Abstract
Background. Children <2 years of age are at high risk of influenza-related mortality and morbidity. However, the appropriate dose of oseltamivir for children <2 years of age is unknown.
Methods. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group evaluated oseltamivir in infants aged <2 years in an age–de-escalation, adaptive design with a targeted systemic exposure.
Results. From 2006 to 2010, 87 subjects enrolled. An oseltamivir dose of 3.0 mg/kg produced drug exposures within the target range in subjects 0–8 months of age, although there was a greater degree of variability in infants <3 months of age. In subjects 9–11 months of age, a dose of 3.5 mg/kg produced drug exposures within the target range. Six of 10 subjects aged 12–23 months receiving the Food and Drug Administration–approved unit dose for this age group (ie, 30 mg) had oseltamivir carboxylate exposures below the target range. Virus from 3 subjects developed oseltamivir resistance during antiviral treatment.
Conclusions. The appropriate twice-daily oral oseltamivir dose for infants ≤8 months of age is 3.0 mg/kg, while the dose for infants 9–11 months old is 3.5 mg/kg.
Clinical Trials Registration. NCT00391768.
Keywords: Oseltamivir, Tamiflu, influenza, antiviral treatment, antiviral resistance
Infants and young children are at greatest risk of mortality during annual epidemics of influenza [1–5]. It was recognized prior to the 2009 pandemic of influenza A virus subtype H1N1 (A[H1N1]pdm09) infection that infants <6 months of age had higher rates of influenza-associated mortality solely because of their young age and that the next highest mortality rates occurred in children 6–23 months of age [1]. During the A(H1N1)pdm09 pandemic, this group continued to be disproportionately impacted as compared to adults [6–13]. Outbreaks in neonatal intensive care units illustrate the dangers posed to the youngest pediatric patients [14–17].
Oseltamivir is one of 2 antiviral drugs recommended for treatment and prophylaxis of influenza virus infection in children and adults [18, 19]. Following metabolism to the active metabolite, oseltamivir carboxylate inhibits viral neuraminidase (NA), blocking release of progeny virions from infected cells and viral entry into uninfected cells [20]. Initiation of oseltamivir therapy within 48 hours of onset of symptoms reduces the duration of influenza illness [21–24] and may decrease influenza-associated complications such as otitis media, pneumonia, and death [23–28]. Only 2 large prospective investigations of oseltamivir have been conducted in children, and neither included children <1 year of age [23, 24].
In 2006, the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) initiated an adaptive pharmacokinetic investigation of oral oseltamivir in infants aged <2 years. During the A(H1N1)pdm09 pandemic, the CASG data set represented the most substantive information available on oseltamivir dosing in infants <1 year of age. Available data were provided to the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention, the European Methods Agency, and health authorities in Canada, Australia, and Japan and served as the basis for oseltamivir emergency use authorization and dosing recommendations made by these agencies [29, 30].
METHODS
Study Population
Children <24 months of age were eligible. Children were required to have confirmation of influenza virus infection by viral culture or rapid influenza virus diagnostic test within 96 hours prior to study enrollment and to have a duration of influenza symptoms of ≤96 hours. Subjects were stratified into 5 age cohorts (0–2 months, 3–5 months, 6–8 months, 9–11 months, and 12–23 months) and could be hospitalized or outpatients.
Study Design and End Points
The study was a prospective, open-label pharmacokinetic/pharmacodynamic and safety evaluation of oseltamivir (oseltamivir powder for oral suspension, 12 mg/mL; F. Hoffmann–La Roche) in young children. The primary objective was to define oseltamivir and oseltamivir carboxylate pharmacokinetics and dosing following oral administration, using a targeted area under the concentration curve (AUC) strategy.
Prior to study initiation, an oseltamivir carboxylate 12-hour AUC (AUC12) of 3800 ng • h/mL was identified as the desired exposure target. The range around this target AUC12 was 2660 ng • h/mL (one SD below the mean) to 7700 ng • h/mL (2 SDs above the mean exposure in adults receiving 150 mg twice daily). This exposure target was selected on the basis of the development of antiviral resistance among 1–2-year-old children receiving 2 mg/kg of oseltamivir (producing an AUC12 of 2800 ng • h/mL) [23, 31]. Conversely, no resistance was observed in 1–2-year-old children receiving 30 mg per dose twice daily [32], which is approximately 3 mg/kg for a 1-year-old with an ideal bodyweight of 10 kg and which produces a modeled AUC12 of 3800 ng • h/mL.
At the outset of the study, the first 9 subjects enrolled in each age cohort were designated to receive 3 mg/kg twice daily (neonates and infants from birth through 11 months of age) or 30 mg twice daily (the FDA-approved dose for toddlers 12–23 months of age) of oseltamivir for 5 days. If the AUC12 from ≥3 of the 9 subjects in a given cohort was <2660 ng • h/mL or >7700 ng • h/mL, the dose was proportionally adjusted, and an additional 9 subjects were enrolled at the new dose.
The primary study end point was achievement of a geometric mean oseltamivir carboxylate AUC12 of 2660–7700 ng • h/mL for each age cohort. Secondary end points were the number and characteristics of neurologic events, adverse events considered to be associated with study therapy, correlation of viral clearance (by polymerase chain reaction [PCR] and culture) with pharmacokinetics and age, and correlation of development of oseltamivir resistance with pharmacokinetics and age.
Pharmacokinetics and Bioanalysis
Oseltamivir and oseltamivir carboxylate concentrations for pharmacokinetic analysis were obtained at steady state on treatment day 3 at 0 hours (ie, before dose receipt), at 1 hour after dose receipt, and between 2–3 hours, 5–7 hours, and 10–12 hours after dose receipt. A minimum of 0.5 mL of whole blood was required for plasma pharmacokinetic analysis at each time point. High performance liquid chromatography with mass spectrometry was used to quantitate oseltamivir and oseltamivir carboxylate (lower limits of detection, 1 and 10 ng/mL, respectively) [33]. Accuracy and precision were approximately ± 3% and ± 6%, respectively, from 5000 quality control samples. Noncompartmental methods were used to derive pharmacokinetic parameters from concentration-time data, using WinNonlin, version 5.2.1 (Pharsight Corporation, Mountain View, CA). Primary parameters included AUC12, maximum concentration (Cmax), half-life (T½), oral clearance (CL/F), time to Cmax (Tmax), apparent distribution volume (V/F), and last measured concentration (Clast). Oseltamivir carboxylate CL/F was calculated as dose/AUC; oseltamivir dose was adjusted for molecular weight differences between the parent and metabolite.
Virologic Assessments
Sample Collection
Copan nasopharyngeal swab specimens for viral detection were obtained on study days 1, 3, 5, and 10. A swab was inserted half the distance from the subject's nostril to the anterior portion of the ear, rotated twice, held in place for 5 seconds, and then removed from the nose. Swabs then were placed in viral transport media and shipped under refrigerated conditions to the CASG Central Unit Laboratory within 32 hours, where they were divided in aliquots and frozen at −80°C.
Reverse Transcription (RT)–PCR for Influenza A and B Virus Detection
Viral load was determined by RT-PCR (performed by Dr Fred Lakeman, Birmingham, AL) [34]. Nucleic acids were isolated from 190 µL of viral specimens, using MagNA Pure LC 1.0 or MagNA Pure LC 2.0. A 5-µL sample of RNA was incubated with SuperScript III reverse transcriptase and specific primers to generate complementary DNA. The lower limit of detection for this assay is 50 copies/mL.
Virus Culture
Frozen aliquots were thawed in batches with 1 freeze-thaw cycle per sample, inoculated in culture tubes containing monolayers of Madin-Darby canine kidney (MDCK) cells, and incubated at 33°C (kindly performed by Dr Marilyn Menegus, Rochester, NY) [35]. Cultures were examined daily for cytopathology, and positive cultures were subsequently titered on MDCK cells.
Sequencing of NA and Hemagglutinin (HA) Genes
For each study participant whose culture(s) grew influenza virus, 1 sample was tested for HA and NA type (eg, H1N1, H3N2, and B). Full-length HA or NA open-reading frames were then amplified by Amplitaq Gold PCR, and resulting DNA fragments were purified by MSB HTS PCRapace kit. Full-length sequencing was performed for HA and NA genes, using the Big Dye Terminator 3.1 Cycle Sequencing kit and sequenced on an ABI 3031 XL automated sequencer. Sequence files were quality checked and contigs assembled in DNAstar. Assembled sequences were then loaded into Vector NTI for translation and alignment with prototype sequences.
Resistance Testing
The first and last specimens from each study participant that yielded influenza virus on culture were tested for oseltamivir susceptibility in vitro, using phenotypic and genotypic analyses.
NA Inhibition Phenotyping
Antiviral phenotypic susceptibility testing was performed at ViroClinics (the Netherlands) [21]. Viral specimens were diluted to a specific level of NA activity in reaction buffer, and a series of oseltamivir carboxylate concentrations were added to samples. MUNANA substrate was then added, and the oseltamivir carboxylate concentration sufficient to reduce NA activity by 50% (IC50) was determined. The control viruses used were A/Puerto Rico/8/34 and B/Lee/40.
Sequence Analyses for Genotyping
Four reference virus strains were used in the genotype comparisons: A/Brisbane/59/2007 for seasonal influenza A virus subtype H1N1, A/California/07/2009 for A(H1N1)pdm09, A/Brisbane/10/2007 for influenza A virus subtype H3N2, and B/Brisbane/60/2008 for influenza B virus. The inferred amino acid sequence from NA or HA nucleotide sequences from study specimens were aligned with the corresponding reference sequences, using the multiple sequence alignment program AlignX in VectorNTI. Amino acid differences between each sequence and the reference were determined. Virus type and subtype were determined by homology to prototype sequences, using BLAST [36]. Known amino acid polymorphisms were defined as sequence variants appearing within the public National Center for Biotechnology Information (NCBI) influenza virus database. All human NA and HA protein sequences were retrieved from the NCBI Influenza Virus Resource (http://www.ncbi.nlm.nih.gov/genomes/FLU/FLU.html). For H1N1 sequences, lineage association was divided further into seasonal H1N1 and A(H1N1)pdm09 isolates that circulated widely late in the study [37]. Mutations in N1 NA H274Y and N294S, using N2 nomenclature (H275Y and N295S, using N1 numbering), were defined as oseltamivir-resistance mutations, while Q136K and K150T were defined as zanamivir-resistance mutations. Mutations in N2 NA R292K, E119V, and N294S were defined as oseltamivir resistance mutations, while R292K, E119A, and E119D were defined as zanamivir resistance mutations. Mutations at position 198 in influenza B NA were defined as possible oseltamivir resistance mutations, and R152K and E119G were defined as zanamivir resistance mutations.
Safety Assessments
Subjects were evaluated on study days 1, 3, 5, 10, and 30, with adverse events documented and neurologic assessments made at each study visit.
Statistical Analysis
With the expected sample size of 12 subjects 12–23 months of age and a SD estimate of 1140 ng • h/mL, the oseltamivir carboxylate AUC12 would have a 95% confidence interval (CI) of ±724 ng • h/mL for AUC estimation purposes. With the expected sample size of 9 subjects for each cohort from birth through 11 months, the oseltamivir carboxylate AUC12 would have a 95% CI of ±744 ng • h/mL for AUC estimation purposes. Other pharmacokinetic parameters also were presented with 95% CIs, for true parameter estimation purposes.
The intent-to-treat population included all subjects who received at least 1 dose of study medication. These subjects were included in all summaries regarding subject accrual, baseline information, and safety parameters. Only subjects with specimens obtained at required intervals for pharmacokinetic measurements were included in those analyses.
RESULTS
Study Population
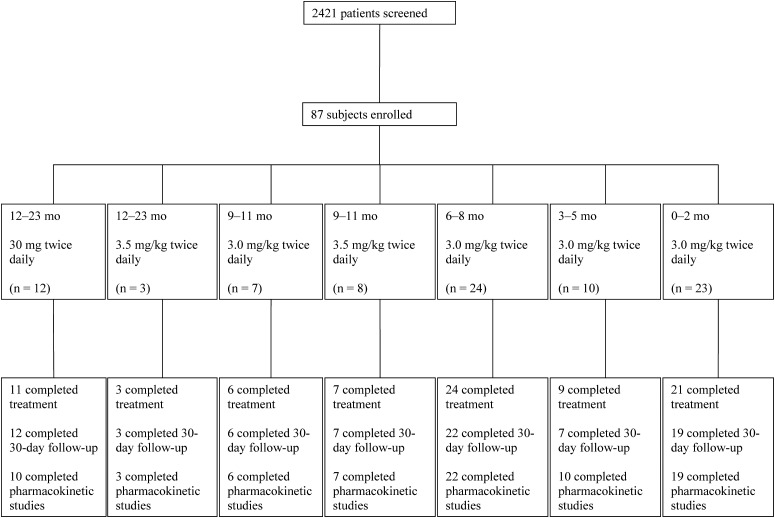
From 2006 to 2010, 87 subjects were enrolled at 16 sites. Sixteen subjects enrolled during the 2006–2007 influenza season, 13 during the 2007–2008 season, 20 during the 2008–2009 season, and 38 during the 2009–2010 season (Figure 1). Demographic data and baseline characteristics are summarized in Table 1.
Figure 1.
Screening, enrollment, and follow-up during the course of the study.
Table 1.
Subject Demographic Data and Baseline Characteristics, by Age and Dosage Cohort
| Twice-Daily Dose, by Age |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | 12–23 mo, 30 mg (n = 12) | 12–23 mo, 3.5 mg/kg (n = 3) | 9–11 mo, 3.0 mg/kg (n = 7) | 9–11 mo, 3.5 mg/kg (n = 8) | 6–8 mo, 3.0 mg/kg (n = 24) | 3–5 mo, 3.0 mg/kg (n = 10) | 0–2 mo, 3.0 mg/kg (n = 23) |
| Sex | |||||||
| Male | 9 (75) | 2 (66.7) | 4 (57.1) | 2 (25) | 11 (45.8) | 8 (80) | 15 (65.2) |
| Female | 3 (25) | 1 (33.3) | 3 (42.9) | 6 (75) | 13 (54.2) | 2 (20) | 8 (34.8) |
| Age, mo | |||||||
| Median | 16.50 | 16 | 10 | 10 | 6 | 4 | 1 |
| Range | 12–22 | 13–21 | 9–10 | 9–11 | 6–8 | 3–5 | 0.43–2 |
| Race | |||||||
| White | 7 (58.3) | 1 (33.3) | 4 (57.1) | 4 (50) | 16 (66.7) | 6 (60) | 17 (73.9) |
| African American | 1 (8.3) | 2 (66.7) | 1 (14.3) | 3 (37.5) | 3 (12.5) | 2 (20) | 3 (13) |
| American Indian/Alaska Native | … | … | … | … | … | 1 (10) | … |
| Asian | … | … | … | … | 1 (4.2) | 1 (10) | … |
| Native Hawaiian | 2 (16.7) | … | 1 (14.3) | … | … | … | 2 (8.7) |
| >1 | 1 (8.3) | … | 1 (14.3) | 1 (12.5) | 2 (8.3) | … | 1 (4.3) |
| Unknown/not reported | 1 (8.3) | … | … | … | 2 (8.3) | … | … |
| Ethnicity | |||||||
| Hispanic/Latino | 6 (50) | 1 (33.3) | … | 2 (25) | 11 (45.8) | 5 (50) | 16 (69.6) |
| Not Hispanic/Latino | 5 (41.7) | 2 (66.7) | 5 (71.4) | 5 (62.5) | 11 (45.8) | 5 (50) | 6 (26.1) |
| Unknown | 1 (8.3) | … | 2 (28.6) | 1 (12.5) | 2 (8.3) | … | 1 (4.3) |
| Symptom duration before enrollment, d | |||||||
| Median | 3 | 2 | 2 | 2.50 | 3 | 2.50 | 2 |
| Range | 1–4 | 2–4 | 2–4 | 1–4 | 1–4 | 1–4 | 1–4 |
| Gestational age, wk | |||||||
| Before term, ≤37 | 4 (33.3) | … | … | 4 (50) | 11 (45.8) | 5 (50) | 5 (21.7) |
| Full term, 38–42 | 8 (66.7) | 2 (66.7) | 7 (100) | 4 (50) | 13 (54.2) | 5 (50) | 18 (78.3) |
| After term, >42 | … | 1 (33.3) | … | … | … | … | … |
| Current weight, kg | |||||||
| Median | 11.39 | 12.70 | 8.50 | 8.50 | 7.35 | 6.20 | 4.30 |
| Range | 8.12–20.60 | 12.20–13.50 | 7.30–10.70 | 5.10–10.40 | 4.62–11.30 | 3.50–8.86 | 3.27–5.60 |
| Confirmation of influenza diagnosis before enrollment | |||||||
| Viral culture | 1 (8.3) | … | … | 1 (12.5) | … | … | 1 (4.3) |
| Rapid influenza diagnostic test | 11 (91.7) | 3 (100) | 7 (100) | 7 (87.5) | 24 (100) | 10 (100) | 22 (95.7) |
| Influenza virus type from isolates obtained after enrollment | |||||||
| Seasonal H1N1 | 1 (8.3) | 0 | 1 (14.3) | 2 (25) | 2 (8.3) | 2 (20) | 0 |
| A(H1N1)pdm09 | 1 (8.3) | 2 (66.7) | 0 | 2 (25) | 6 (25) | 5 (50) | 21 (91.3) |
| H3N2 | 4 (33.3) | 0 | 3 (42.9) | 0 | 10 (41.7) | 1 (10) | 0 |
| B | 3 (25) | 0 | 1 (14.3) | 1 (12.5) | 0 | 0 | 0 |
| Negative culture result | 3 (25) | 1 (33.3) | 2 (28.6) | 3 (37.5) | 6 (25) | 2 (20) | 2 (8.7) |
| Location of subject | |||||||
| Inpatient, non-ICU | 8 (66.7) | 1 (33.3) | 3 (42.9) | 2 (25) | 6 (25) | 2 (20) | 18 (78.3) |
| Inpatient, ICU | 1 (8.3) | 1 (33.3) | … | 2 (25) | … | … | 5 (21.7) |
| Outpatient | 3 (25) | 1 (33.3) | 4 (57.1) | 4 (50) | 18 (75) | 8 (80) | … |
Data are no. (%) of subjects, unless otherwise indicated.
Abbreviations: A(H1N1)pdm09, 2009 pandemic influenza A virus subtype H1N1; H1N1, influenza A virus subtype H1N1; H3N2, influenza A virus subtype H3N2; B, influenza B virus; ICU, intensive care unit.
Pharmacokinetic Results
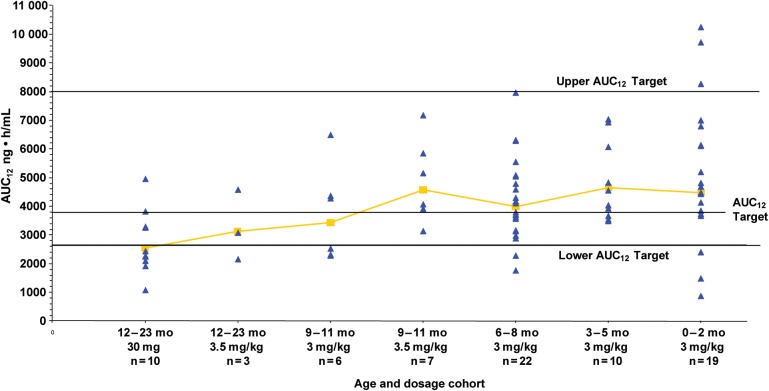
Oseltamivir carboxylate pharmacokinetic parameters for the 77 subjects who completed pharmacokinetic sampling are presented in Table 2. Reasons for the lack of pharmacokinetic assessments in the remaining 10 subjects are as follows: early termination (n = 2), failure to return for specimen collection for laboratory-based pharmacokinetic analysis (n = 2), parental refusal to allow completion of pharmacokinetic sampling (n = 3), and collection of an insufficiently large blood sample (n = 3). An oseltamivir dose of 3.0 mg/kg produced exposures within the target range in subjects 0–8 months of age, although a greater degree of variability in the very young subjects (ie, those <3 months old) was seen (Figure 2). In subjects 9–11 months of age, a dose of 3.0 mg/kg was not adequate to reproducibly attain exposure above the predetermined minimum target, with 3 of 6 subjects having an AUC12 below the lower limit of 2660 ng • h/mL. All subjects in this age cohort receiving an adjusted dose of 3.5 mg/kg achieved an AUC12 within the targeted range (Figure 2). Likewise, 6 of 10 subjects 12–23 months of age receiving the FDA-approved unit dose of 30 mg had oseltamivir carboxylate AUC12 values below the target range, but an adjusted dose of 3.5 mg/kg produced a drug exposure within the target range in 2 of 3 recipients. These observations correlate with CL/F increasing (and therefore AUC12 values decreasing) with increasing subject age (r = −0.69; P ≤ .0001; Table 2). The average concentration-time curves for oseltamivir and oseltamivir carboxylate by cohort demonstrate that although there is a delay in the formation of the metabolite, especially in the younger babies, the overall oseltamivir carboxylate exposures were similar (data not shown).
Table 2.
Oseltamivir Carboxylate Pharmacokinetic Parameters, by Age and Dosage Cohort
| Variable | Twice-Daily Dose, by Age |
||||||
|---|---|---|---|---|---|---|---|
| 12–23 mo, 30 mg (n = 10) | 12–23 mo, 3.5 mg/kg (n = 3) | 9–11 mo, 3.0 mg/kg (n = 6) | 9–11 mo, 3.5 mg/kg (n = 7) | 6–8 mo, 3.0 mg/kg (n = 22) | 3–5 mo, 3.0 mg/kg (n = 10) | 0–2 mo, 3.0 mg/kg (n = 19) | |
| AUC0–12, ng • h/mL | |||||||
| Geometric mean | 2534.39 | 3114.66 | 3426.63 | 4575.56 | 3991.74 | 4644.00 | 4475.46 |
| Median | 2354.38 | 3072.81 | 3401.33 | 4067.73 | 3948.74 | 4291.78 | 4688.04 |
| Range | 1073.31–4948.63 | 2148.59–4576.58 | 2276.91–6487.14 | 3146.37–7171.09 | 1758.56–7957.98 | 3497.34–7027.59 | 872.54–10 241.73 |
| Cmax, ng/mL | |||||||
| Median | 262 | 299 | 347.50 | 497 | 440.50 | 427 | 535 |
| Range | 101–526 | 213–506 | 200–705 | 338–747 | 169–864 | 361–807 | 103–1120 |
| Tmax, h | |||||||
| Median | 3.93 | 2.83 | 5.14 | 5.52 | 3.93 | 5.04 | 5.13 |
| Range | 0–5.67 | 2.20–5.08 | 2.22–5.73 | 2.17–10.52 | 1.03–10.02 | 2.08–6.18 | 2.17–6.97 |
| T1/2, h | |||||||
| Median | 7.98 | 14.82 | 11.13 | 14.56 | 10.29 | 9.09 | 6.64 |
| Range | 4.49–17.11 | 8.13–20.16 | 5.40–51.86 | 7.22–25.67 | 1.02–78.26 | 6.25–19 | 4.65–28.71 |
| CL/F, L/h | |||||||
| Median | 11.60 | 12.64 | 7.05 | 5.21 | 4.90 | 3.81 | 2.30 |
| Range | 5.51–25.42 | 9.39–18.81 | 3.57–9.49 | 2.81–8.72 | 2.45–15.00 | 1.43–6.83 | 1.04–13.79 |
| V/F, L/kg | |||||||
| Median | 13.92 | 24.36 | 15.13 | 10.41 | 10.19 | 10.40 | 5.80 |
| Range | 5.89–53.31 | 8.97–47.37 | 5.46–95.30 | 7.04–21.56 | 1.39–107.59 | 4.03–13.94 | 2.97–44.91 |
Abbreviations: AUC0–12, 0–12-h area under the concentration curve; Clast, last measured concentration; CL/F, oral clearance rate; Cmax, maximum concentration; Tmax, time to Cmax; T½, half-life; V/F, apparent distribution volume.
Figure 2.
Oseltamivir carboxylate geometric mean 12-hour area under the concentration curve (AUC12), by age and dosage cohort, with target and range.
Virologic Results
Of the 87 enrolled subjects, 84 had a positive rapid influenza virus diagnostic test, and 3 had a positive influenza virus culture to qualify for enrollment (Table 1). Of the 84 with rapid diagnostic testing, 19 subsequently were culture negative for influenza virus by the research laboratory, 3 of whom were PCR positive but had low viral loads (range 134 to 1117 copies per mL); the remaining 16 were PCR-negative. Of the 68 subjects who were culture-positive in the research laboratory, 37 had A(H1N1)pdm09, 8 had seasonal H1N1, 18 had H3N2, and 5 had influenza B.
Pharmacodynamic Analyses
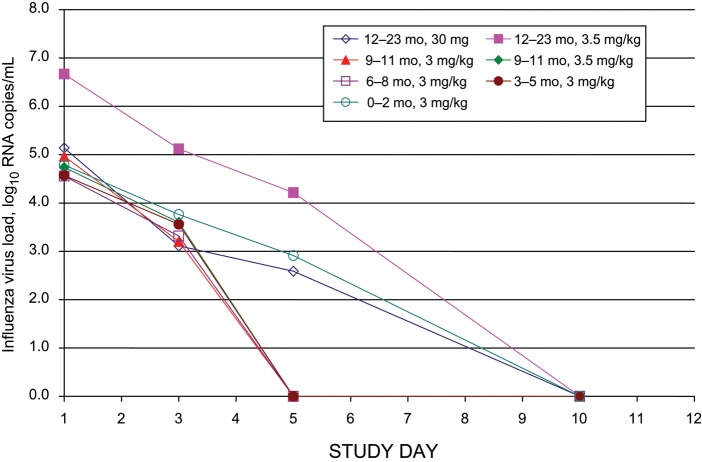
Median viral loads by study day and age cohort, as measured by PCR, are presented in Figure 3 and Table 3. The amount of virus by study day and age cohort, as measured by viral culture, is presented in Table 4. The quantity of virus detectable by PCR correlated highly with the amount of virus detected by culture, with Spearman correlation coefficients of 0.83 353 (P < .0001) for day 1, 0.74 543 (P < .0001) for day 3, 0.82 836 (P < .0001) for day 5, and 0.22 631 (P = .0615) for day 10. The median days to viral loads <50 copies/mL, by cohort, were 3 days (3–5 months), 4 days (6–8 and 9–11 months), and 5 days (0–2 months and 12–23 months). Clearance of virus by PCR or culture did not correlate with oseltamivir carboxylate pharmacokinetic parameters (data not shown). Neither virus type nor viral RNA load correlated with the number of symptoms at baseline or the duration of symptoms prior to enrollment (data not shown).
Figure 3.
Decline in nasopharyngeal influenza virus load over time, by age and dosage cohort.
Table 3.
Influenza Viral Load Detected by Polymerase Chain Reaction, by Age and Dosage Cohort and Study Day
| Variable | Twice-Daily Dose, by Age |
||||||
|---|---|---|---|---|---|---|---|
| 12–23 mo, 30 mg | 12–23 mo, 3.5 mg/kg | 9–11 mo, 3.0 mg/kg | 9–11 mo, 3.5 mg/kg | 6–8 mo, 3.0 mg/kg | 3–5 mo, 3.0 mg/kg | 0–2 mo, 3.0 mg/kg | |
| Day 1 | |||||||
| Median | 5.14 | 6.67 | 4.97 | 4.98 | 4.56 | 4.57 | 4.79 |
| Range | 0–6.59 | 0–7.03 | 0–6.41 | 0–6.97 | 0–7.01 | 0–6.35 | 0–6.94 |
| Subjects, no | 12 | 3 | 7 | 7 | 24 | 10 | 23 |
| Day 3 | |||||||
| Median | 3.11 | 5.12 | 3.20 | 3.60 | 3.32 | 3.68 | 3.76 |
| Range | 0–5.90 | 0–5.14 | 0–4.50 | 0–4.16 | 0–5.17 | 0–6.51 | 0–5.79 |
| Subjects, no | 10 | 3 | 6 | 7 | 24 | 10 | 22 |
| Day 5 | |||||||
| Median | 2.59 | 4.22 | 0 | 0 | 0 | 0 | 2.91 |
| Range | 0–4.47 | 0–6.27 | 0–5.72 | 0–4.24 | 0–6.09 | 0–3.95 | 0–6.23 |
| Subjects, no | 12 | 3 | 7 | 7 | 23 | 10 | 21 |
| Day 10 | |||||||
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Range | 0–3.76 | 0–0 | 0–0 | 0–0 | 0–2.63 | 0–0 | 0–3.38 |
| Subjects, no | 11 | 3 | 7 | 7 | 22 | 9 | 20 |
Data are log10 influenza virus RNA copies per milliliter, unless otherwise indicated.
Table 4.
Median Tissue Culture Infective Dose (TCID50) of Influenza Virus Detected by Culture, by Age and Dosage Cohort and Study Day
| Variable | Twice-Daily Dose, by Age |
||||||
|---|---|---|---|---|---|---|---|
| 12–23 mo, 30 mg | 12–23 mo, 3.5 mg/kg | 9–11 mo, 3.0 mg/kg | 9–11 mo, 3.5 mg/kg | 6–8 mo, 3.0 mg/kg | 3–5 mo, 3.0 mg/kg | 0–2 mo, 3.0 mg/kg | |
| Day 1 | |||||||
| Median | 4.15 | 4.85 | 3.80 | 4.49 | 3.45 | 3.28 | 3.80 |
| Range | 0–7.29 | 4.15–5.54 | 3.45–6.94 | 4.15–5.54 | 0–7.64 | 0–4.49 | 1.70–6.24 |
| Subjects, no | 11 | 2 | 5 | 5 | 20 | 10 | 21 |
| Day 3 | |||||||
| Median | 1.53 | 3.80 | 1.88 | 2.40 | 1.53 | 2.40 | 2.93 |
| Range | 0–6.24 | 3.45–4.15 | 1–3.45 | 0–4.84 | 0–4.84 | 0–5.54 | 0–5.54 |
| Subjects, no | 10 | 2 | 4 | 5 | 20 | 10 | 20 |
| Day 5 | |||||||
| Median | 1 | 3.80 | 2.40 | 0 | 0 | 0 | 2.40 |
| Range | 0–3.45 | 2.75–4.84 | 0–4.15 | 0–3.80 | 0–4.84 | 0–3.80 | 0–4.49 |
| Subjects, no | 11 | 2 | 5 | 5 | 19 | 10 | 19 |
| Day 10 | |||||||
| Median | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Range | 0–1.35 | 0–0 | 0–1 | 0–0 | 0–0 | 0–0 | 0–1.35 |
| Subjects, no | 11 | 2 | 5 | 5 | 18 | 9 | 19 |
Data are TCID50, unless otherwise indicated.
Resistance Testing
No known zanamivir resistance mutations were observed in any of the samples with sequence information.
A(H1N1)pdm09 Resistance Testing
The mean phenotypic IC50 values ( ± SD) for oseltamivir carboxylate in the 37 subjects with pandemic H1N1 were 4.0 ± 25 nM (median, 0.6 nM [range, 0.04–205 nM]; 1 nM = 0.28 ng/mL). Further analysis showed that the mean IC50 values ( ± SD) from baseline samples were 0.65 ± 0.48 nM (median, 0.56 nM [range, 0.03–1.86 nM]). Isolates from 2 subjects acquired reduced susceptibility to oseltamivir carboxylate during therapy, with IC50 values increasing from 0.55 nM at baseline to 30 nM on day 2 of treatment in one subject and from 1.75 nM at baseline to 205 nM on study day 9 (4 days after treatment was discontinued) in the other.
No H275Y mutations were identified at baseline. Specimens from 3 subjects acquired the H275Y mutation during therapy. In 2 of these subjects, including the subject with an IC50 of 205 nM on study day 9 and another subject with an IC50 of 3.83 on study day 5, the entire viral population had the H275Y mutation, on the basis of population sequencing. The third subject, who had an IC50 of 30 nM, had a mixed viral population, in which a detectable minority carried the H275Y mutation. Two of these subjects were in the 0–2-month age cohort (IC50 values, 30 nM and 205 nM), and 1 was in the 6–8-month age cohort (IC50, 3.83 nM).
Seasonal H1N1 Resistance Testing
Among the 8 subjects with seasonal H1N1 infection, the mean phenotypic IC50 values ( ± SD) were 354 ± 302 nM (median, 385 nM [range, 0.5–852 nM]) for oseltamivir carboxylate. Isolates obtained during the 2006–2007 influenza season were fully susceptible, with mean IC50 values ( ± SD) of 1.44 ± 0.36 nM (median, 1.47 nM [range, 1.05–1.78 nM]). By the 2008–2009 influenza season, however, mean oseltamivir carboxylate IC50 values ( ± SD) were 512 ± 183 nM (median, 581 nM [range, 192–672 nM]).
As expected, all seasonal H1N1 influenza isolates obtained in 2009 carried the H275Y mutation, as did 1 isolate obtained in 2008. The mutation was present in each of these specimens at baseline and therefore reflects the genotype of the circulating strain. The H275Y-associated change D344N also was observed in these samples. All the viruses carrying the H275Y mutation exhibited reduced susceptibility to oseltamivir carboxylate.
H3N2 Resistance Testing
Among the 18 subjects with H3N2 infection, mean phenotypic IC50 values ( ± SD) were 0.8 ± 0.59 nM (median, 0.62 nM [range, 0.04–2.09 nM]) for oseltamivir carboxylate, indicating that all specimens were fully susceptible in phenotypic analysis. In genotypic analysis, no mutations associated with oseltamivir resistance were identified.
Influenza B Resistance Testing
Among the 5 subjects with influenza B infection, mean phenotypic IC50 values ( ± SD) were 28 ± 13 nM (median, 26 nM [range, 12–50 nM]) for oseltamivir carboxylate, indicating that all specimens were fully susceptible in phenotypic analysis. In genotypic analysis, polymorphisms were identified at position 198, but there was no correlation with oseltamivir susceptibility.
Safety Assessments
Eighty-one of 87 subjects (93%) received ≥8 doses of study medication (Figure 1), and no subject had study drug discontinued early due to tolerability issues. No unexpected safety concerns were observed during oseltamivir therapy, and there were no seizures. Seven adverse events were considered related to study medication, including emesis (n = 5) and rash (n = 2). One serious adverse event (cutaneous hypersensitivity reaction) was considered to be associated with study medication.
DISCUSSION
These data provide the most robust information to date on dosing of an influenza antiviral drug in young children. In infants from birth through 8 months of age, oseltamivir suspension administered orally at 3.0 mg/kg/dose twice daily provides systemic oseltamivir carboxylate exposures within the targeted range selected to maximize effectiveness and minimize development of resistance. A higher dose of 3.5 mg/kg/dose twice daily is required for infants 9–11 months of age to achieve the targeted exposure. Children aged 12–23 months have suboptimal exposure when administered the FDA-approved unit dose of 30 mg, according to our study criteria, and underexposure can lead to development of antiviral resistance [38]. However, with only 3 study subjects in this age group receiving 3.5 mg/kg/dose twice daily (one of whom had a level below the target range), we are unable to recommend this as the appropriate dose for this age group. We previously have estimated the appropriate oseltamivir treatment dose in premature neonates to be 1.0 mg/kg/dose twice daily [14].
A recent supplemental new drug application (sNDA) has been submitted to the FDA by Roche on the basis of this study and a Roche-sponsored trial (WP22849). Modeling and simulation from these studies led to dosing recommendations in this sNDA of 3.0 mg/kg/dose twice daily for all infants, from birth through 11 months of age. Another recent study, also based on modeled data but from a much smaller number of subjects (n = 9), has recommended a dose of 2.0 mg/kg for term infants [39]. While modeling can be useful in estimating dosing in different populations, we believe the doses of 3.0 mg/kg (for birth through 8 months) and 3.5 mg/kg (for 9–11 months) identified in this large pharmacokinetic study more accurately reflect the correct doses in this population.
With decreasing age, the variability around the oseltamivir carboxylate AUC12 increases (Figure 2), and clearance decreases (Table 2). Two major human carboxylesterases (HCE1 and HCE2) are abundantly expressed in the liver. These enzymes differ in the hydrolysis of certain drugs. HCE1 rapidly converts oseltamivir phosphate to oseltamivir carboxylate. One recent analysis of 104 liver samples for the expression patterns of both carboxylesterases in fetuses (82–224 gestation days), children (aged 0 days through 10 years), and adults (aged >18 years) [40] reported the variability in relative HCE1 messenger RNA as ranging from 12-fold in adults to 218-fold in children and 431-fold in fetuses. These data help explain, in part, why the variability in oseltamivir carboxylate exposure is high in our youngest age cohort.
This is the first large study to document rates of oseltamivir resistance in young children before and after the 2009 pandemic. In our trial, 3 subjects (all with A[H1N1]pdm09 infection) who had an oseltamivir-susceptible strain at baseline had virus that developed oseltamivir resistance during treatment. Thus, our overall rate for development of oseltamivir resistance was 4.4% (3 of 68 subjects with culture-confirmed influenza) but was 8.1% (3 of 37) among those with A(H1N1)pdm09 infection. These resistance rates are relatively consistent with prior studies, which have reported resistance rates of 6.5% in the pediatric population [23, 31, 41, 42]. However, previously published studies of immunocompetent adults with A(H1N1)pdm09 infection reported a lower rate of oseltamivir resistance of 1.6% [41, 43–45], suggesting that resistance patterns in children continue to need close monitoring.
The lack of correlation between oseltamivir carboxylate pharmacokinetics and influenza virus load likely reflected all subjects receiving the same therapeutic intervention. The relatively narrow range of oseltamivir carboxylate exposures produced similar drug exposures, which precluded the establishment of concentration-response relationships.
In adults, influenza virus load correlates with disease severity and rate of clinical improvement [46–49]. We did not demonstrate a relationship between viral quantity and clinical disease severity. This may relate to different disease pathogenesis between children and adults or to sampling issues correlating with difficulty obtaining adequate nasopharyngeal samples in children.
In conclusion, the optimal oseltamivir dose is 3.0 mg/kg/dose twice daily for infants through 8 months of life and is 3.5 mg/kg/dose twice daily for infants 9–11 months of age. Additional study of weight-based dosing of oseltamivir in children 12–23 months of age is warranted.
Supplementary Material
Notes
Acknowledgments. The members of the CASG 114 Data and Safety Monitoring Board were Charles Grose, MD (chair; University of Iowa); Jon S. Abramson, MD (Wake Forest University); Courtney V. Fletcher, PharmD (University of Nebraska); Flor M. Munoz, MD (Baylor College of Medicine); and Mark VanRaden, PhD (Biostatistics Research Branch, NIAID).
Study personnel at each site who participated significantly in the conduct of this multiinstitutional clinical trial include Dusty Giles and Bari Cotton (University of Alabama at Birmingham); Sharon Judy and Margaret Cowie (University of Texas Southwestern Medical Center, Dallas); Jeanne Francis (University of Utah, Salt Lake City); Candice Evans and Nan O'Donnell (Miller Children's Hospital, Long Beach, CA); Ofelia Vargas Shiraishi (Children's Hospital of Orange County, Orange, CA); Lisa Latiolais (Louisiana State University Health Sciences Center, Shreveport); Valeri Aymami and Ken Dole (University of Colorado School of Medicine, Aurora); Julie Gaultier (Case Western Reserve University, Cleveland, OH); Gerry Lofthus (University of Rochester School of Medicine and Dentistry, NY); Diane Kinnunen and Kirsten Lacombe (Seattle Children's Hospital, WA); Nancy Stellato (Cohen Children's Medical Center, Hofstra North Shore-LIJ School of Medicine, Hempstead, NY); Julie Denlinger (Cincinnati Children's Medical Center, OH); Sara Hingtgen (Rady Children's Hospital, San Diego, CA); Christina Mason (University of Florida, Gainesville); and Noreen Jeffrey (University of Pittsburgh School of Medicine, PA).
Financial support. This work was supported by the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (contracts N01-AI-30025, N01-AI -65306, N01-AI -15113, and N01-AI-62554). Assays of oseltamivir and oseltamivir carboxylate concentrations in plasma were provided by F. Hoffman–La Roche.
Potential conflicts of interest. R. J. W. serves on the board of directors of Gilead, which licensed oseltamivir to F. Hoffman–La Roche. His involvement in this study was overseen by the University of Alabama at Birmingham Conflict of Interest Review Board. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003—2004. N. Engl. J. Med. 2005;353:2559–67. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 2.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N. Engl. J. Med. 2000;342:232–9. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 2000;342:225–31. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 4.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–64. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004;113:585–93. doi: 10.1542/peds.113.3.585. [DOI] [PubMed] [Google Scholar]
- 6.Hackett S, Hill L, Patel J, et al. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet. 2009;374:605. doi: 10.1016/S0140-6736(09)61511-7. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N. Engl. J. Med. 2009;361:1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 8.Libster R, Bugna J, Coviello S, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N. Engl. J. Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 9.Lister P, Reynolds F, Parslow R, et al. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet. 2009;374:605–7. doi: 10.1016/S0140-6736(09)61512-9. [DOI] [PubMed] [Google Scholar]
- 10.Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet. 2010;376:1846–52. doi: 10.1016/S0140-6736(10)61195-6. [DOI] [PubMed] [Google Scholar]
- 11.Cox CM, Blanton L, Dhara R, Brammer L, Finelli L. 2009 pandemic influenza A (H1N1) deaths among children—United States, 2009–2010. Clin. Infect. Dis. 2011;52(Suppl 1):S69–74. doi: 10.1093/cid/ciq011. [DOI] [PubMed] [Google Scholar]
- 12.Randolph AG, Vaughn F, Sullivan R, et al. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–e1458. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Medina E, Ardura MI, Siegel JD, Brock E, Sanchez PJ. 2009 influenza A in infants hospitalized at younger than 6 months. J. Pediatr. 2012;160:626–631. doi: 10.1016/j.jpeds.2011.09.060. e1. [DOI] [PubMed] [Google Scholar]
- 14.Acosta EP, Jester P, Gal P, et al. Oseltamivir dosing for influenza infection in premature neonates. J. Infect. Dis. 2010;202:563–6. doi: 10.1086/654930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson DJ, Buttery JP, Andersen CC. Influenza in the neonatal intensive care unit. J. Perinatol. 2006;26:772–6. doi: 10.1038/sj.jp.7211625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sert A, Yazar A, Odabas D, Bilgin H. An unusual cause of fever in a neonate: influenza A (H1N1) virus pneumonia. Pediatr. Pulmonol. 2010;45:734–6. doi: 10.1002/ppul.21245. [DOI] [PubMed] [Google Scholar]
- 17.Munoz FM, Campbell JR, Atmar RL, et al. Influenza A virus outbreak in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 1999;18:811–5. doi: 10.1097/00006454-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2011–2012. Pediatrics. 2011;128:813–25. doi: 10.1542/peds.2011-2295. [DOI] [PubMed] [Google Scholar]
- 19.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 20.He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64–0802. Clin. Pharmacokinet. 1999;37:471–84. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–50. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 22.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 23.Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 2001;20:127–33. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin. Infect. Dis. 2010;51:887–94. doi: 10.1086/656408. [DOI] [PubMed] [Google Scholar]
- 25.McGeer A, Green KA, Plevneshi A, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin. Infect. Dis. 2007;45:1568–75. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 26.Hanshaoworakul W, Simmerman JM, Narueponjirakul U, et al. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS One. 2009;4:e6051. doi: 10.1371/journal.pone.0006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. doi: 10.1136/bmj.c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernan MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin. Infect. Dis. 2011;53:277–9. doi: 10.1093/cid/cir400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. FDA issuance letter for Tamiflu. 2009 Accessed 8 September 2009. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM153547.pdf . [Google Scholar]
- 30.Centers for Disease Control and Prevention. Recommendations for use of antiviral medications for the management of influenza in children and adolescent for the 2009–2010 season—pediatric supplement for health care providers. 24 December 24 2009. http://www.cdc.gov/h1n1flu/recommendations_pediatric_supplement.htm . Accessed 29 December 2011. [Google Scholar]
- 31.Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–65. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- 32.Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J. Infect. Dis. 2004;189:440–9. doi: 10.1086/381128. [DOI] [PubMed] [Google Scholar]
- 33.Wiltshire H, Wiltshire B, Citron A, et al. Development of a high-performance liquid chromatographic-mass spectrometric assay for the specific and sensitive quantification of Ro 64–0802, an anti-influenza drug, and its pro-drug, oseltamivir, in human and animal plasma and urine. J. Chromatogr. B. Biomed. Appl. 2000;745:373–88. doi: 10.1016/s0378-4347(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 34.Ward CL, Dempsey MH, Ring CJ, et al. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J. Clin. Virol. 2004;29:179–88. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton DW, Mellen CF, Baxter BD, Atmar RL, Menegus MA. Practical and sensitive screening strategy for detection of influenza virus. J. Clin. Microbiol. 2002;40:4353–6. doi: 10.1128/JCM.40.11.4353-4356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 37.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson I, Democratis J, Lackenby A, et al. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin. Infect. Dis. 2009;48:389–96. doi: 10.1086/596311. [DOI] [PubMed] [Google Scholar]
- 39.Standing JF, Nika A, Tsagris V, et al. Oseltamivir pharmacokinetics and clinical experience in neonates and infants during an outbreak of H1N1 influenza a virus infection in a neonatal intensive care unit. Antimicrob. Agents Chemother. 2012;56:3833–40. doi: 10.1128/AAC.00290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D, Pearce RE, Wang X, Gaedigk R, Wan YJ, Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem. Pharmacol. 2009;77:238–47. doi: 10.1016/j.bcp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorlund K, Awad T, Boivin G, Thabane L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infect Dis. 2011;11:134. doi: 10.1186/1471-2334-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatakeyama S, Sugaya N, Ito M, et al. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA. 2007;297:1435–42. doi: 10.1001/jama.297.13.1435. [DOI] [PubMed] [Google Scholar]
- 43.Harvala H, Gunson R, Simmonds P, Hardie A, Bennett S, Scott F, Roddie H, McKnight J, Walsh T, Rowney D, Clark A, Bremner J, Aitken C, Templeton K. The emergence of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus amongst hospitalised immunocompromised patients in Scotland, November-December, 2009. Euro Surveill. 2010;15(14) pii=19536. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19536 . [PubMed] [Google Scholar]
- 44.Wang B, Dwyer DE, Blyth CC, et al. Detection of the rapid emergence of the H275Y mutation associated with oseltamivir resistance in severe pandemic influenza virus A/H1N1 09 infections. Antiviral Res. 2010;87:16–21. doi: 10.1016/j.antiviral.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Winzer R, Kanig N, Schneitler S, et al. Early clinical experiences with the new influenza A (H1N1/09) Dtsch Arztebl Int. 2009;106:770–6. doi: 10.3238/arztebl.2009.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boivin G, Coulombe Z, Wat C. Quantification of the influenza virus load by real-time polymerase chain reaction in nasopharyngeal swabs of patients treated with oseltamivir. J. Infect. Dis. 2003;188:578–80. doi: 10.1086/377046. [DOI] [PubMed] [Google Scholar]
- 47.To KK, Hung IF, Li IW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 2010;50:850–9. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ison MG, de Jong MD, Gilligan KJ, et al. End points for testing influenza antiviral treatments for patients at high risk of severe and life-threatening disease. J. Infect. Dis. 2010;201:1654–62. doi: 10.1086/652498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.