Figure 3B). Therefore, we found that ATP sulfurylase was a highly protective component of M. tuberculosis.
Figure 3.
Protection afforded by DNA vaccines encoding members of the sulfate-activation complex following Mycobacterium tuberculosis challenge. C57BL/6 mice (n = 5) were immunized 3 times by intramuscular injection at 2-week intervals with either pCDNA3, DNA-cysD, DNA-cysNC, or DNA-cysD combined with DNA-cysNC. At the time of the first injection of DNA vaccines, mice were immunized once, by subcutaneous injection, with 5 × 105 colony-forming units (CFU) of BCG. Four weeks following the third immunization, mice were challenged with aerosolized M. tuberculosis, and bacterial load in the lung (A) and the spleen (B) was determined 4 weeks later. These data are shown as the mean CFU (±standard error of the mean) per organ and are representative of 1 of 3 individual experiments for all groups. The significance of the differences between unvaccinated and immunized groups in the lung and spleen were determined by analysis of variance; *P < .001.
Downstream Enzymes of M. tuberculosis SAP Are Immunogenic Components of M. tuberculosis
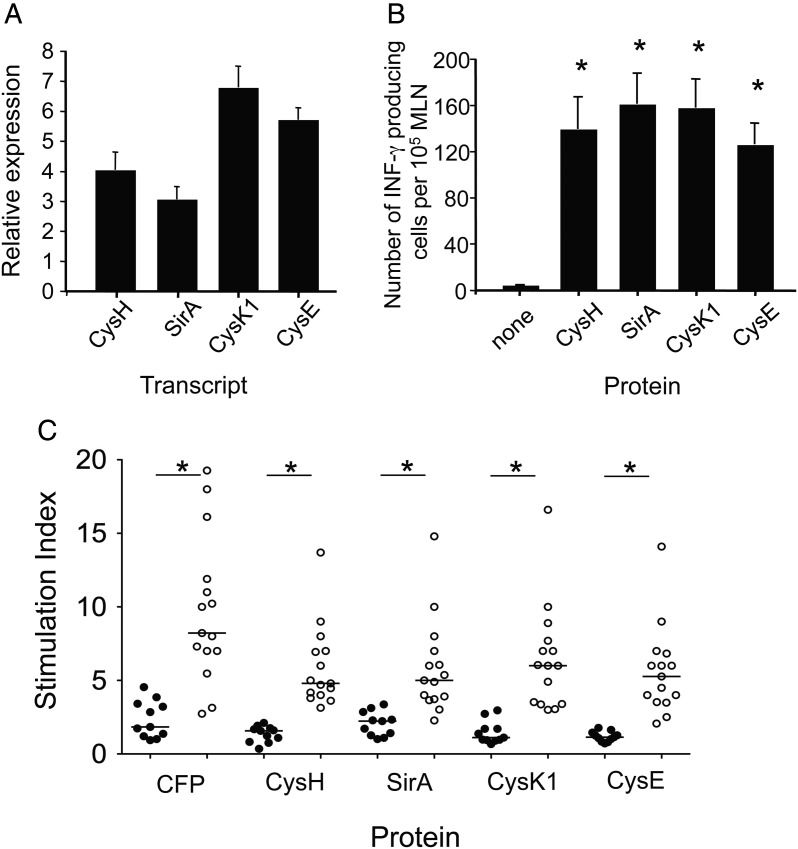
The promising results obtained with M. tuberculosis ATP sulfurylase (CysDNC) led us to question whether other members of the SAP are targets of the host immune response. We found that all SAP proteins tested were significantly upregulated in the intracellular environment, with cysK1 mRNA displaying the highest induction, at approximately 6.7-fold (Figure 4A). The level of induction was similar for SirA and cysH, while cysK1 and cysE also displayed similar levels of intracellular upregulation. This is in agreement with the fact the sirA and cysH are located within the same operon in the M. tuberculosis genome [28], while cysK1 and cysE lie adjacent in the genome [23, 28]. We also determined that these proteins are recognized in M. tuberculosis–infected mice, as all proteins induced IFN-γ secreting T cells at 8 weeks after infection (Figure 4B). Similar to CysDNC findings (Figure 2C), lymphocyte proliferation assays of human PBMCs revealed that all SAP enzymes studied were recognized during human M. tuberculosis infection (Figure 4C).
Figure 4.
Upregulation and host immune recognition of downstream enzymes in the Mycobacterium tuberculosis sulfate-assimilation pathway (SAP). A, Relative upregulation of M. tuberculosis SAP genes within RAW cells as compared to in vitro–grown bacilli. Total RNA extracted from in vitro grown bacilli or M. tuberculosis–infected RAW cells at 48 hours after infection was reverse transcribed, and quantitative real-time reverse transcription polymerase chain reaction was used to determine expression of M. tuberculosis cysH, sirA, cysK1, and cysE. Data are the mean relative expression ± standard error of the mean (SEM) measured in triplicate and are representative of 2 independent experiments. B, Antigen-specific T-cell responses in the mediastinal lymph node (MLN) of mice were measured 8 weeks following M. tuberculosis challenge. Interferon γ (IFN-γ)–secreting cells were enumerated by enzyme-linked immunosorbent spot analysis following recall to CysH, SirA, CysK1, and CysE (10 μg/mL). Data are the means ± SEM for 4 mice and are representative of duplicate experiments. The significance of differences between protein-stimulated and unstimulated cells was determined by analysis of variance; *P < .001. C, Recognition of SAP proteins by M. tuberculosis-infected individuals. Antigen-specific T-cell responses were measured in the peripheral blood of M. tuberculosis–infected patients (open circles; n = 15) and TST-negative individuals (filled circles; n = 11). T-cell proliferation in response to M. tuberculosis CFP, CysH, SirA, CysK1, and CysE proteins at 10 μg/mL was measured as described in Figure 2C. Significance of the differences between M. tuberculosis–infected patients and TST-negative individuals was determined by a Mann–Whitney U test; *P < .001.
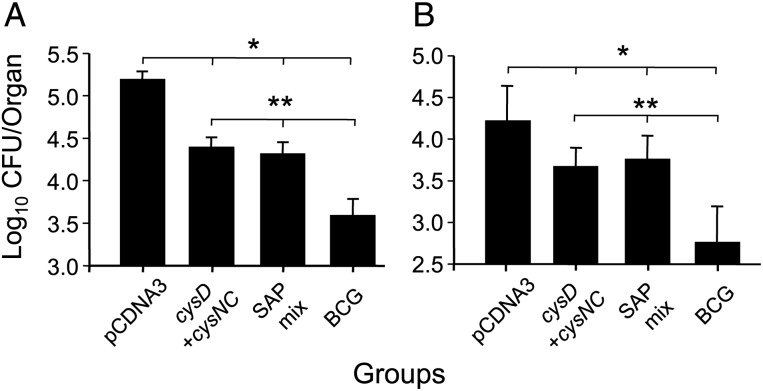
Because expression of all SAP enzymes was upregulated in vivo and the proteins were recognized in mice and humans, we assessed whether they could improve the protective efficacy afforded by DNA-cysDNC. When mice were vaccinated with DNA-cysDNC together with DNA encoding cysH, sirA, cysK1, and cysE, we did not observe increases in protective efficacy, compared with the efficacy of DNA-cysDNC alone, in both the lung (Figure 5A) and spleen (Figure 5B). Therefore, although all SAP members were recognized by the immune response in M. tuberculosis–infected humans and mice, CysDNC alone afforded maximal protective efficacy in the mouse model used here.
Figure 5.
Protective efficacy of downstream enzymes of the sulfate-assimilation pathway. C57BL/6 mice (n = 5) were immunized 3 times by intramuscular injection with either pCDNA3, DNA-cysD combined with DNA-cysNC, or a mix of DNA vaccines expressing cysH, sirA, cysKI, and cysE (SAP mix). At the time of the first injection of DNA vaccines, mice were immunized once by subcutaneous injection with 5 × 105 colony-forming units (CFU) of BCG. Four weeks following the third immunization, mice were challenged by aerosolized M. tuberculosis, and bacterial load was determined in the lung (A) and the spleen (B) 4 weeks later. These data are shown as the mean CFU (±standard error of the mean) per organ and are representative of 1 of 3 individual experiments for all groups. The significance of the differences between unvaccinated and immunized groups in the lung and spleen (*P < .001) and the differences between BCG immunized animals and other immunized groups (**P < .001) were determined by analysis of variance.
Boosting BCG-Vaccinated Mice With ATP Sulfurylase Improves Protection Afforded by BCG Against Challenge With M. tuberculosis
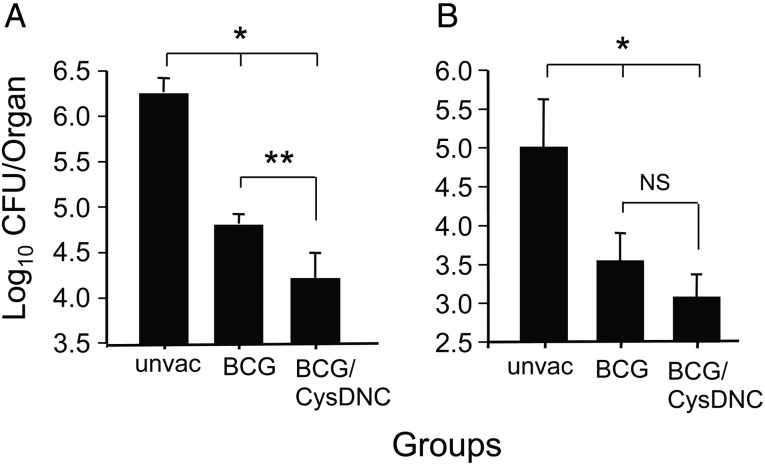
Considering the strong recognition of ATP sulfurylase by tuberculosis patients and its protective effect in mice, we determined whether this protein complex is a suitable candidate to boost the protective effect of BCG after M. tuberculosis challenge. After aerosol-based delivery of a low dose of M. tuberculosis, naive mice demonstrated substantial bacterial growth in the lungs and dissemination to spleens (Figure 6A and 6B). In contrast, immunization with BCG alone resulted in significant protection against M. tuberculosis challenge, with an approximate 1.5-log10 reduction in the M. tuberculosis load in the lung and spleen (Figure 6A and 6B). Boosting with CysDNC protein led to a further significant reduction of 0.5-log10 M. tuberculosis in the lung, compared with vaccination with BCG alone (Figure 6A). While the bacterial burden was also reduced in the spleen with boosting, this difference did not achieve significance (Figure 6B). Therefore CysDNC improved the protective effect of the BCG against M. tuberculosis infection, and this effect was strongest in the lung.
Figure 6.
Boosting of BCG-induced protective immunity with the sulfate-activation complex. Groups of mice were immunized with 5 × 105 colony-forming units (CFU) of BCG by subcutaneous injection, and after 24 weeks mice received CysDNC protein in monophosphoryl lipid A (MPL)–dimethyl dioctadecyl ammonium bromide (DDA; subcutaneously, 3 times at 2-week intervals). After 6 weeks, mice were challenged with aerosolized Mycobacterium tuberculosis. Control mice were unvaccinated (unvac) or were immunized with BCG and boosted with MPL-DDA adjuvant alone. Four weeks after challenge, the bacterial loads in the lung (A) and spleen (B) were determined. These data are presented as the mean bacterial number ± standard error of the mean per organ for 6–10 mice per group. Data are representative of 2 independent experiments. The significance of the differences between unvaccinated mice and other groups (*P < .001) and between BCG/MPL-DDA–immunized animals and other groups (**P < .001) were determined by analysis of variance. Abbreviation: NS, not significant.
DISCUSSION
The identification of new targets of host immunity would markedly aid efforts to develop more effective tuberculosis vaccines. In this report, we identified the SAC of M. tuberculosis as a major antigenic component of the bacillus. The SAC is an enzyme complex with 3 catalytic activities [9, 29]. This complex is predicted to play a role in adaption of M. tuberculosis to the host cell environment because of the upregulation of CysDNC within macrophages (Figure 1B) [10] and its response to a number of in vitro stress conditions, including nutrient starvation and oxidative stress [9, 30]. Therefore, it is possible that strong recognition of CysDNC by tuberculosis patients (Figure 2C) and M. tuberculosis–infected mice (Figure 2A and 2B) may be due to the enhanced expression of CysDNC within the host. Intriguingly, all components of the SAP tested displayed upregulation within the macrophage cell line used. This adaptation to the phagosome environment is an indication of the bacterium's requirement for cysteine; for example, cysteine is incorporated into acetyl coenzyme A, the building block for lipids in the organism's cell wall and a substrate for the glyoxylate shunt, a pathway required for M. tuberculosis to persist in macrophages and in mice [31]. It is also possible that certain enzymes of the SAP regulate expression of other members of the pathway, resulting in the coordinated induction of gene expression within the host. For instance, serine acetyl transferase of E. coli has been shown to associate and form a complex with the last enzyme in the pathway, O-acetylserine sulfhydrylase [32]. E. coli ATP sulfurylase additionally forms a tight complex with O-acetylserine sulfhydrylase [33], and it is this complex that can activate sulfate to produce APS. It has been suggested that, given the similarities between E. coli and M. tuberculosis ATP sulfurylases, the mycobacterial system also forms a higher-order complex, linking catalytic functions with other enzymes in the SAP [29]. This has not been formally tested. However, it may be one reason why all of the enzymes of the SAP induce robust host immune responses in M. tuberculosis–infected mice and humans (Figure 2 and Figure 4B and 4C). Furthermore, because ATP sulfurylase differs significantly from its human counterpart, it represents both a suitable vaccine and a drug target for application in humans [21]. Sulfur-metabolism genes display enhanced transcription after exposure to various antibiotics, and this may influence the response of M. tuberculosis to these compounds [30]. Because drug-responsive genes encode proteins that may be relevant to the drug's mode of action, sulfur-metabolizing enzymes have been proposed as potential drug targets [21].
Enhanced recognition of mycobacterial antigens by the host immune response does not always correlate with protection against challenge with virulent M. tuberculosis in animal models [34–36]. We therefore assessed whether CysDNC was protective in our low-dose murine model of aerosol-induced M. tuberculosis infection. When delivered as DNA vaccines, CysD and CysNC were protective as single components in both the lung and spleen, and a combination of the 2 constructs achieved a level of protection similar to that induced by BCG (Figure 3). Thus, the strong expression of genes encoding CysDNC correlates with the protective effect of the antigenic complex in the models used here. However, addition of DNA vaccines encoding other components of the SAP did not improve the protective effect of CysDNC, despite the fact that proteins were recognized by PBMCs from tuberculosis patients, were upregulated within macrophages, and induced strong IFN-γ responses from T cells of M. tuberculosis–infected mice (Figure 4). It is unclear why CysDNC appears to be dominant member of the SAP in terms of protective efficacy, but this may relate to major histocompatibility complex–restricted T-cell responses in the mouse strain used in this study. It is also of interest to note that the SAP antigens are proposed to be intracellular or membrane-associated proteins, owing to their function in sulfur metabolism [22], and we have confirmed this for some of the members, by immunoblotting (data not shown). At present, all M. tuberculosis antigens in clinical trials are secreted proteins, because these are predicted to be the early targets of host immunity [2]. This study suggests that nonsecreted proteins may also be suitable components of new tuberculosis vaccine formulations.
An important property of potential subunit vaccines is the capacity to “boost” protection with prior BCG immunization, because this is the proposed role for such vaccines in new tuberculosis vaccine schedules [2]. A small number of proteins have demonstrated an ability to boost BCG-induced protection in experimental M. tuberculosis infection [37–39]. CysDNC can now be added to this list, as the antigen complex was able to significantly increase the protective effect of BCG alone in the lung, the primary site of infection in humans and this model (Figure 6). Although protection was improved in the spleen, it did not reach statistical significance. This has also been observed in other studies, suggesting that boosting with certain subunit protein vaccines may have maximum effect at improving protection in the lungs [40]. It is possible that enhancing the expression of CysDNC within BCG may further improve the protective effect of this prime-boost protocol, and this is an approach we are actively pursing.
In summary, we have identified components of the SAP as host cell–induced proteins that are major antigenic components of M. tuberculosis. Because these proteins are recognized the immune response of M. tuberculosis-infected individuals, they warrant further assessment for potential incorporation into new-generation vaccines to control tuberculosis in human populations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Meihao Sun and Joseph Harrison, for the purification of M. tuberculosis CysH and CysE, respectively; and Dr H. Goldberg, Prof P. Seale, and patients at the Chest Clinic, RPAH, for their participation in the study.
Financial support. This work was supported in part by the National Health and Medical Research Council of Australia (grant 570768 to J. A. T. and W. J. B), the University of Sydney (research and development grant to J. A. T.), the National Institutes of Health (grant GM54469 to T. S. L), and the New South Wales Department of Health (research infrastructure grant to the Centenary Institute).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–61. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect Dis. 2011;11:633–40. doi: 10.1016/S1473-3099(11)70146-3. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Shiloh M. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. PNAS. 2000;97:8841–8. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timm J, Post FA, Bekker LG, et al. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. PNAS. 2003;100:14321–6. doi: 10.1073/pnas.2436197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–31. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 6.Hampshire T, Soneji S, Bacon J, et al. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis (Edinb) 2004;84:228–38. doi: 10.1016/j.tube.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manganelli R, Voskuil MI, Schoolnik GK, Dubnau E, Gomez M, Smith I. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Mol Microbiol. 2002;45:365–74. doi: 10.1046/j.1365-2958.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 8.Muttucumaru DG, Roberts G, Hinds J, Stabler RA, Parish T. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb) 2004;84:239–46. doi: 10.1016/j.tube.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Pinto R, Tang QX, Britton WJ, Leyh TS, Triccas JA. The Mycobacterium tuberculosis cysD and cysNC genes form a stress-induced operon that encodes a tri-functional sulfate-activating complex. Microbiology. 2004;150:1681–6. doi: 10.1099/mic.0.26894-0. [DOI] [PubMed] [Google Scholar]
- 10.Schnappinger D, Ehrt S, Voskuil MI, et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee KY, Erdjument-Bromage H, Tempst P, Nathan CF. S-nitroso proteome of Mycobacterium tuberculosis: enzymes of intermediary metabolism and antioxidant defense. PNAS. 2005;102:467–72. doi: 10.1073/pnas.0406133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan F, Vetting MW, Frantom PA, Blanchard JS. Structures and mechanisms of the mycothiol biosynthetic enzymes. Curr Opin Chem Biol. 2009;13:451–9. doi: 10.1016/j.cbpa.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchmeier NA, Newton GL, Koledin T, Fahey RC. Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol Microbiol. 2003;47:1723–32. doi: 10.1046/j.1365-2958.2003.03416.x. [DOI] [PubMed] [Google Scholar]
- 14.Rawat M, Newton GL, Ko M, Martinez GJ, Fahey RC, Av-Gay Y. Mycothiol-deficient Mycobacterium smegmatis mutants are hypersensitive to alkylating agents, free radicals, and antibiotics. Antimicrob Agents Chemother. 2002;46:3348–55. doi: 10.1128/AAC.46.11.3348-3355.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchmeier N, Fahey RC. The mshA gene encoding the glycosyltransferase of mycothiol biosynthesis is essential in Mycobacterium tuberculosis Erdman. FEMS Microbiol Lett. 2006;264:74–9. doi: 10.1111/j.1574-6968.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 16.Newton GL, Fahey RC. Mycothiol biochemistry. Arch Microbiol. 2002;178:388–94. doi: 10.1007/s00203-002-0469-4. [DOI] [PubMed] [Google Scholar]
- 17.Sareen D, Newton GL, Fahey RC, Buchmeier NA. Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman. J Bacteriol. 2003;185:6736–40. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senaratne RH, De Silva AD, Williams SJ, et al. 5'-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol Microbiol. 2006;59:1744–53. doi: 10.1111/j.1365-2958.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- 19.Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992;36:823–31. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AD, Sonnenberg MG, Ordway DJ, et al. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Bhave DP, Muse WB, 3rd, Carroll KS. Drug targets in mycobacterial sulfur metabolism. Infectious Disorders Drug Targets. 2007;7:140–58. doi: 10.2174/187152607781001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza GA, Leversen NA, Malen H, Wiker HG. Bacterial proteins with cleaved or uncleaved signal peptides of the general secretory pathway. J Proteomics. 2011;75:502–10. doi: 10.1016/j.jprot.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Schelle MW, Bertozzi CR. Sulfate metabolism in mycobacteria. ChemBioChem. 2006;7:1516–24. doi: 10.1002/cbic.200600224. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Banaiee N, Jacobs WR, Jr., Ernst JD. Regulation of Mycobacterium tuberculosis whiB3 in the mouse lung and macrophages. Infect Immun. 2006;74:6449–57. doi: 10.1128/IAI.00190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palendira U, Kamath AT, Feng CG, et al. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect Immun. 2002;70:1949–56. doi: 10.1128/IAI.70.4.1949-1956.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto R, Saunders BM, Camacho LR, et al. Mycobacterium tuberculosis defective in phthiocerol dimycocerosate translocation provides greater protective immunity against tuberculosis than the existing bacille Calmette-Guerin vaccine. J Infect Dis. 2004;189:105–12. doi: 10.1086/380413. [DOI] [PubMed] [Google Scholar]
- 28.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, Andreassi JL, 2nd, Liu S, Pinto R, Triccas JA, Leyh TS. The trifunctional sulfate-activating complex (SAC) of Mycobacterium tuberculosis. J Biol Chem. 2005;280:7861–6. doi: 10.1074/jbc.M409613200. [DOI] [PubMed] [Google Scholar]
- 30.Hatzios SK, Bertozzi CR. The regulation of sulfur metabolism in Mycobacterium tuberculosis. PLoS Pathog. 2011;7:e1002036. doi: 10.1371/journal.ppat.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–8. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 32.Droux M, Ruffet ML, Douce R, Job D. Interactions between serine acetyltransferase and O-acetylserine (thiol) lyase in higher plants—structural and kinetic properties of the free and bound enzymes. Eur J Biochem. 1998;255:235–45. doi: 10.1046/j.1432-1327.1998.2550235.x. [DOI] [PubMed] [Google Scholar]
- 33.Wei J, Tang QX, Varlamova O, Roche C, Lee R, Leyh TS. Cysteine biosynthetic enzymes are the pieces of a metabolic energy pump. Biochemistry. 2002;41:8493–8. doi: 10.1021/bi025953j. [DOI] [PubMed] [Google Scholar]
- 34.Gartner T, Baeten M, Otieno S, Revets H, De Baetselier P, Huygen K. Mucosal prime-boost vaccination for tuberculosis based on TLR triggering OprI lipoprotein from Pseudomonas aeruginosa fused to mycolyl-transferase Ag85A. Immunol Lett. 2007;111:26–35. doi: 10.1016/j.imlet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Kamath AT, Hanke T, Briscoe H, Britton WJ. Co-immunization with DNA vaccines expressing granulocyte-macrophage colony-stimulating factor and mycobacterial secreted proteins enhances T-cell immunity, but not protective efficacy against Mycobacterium tuberculosis. Immunology. 1999;96:511–6. doi: 10.1046/j.1365-2567.1999.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner MA, Ramsay AJ, Buchan GS, et al. A DNA prime-live vaccine boost strategy in mice can augment IFN-gamma responses to mycobacterial antigens but does not increase the protective efficacy of two attenuated strains of Mycobacterium bovis against bovine tuberculosis. Immunology. 2003;108:548–55. doi: 10.1046/j.1365-2567.2003.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dey B, Jain R, Khera A, et al. Latency antigen alpha-crystallin based vaccination imparts a robust protection against tuberculosis by modulating the dynamics of pulmonary cytokines. PLoS One. 2011;6:e18773. doi: 10.1371/journal.pone.0018773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Wang C, Zhou Z, et al. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin Dev Immunol. 2011;2011:617892. doi: 10.1155/2011/617892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouanet C, Debrie AS, Lecher S, Locht C. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microb Infect. 2009;11:995–1001. doi: 10.1016/j.micinf.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Guerrero GG, Locht C. Recombinant HBHA boosting effect on BCG-induced immunity against Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:730702. doi: 10.1155/2011/730702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.