Abstract
Background. We hypothesized that tacrolimus, an inhibitor of the calcineurin pathway, would enhance the in vivo activity of posaconazole against Rhizopus oryzae, the Mucorales species most commonly associated with mucormycosis.
Methods. We examined patterns of growth inhibition and fungicidal activity of posaconazole and tacrolimus, alone and in combination, against R. oryzae in vitro, using multiple methods (ie, hyphal metabolic and fluorescent vital dye reduction assays and measurement of chitin concentrations), and in vivo, using 2 mucormycosis models: an invertebrate model (Drosophila) and a nonlethal murine model of cutaneous mucormycosis.
Results. Combinations of posaconazole and tacrolimus were synergistic in checkerboard assays for 4 clinical isolates of R. oryzae (48-hour fractional inhibitory concentration index, 0.187–0.281). Pharmacodynamic analysis of the combination revealed that the 90% effective concentration threshold of posaconazole activity against R. oryzae could be achieved with 2-fold lower drug concentrations (0.5–1 mg/L) when administered with tacrolimus (0.007–2 mg/L). In vivo, combination therapy was associated with improved survival in the fly model of mucormycosis (65% vs 57% posaconazole alone) and with significant reductions in cutaneous lesions and R. oryzae fungal burden, compared with animals that received posaconazole monotherapy, in the cutaneous model of mucormycosis.
Conclusions. Combination posaconazole-tacrolimus therapy displays synergism in vitro and improved antifungal efficacy in vivo in 2 phylogenetically distinct models of mucormycosis.
Keywords: Mucormycosis, tacrolimus, posaconazole, animal models, subcutaneous, immunocompromised
Mucormycosis is an aggressive and often lethal opportunistic fungal infection that is increasing in frequency among immunocompromised populations, especially patients with leukemia who undergo hematopoietic stem cell transplantation (HSCT) [1–3]. The widespread use of anti-Aspergillus active antifungals with limited activity against Mucorales (ie, voriconazole) [1], transfusional hemochromatosis, and corticosteroid-associated hyperglycemia are risk factors that may predispose a subset of hematology patients to this infection [4–6]. Mucorales are resistant to many antifungal agents and can only be controlled with timely administration of amphotericin B (occasionally used in combination with echinocandins) and posaconazole. Unfortunately, many patients still die from mucormycosis despite intensive medical and surgical interventions, thus highlighting an urgent need for new treatment strategies [7].
Tacrolimus is a macrolide lactone immunosuppressive agent widely used after solid-organ and allogeneic HSCT to prevent graft rejection and modulate graft versus host disease [8]. Tacrolimus binds to the intracellular protein immunophilin FKB12, thereby inhibiting activation of the calcineurin pathway and interleukin 2 transcription in T cells [8]. In pathogenic fungi, the evolutionarily conserved calcineurin pathway functions as an important “circuit” for fungal homeostatic cell responses, which counteract the damaging effects of antifungals at the cell membrane and cell wall, as well as contribute to the development and maintenance of antifungal resistance [9–11].
Recent genomic analysis of Rhizopus oryzae, the most common cause of mucormycosis in US transplant recipients [1], revealed evidence of an ancestral whole-genome duplication event that expanded gene families related to cell wall synthesis and homeostatic cell responses, compared with more common molds, such as Aspergillus [12]. This evolutionary pathway also led to the duplication of genes involved in the ergosterol biosynthesis pathway—the principle target for posaconazole [12]. As a consequence, R. oryzae appears to be genetically well equipped to overcome the toxic effects of antifungals such as posaconazole [13]. Therefore, simultaneous targeting of both ergosterol synthesis and homeostatic calcineurin pathways in R. oryzae could be an important strategy for enhancing the potency of antifungal agents against this devastating pathogen.
Although in vitro reports [14–16] have suggested possible synergy between triazoles and calcineurin inhibitors against some Aspergillus species and Mucorales, and although a recent study reported the antifungal efficacy of the mTOR inhibitor rapamycin in the Galleria mellonella (wax moth) model of mucormycosis [17], no published reports have examined the efficacy of posaconazole-calcineurin inhibitor combinations in animal models of mucormycosis [18]. Here, we examined the effects of tacrolimus on posaconazole activity, using several in vitro and in vivo methods for characterizing antifungal activity against R. oryzae. We found that tacrolimus significantly enhanced the potency of posaconazole in vitro and improved survival in posaconazole-treated flies with invasive mucormycosis. Combination tacrolimus-posaconazole therapy also improved control of invasive, necrotizing cutaneous mucormycosis in immunosuppressed mice, compared with posaconazole monotherapy.
METHODS
Study Isolates
Four clinical isolates of R. oryzae recovered from patients at The University of Texas M. D. Anderson Cancer Center (Houston, TX) were selected for testing. Isolates were identified by standard morphological criteria, and the genus identification was confirmed by sequencing of the ITS1-5.8S-ITS2 region of the fungal ribosomal RNA genes as previously described [19]. R. oryzae 557969, a clinical strain that was previously tested by our laboratory in a murine model of pulmonary infection [20], was selected for additional in vitro and in vivo analysis. All isolates were grown on yeast extract agar glucose (YAG) plates for 48–72 hours at 37°C. Sporangiospores were collected in sterile normal saline with 0.08% Tween 20, washed twice in normal saline, passed through 40-µm nylon filters (BD Biosciences, Franklin Lakes, NJ), and enumerated in a hemocytometer, to standardize the inoculum for in vitro testing.
Drugs and Susceptibility Testing
For in vitro experiments, stock solutions of posaconazole (20 mg/mL; Merck, Rahway, NJ) and tacrolimus (1 mg/mL; Astellas Pharma, Deerfield, IL) were prepared at 100-fold higher concentrations than the highest test concentration in 100% analytical-grade dimethyl sulfoxide (DMSO), according to CLSI recommendations [21], and were stored in light-protected containers at 4°C until use. The final test concentration of DMSO did not exceed 1% in any experimental group, and solvent controls were included for in vitro tests.
Posaconazole and tacrolimus minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) were determined using methods outlined by the CLSI [21] and by Espinel-Ingroff et al [22, 23]. Serial dilutions of posaconazole and tacrolimus (0.002–4 µg/mL) along with drug-free controls were prepared in Roswell Park Memorial Institute (RPMI) 1640 medium buffered with MOPS (3-[N-morpholino]propanesulfonic acid) at a final concentration of 0.165 mol/L at pH 7.0, with glutamine and without bicarbonate. Spores collected on the day of testing were suspended in the test medium at 0.5–4 × 104 spores/mL, and 100 µL of the suspension was inoculated into flat-bottom microtiter trays containing drug and drug-free control wells. Plates were incubated at 37°C for 48 hours, and the MIC was determined at 24 and 48 hours visually and by spectrophotometry as the lowest drug concentration resulting in 100% growth inhibition. MFCs were determined at 48 hours as described previously [22] and were defined as the lowest drug concentration at which <3 colonies were observed, which corresponded to a killing activity of ≥99.9%.
In Vitro Drug Combination Studies
The combined activity of posaconazole and tacrolimus was initially screened in clinical isolates in triplicate on different experimental days, using the XTT assay in a checkerboard dilution array as previously described [24]. To better understand the magnitude of synergy, the combination index was calculated for each ratio of the drug combination tested at the 90% effective concentration (EC90) for R. oryzae 557969, using the median-effect equation described by Chou and Talalay, as implemented in the Calcusyn 2.0 software package (Biosoft, Cambridge, United Kingdom) [25]. Briefly, the method is derived from mass-action law models and accounts for both the potency (eg, EC50) and shape of the dose-effect curve for each drug individually and at each ratio of drug concentrations tested in the checkerboard array, to derive a combination index that can be interpreted as antagonism (if >1), synergism (if 0.3–0.9), and strong synergism (if <0.3) [25, 26].
We also directly examined R. oryzae 557969 hyphae cell injury following exposure to each drug, alone or in combination, using vital dye staining with mortal cell injury dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC; Molecular Probes, Carlsbad, CA) [27]. A standardized suspension of 1 × 105 R. oryzae spores were grown to hyphae in microcentrifuge tubes with RPMI 1640 medium plus 0.15% (wt/vol) Junlon (Nihon Junyaku, Tokyo, Japan) at 37°C with shaking for 18 hours. Tubes were centrifuged at 13 000 × g to remove the media, and hyphae were resuspended in RPMI medium containing 2 μg/mL posaconazole, 2 μg/mL tacrolimus, a combination of posaconazole and tacrolimus, amphotericin B 2 μg/mL (positive control), or drug-free medium (negative control). Tubes were then incubated for 6 hours at 37°C with shaking. Hyphae were then washed twice in 3-(N-morpholino) propanesulfonic acid (MOPS) at pH 7 (designated MOPS7), and DiBAC was added from 1 mg/mL stock in 100% ethanol at 2 μg/mL in MOPS7. Tubes were incubated with gentle shaking at ambient temperature in the dark for 1 hour. Samples then were washed twice in MOPS7 and stored on ice until fluorescent microscopy was performed. Images of 5 separate microscopic fields containing hyphae were acquired at 200× magnification under a triple-band fluorescent microscope (Olympus BX-51; Olympus, Melville, NY), using fluorescein isothiocyanate (FITC). Analysis of hyphal staining was qualitative in nature relative to untreated positive and negative controls.
Finally, the capacity of the combination to reduce fungal cell biomass in vitro was analyzed using the chitin assay. Hyphae were grown as described above in a subinhibitory concentration of posaconazole (0.5 mg/L), alone or in combination with low (0.015 mg/L) or high (2 mg/L) concentrations of tacrolimus. The chitin concentrations were then determined using methods described by Lehmann and White [28]. Experiments were performed in 4 replicates.
In Vivo Toll-Deficient Fly Drug Protection Assay
An established Toll-deficient Drosophila model of invasive mucormycosis [29] was used to screen the protective efficacy of posaconazole, alone or in combination with tacrolimus, following a lethal challenge with R. oryzae 557969 sporangiospores. A sterile spatula was used to prepare horizontal and vertical wells in fly media that were filled (200 μL) with a drug stock solution containing posaconazole 2 μg/mL, tacrolimus 1 μg/mL, or both drugs in combination. Test concentrations (doses) were selected on the basis of preliminary studies exhibiting 40%–60% fly survival of monotherapy regimens without toxicity. Yeast particles were then added to each trough to absorb the drug solution and were fed to Tl− flies for 12 hours following an 8-hour starvation period. Next, non-drug-treated and drug-treated Tl− flies (n = 240) were infected by pricking under CO2 anesthesia with a 10-μm needle that was dipped in an inoculum of 1 × 108 sporangiospores/mL. Serial dilutions of each inoculum suspension were plated on YAG and incubated for 18 hours at 30°C to determine the viable spore count. The flies were then returned to vials containing antifungal-spiked food that was changed daily for a total of 10 days.
Cutaneous Murine Model of Mucormycosis
Antifungal synergism may be difficult to assess in an acutely fatal model of mucormycosis. Therefore, we developed a novel nonlethal skin and soft tissue model of mucormycosis in neutropenic mice that was similar to the cutaneous aspergillosis model previously described by our group [30]. The animal model and experiments were developed in accordance with guidelines and oversight provided by veterinary staff and the animal use and care and biosafety committee at the University of Texas M.D. Anderson Cancer Center.
Eight-week-old female BALB/c mice (National Cancer Institute, Bethesda, MD) weighing 18–20 g were used in all experiments. Mice were immunosuppressed with 3 intraperitoneal injections of cyclophosphamide (100 mg/kg), 1 each on days 4 and 1 before infection and 1 on day 2 after infection, to maintain neutropenia for 5 days after animal inoculation. Additionally, a subcutaneous injection of cortisone acetate (250 mg/kg) was administered on day 1 before infection, to impair tissue macrophage clearance of sporangiospores.
Fur was shaved along the right thigh 1 day before infection. On the day of inoculation, the mice were anesthetized by inhalation of 2% isoflurane and received a subcutaneous injection of 200 μL of R. oryzae conidial suspension (2.5 × 108/mL), using a sterile 26-gauge needle, in the right thigh area. Antifungal treatment was started within 30 minutes of infection, with saline (control), posaconazole 40 mg/kg in an oral suspension by gavage once daily, tacrolimus 1 mg/kg by intraperitoneal injection once daily, and posaconazole plus tacrolimus once daily. Treatment was continued for 7 days with daily monitoring of animal health and skin lesion size until the animals were euthanized on day 7 by CO2 asphyxiation. Immediately after euthanization, thigh tissue was excised, homogenized, and analyzed for tissue fungal burden by determining tissue chitin concentrations according to methods described by Lehmann and White [28].
Because of the nonlethal nature of the infection model, no animals required euthanization for distress or moribund state before the terminal end point of the study. The posaconazole dose selected for testing was based on an established dose for aspergillosis in immunosuppressed murine models [31] and on preliminary pharmacokinetic studies in our laboratory that demonstrated that a once-daily 40 mg/kg oral dose maintained plasma posaconazole trough concentrations of >1 mg/L (data not shown).
Infection severity was assessed by daily measurement of skin lesions by using digital calipers to measure the longest and shortest diameters of the lesions. The lesion area was approximated using the following formula: lesion area = π × 0.5w × 0.5l, where w and l equal the width and length (in millimeters) of the lesion, respectively.
Histopathologic examination of thigh tissue was performed in resected tissue to assess patterns of tissue invasion and fungal burden. Briefly, necrotic thigh tissue and healthy margins were resected immediately from euthanized animals, fixed in 10% formalin, and embedded in paraffin. Tissue sections were stained with Grocott-Gomori methenamine silver to aid visualization of hyphae in tissue.
Statistical Analysis
All graphical data were expressed as mean ± standard error of the mean and compared by the Mann–Whitney U test or the Kruskal-Wallis test, with Dunn's posttest for multiple samples performed when appropriate. Survival curves were compared by the Mantel-Cox (log-rank) test. Differences were considered statistically significant when P values were < .05. The combination index was determined as described previously [25]. All analyses were performed using JMP 9 (SAS Institute, Chicago, IL) and the Calcusyn 2.0 software packages (Biosoft).
RESULTS
Posaconazole and Tacrolimus Are Synergistic In vitro Against R. oryzae
Posaconazole consistently demonstrated 4-fold higher potency against clinical isolates of R. oryzae, compared with tacrolimus, with 24-hour median MICs of 0.25 mg/L versus 1.0 mg/L for tacrolimus (Table 1). Median 48-hour FICs of posaconazole were 8-fold higher than 24-hour MICs, and 2-fold higher than corresponding 48-hour MICs. No fungicidal activity was observed in tacrolimus-exposed isolates over the range of concentrations tested. Checkerboard arrays suggested synergism between posaconazole and tacrolimus against clinical isolates of R. oryzae, with a 48-hour fractional inhibitory concentration index of 0.187–0.281.
Table 1.
In Vitro Activity of Posaconazole (POS)–Tacrolimus (TCR) Combinations Against Clinical Isolates of Rhizopus oryzae
| Isolate, Drug | MIC, μg/mL, Media n |
48-Hour MFC, μg/mL, Median (Range) | 48-Hour FIC Index | |
|---|---|---|---|---|
| 24 hours | 48 hours | |||
| R. oryzae 557969 | ||||
| POS | 0.25 | 1.0 | 2.0 (0.5–4) | 0.187 |
| POS-TCR | … | 0.0625 | … | … |
| TCR | 1.0 | 1.0 | >2 | … |
| TCR-POS | … | 0.125 | … | … |
| R. oryzae 544946 | ||||
| POS | 0.25 | 1.0 | 2.0 (0.5–4) | 0.187 |
| POS-TCR | … | 0.0625 | … | … |
| TCR | 1.0 | 1.0 | >2 | … |
| TCR-POS | … | 0.125 | … | … |
| R. oryzae 570983 | ||||
| POS | 0.25 | 1.0 | 2.0 (0.5–4) | 0.187 |
| POS-TCR | … | 0.0625 | … | … |
| TCR | 1.0 | 1.0 | >2 | … |
| TCR-POS | … | 0.125 | … | … |
| R. oryzae 529120 | ||||
| POS | 0.25 | 1.0 | 2.0 (0.5–4) | 0.281 |
| POS-TCR | … | 0.0315 | … | … |
| TCR | 1.0 | 1.0 | >2 | … |
| TCR-POS | … | 0.25 | … | … |
Abbreviations: MFC, median fungicidal concentration; MIC, minimum inhibitory concentration.
a The fractional inhibitory concentration (FIC) index was calculated as (MIC of POS alone / MIC of POS + TCR combination) + (MIC of TCR alone/MIC of TCR + POS combination). An index of < 0.5 is indicative of synergy.
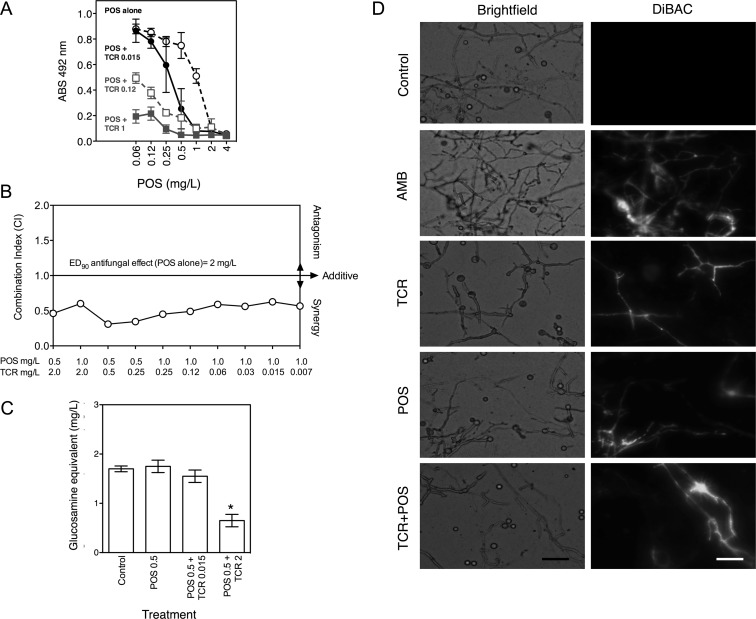
Tacrolimus enhanced the antifungal potency of posaconazole against R. oryzae hyphae, as determined by the checkerboard XTT reduction assay (Figure 1A and 1B). Specifically, when tested alone, posaconazole reduced R. oryzae hyphal viability by ≥90% at 2 mg/L. A similar magnitude of effect was achieved with 0.5 μg/mL of posaconazole in the presence of 1 μg/mL of tacrolimus (Figure 1A). Notably, a concentration of tacrolimus typically encountered in transplant recipients (0.015 mg/L) significantly enhanced the activity of posaconazole concentrations (0.5–1 mg/L) reported in patients receiving oral posaconazole therapy (Figure 1A) [32].
Figure 1.
Posaconazole (POS) and tacrolimus (TCR) display synergistic interactions in vitro against Rhizopus oryzae. A, Fixed concentrations of TCR enhance POS inhibition of R. oryzae hyphal viability, as assessed by the XTT reduction assay at 24 hours. Data points represent the mean ± SD of hyphal viability data from 4 experiments (indexed to drug-free control) for increasing concentrations of POS alone, POS + 0.015 μg/mL TCR, POS + 0.12 μg/mL TCR, and POS + 1 μg/mL TCR. B, POS and TCR exposures display synergistic interactions at concentrations below the 90% effective concentration (EC90) of POS monotherapy. Combination indexes were calculated from XTT checkerboard arrays at 24 hours, using the multiple drug effect equations of Chou and Talalay [25, 26]. C, TCR (0.015 and 2 mg/L) enhances the activity of a subinhibitory POS concentration (0.5 mg/L) to reduce R. oryzae biomass, as determined by measurement of total cell chitin level. Data are presented as mean ± SD of the glucosamine equivalent standard concentration of chitin analyzed in the assay from experiments performed in 4 replicates. *P < .05 vs control, by the Kruskal-Wallis test with Dunn's test. D, TCR 2 mg/L enhances POS 2 mg/L hyphal damage, as visualized using DiBAC fluorescent viability dye. Representative micrographs were obtained 24 hours after drug exposure. Amphotericin B (AMB) 2 μg/mL is a positive control. Increasing fluorescence in DiBAC panels is indicative of membrane damage and loss of cellular integrity. Images are at original magnification ×200. Scale bar = 15 μm. Abbreviation: OD, optical density.
Derivation of the combination index for fractional drug exposures tested in the checkerboard array confirmed consistent synergism between sub-EC90 posaconazole concentrations (0.5–1 mg/L) over a wide range of tacrolimus concentrations (0.007–2 mg/L) against R. oryzae (Figure 1B). The EC90 combination index for posaconazole-tacrolimus concentrations against R. oryzae 557969 was 0.31–0.62, with a mean combination index of 0.5, confirming that an EC90 effect observed with posaconazole 2 mg/L could be maintained at ≥2-fold lower posaconazole concentrations (0.5–1 mg/L) when administered with 0.007–2 mg/L of tacrolimus against R. oryzae 557969. This synergy was also confirmed through analysis of total cellular chitin concentrations, which were lowest with the combination regimen versus either drug alone (Figure 1C).
Analysis of synergistic interactions at or just above the EC90 or MFC of posaconazole and tacrolimus are limited with standard XTT assay procedures, which detect impaired metabolic activity but not necessarily lethal effects. By using the DiBAC mortal cell injury dye, we qualitatively observed increased hyphal damage when R. oryzae was exposed to 2 mg/L of posaconazole and 2 mg/L of tacrolimus in combination (Figure 1D), suggesting that synergistic lethality may persist at or above the in vitro EC90 threshold of posaconazole.
Tacrolimus Enhances the In Vivo Activity of Posaconazole in Experimental Mucormycosis
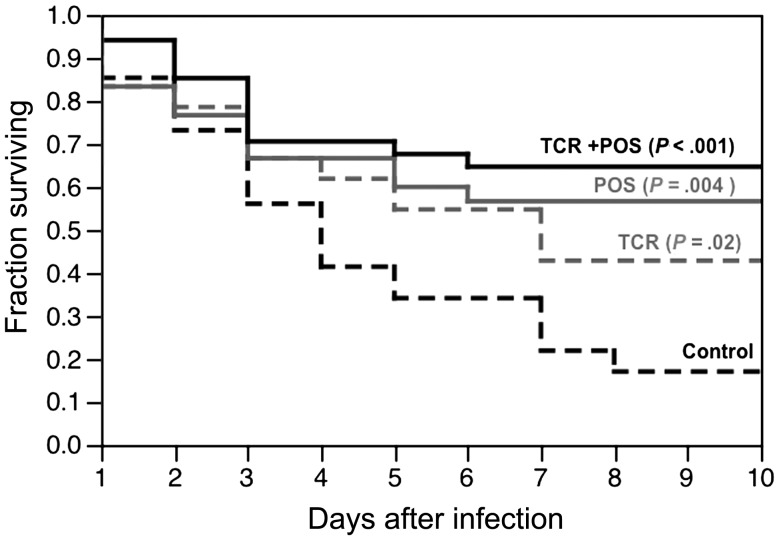
Monotherapy with posaconazole or tacrolimus significantly prolonged the median survival time of Tl− deficient flies challenged with a lethal inoculum of R. oryzae, compared with untreated control flies (Figure 2). The highest survival rate at 10 days (65%) was observed in infected flies that were fed the combination of posaconazole and tacrolimus (P < .001 vs control) followed by posaconazole alone (57%; P = .004 vs control) and tacrolimus alone (43%; P = .02 vs control). Mortality curves were not statistically different, however, when the combination group was compared to the groups that received posaconazole alone (P = .47) or tacrolimus alone (P = .08).
Figure 2.
Combination posaconazole (POS) and tacrolimus (TCR) improves survival of Tl-deficient Drosophila challenged with a lethal inoculum of Rhizopus oryzae. Each treatment group contained 30–40 flies. Median survival time was 4 days in controls, 7 days in flies fed TCR alone, and > 10 days in flies fed POS alone, or POS plus TCR. P values represent comparison versus controls using the Mantel–Cox (log-rank) test.
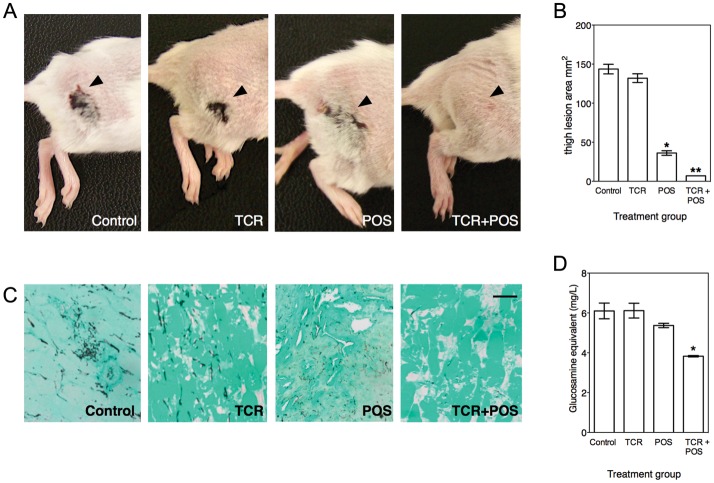
In the neutropenic murine cutaneous model of mucormycosis, inoculation with R. oryzae 557969 resulted in necrotic skin lesions at the site of inoculation within 48 hours. Lesions were typically erythematous macules with peripheral enhancement that frequently developed central eschars (Figure 3A). Skin lesions progressed over 7 days of follow-up and were not associated with animal death. Mice generally lost up to 10% of their body weight during the initial 4 days of immunosuppression, but body weight subsequently stabilized or increased during the remainder of the experiment. No significant differences in animal weights were observed between the control group and mice that received tacrolimus or posaconazole, either alone or in combination.
Figure 3.
Combinations of posaconazole (POS) and tacrolimus (TCR) display enhanced activity over either drug alone in an immunosuppressed murine model of cutaneous mucormycosis (n = 10 mice per treatment group). Necrotic skin lesions were significantly reduced, as revealed by gross examination (A) and digital caliper measurement (B) in animals that received the POS 40 mg/kg plus TCR 1 mg/kg combination regimen daily versus controls or animals that received either drug alone. *P = .001 vs control; **P = .02 vs POS alone. C, Smaller lesion area was associated with reduced hyphal invasion of subcutaneous tissue and thigh muscle, by histologic analysis. Representative images of tissue collected from necrotic lesions and stained by Grocott–Gomori methenamine silver before imaging at an original magnification of 200×. Scale bar = 20 μm. D, Combination therapy resulted in a significantly lower tissue fungal burden, as reflected by analysis of chitin concentrations in tissue. Data are presented as mean values ± SD of glucosamine equivalents. *P < .05, by the Kruskal–Wallis test with Dunn's test.
Treatment of infected animals with oral posaconazole 40 mg/kg significantly reduced the severity (Figure 3A) and area (Figure 3B) of thigh lesions. The reduction in the thigh lesion severity correlated with reduced hyphal invasion of subcutaneous tissue and muscle, as revealed by histologic analysis (Figure 3C), and with modest reduction in tissue chitin concentrations (Figure 3D). Tacrolimus 1 mg/kg day intraperitoneally alone was minimally effective at reducing the severity and size of skin lesions. Thigh tissue of tacrolimus-treated mice showed extensive hyphal invasion in subcutaneous tissue and muscle, and tissue chitin concentrations were similar to those of untreated controls.
In contrast to animals that received monotherapy regimens, treatment with a combined regimen of posaconazole and tacrolimus resulted in near complete prevention or resolution of erythematous or necrotic macules (Figure 3A), significantly reduced lesion area as compared to posaconazole alone (Figure 3B), minimal hyphal invasion and destruction of subcutaneous tissue (Figure 3C), and significant reductions in tissue chitin concentrations (Figure 3D).
DISCUSSION
The calcineurin pathway regulated by the molecular chaperone Hsp90 has been suggested to be the “Achilles heel” of pathogenic fungi, because of its central role in a plethora of cell processes, including morphogenetic transition and the development/maintenance of antifungal tolerance and resistance [33–35]. Consequently, selective inhibition of Hsp90 or the calcineurin pathway in pathogenic fungi could have broad therapeutic potential, especially if an inhibitor of this pathway is used in combination with classical antifungal agents that target the cell membrane or cell wall [34, 36].
Mucorales such as R. oryzae possess an ergosterol biosynthesis pathway that is genetically similar to that of Aspergillus species [12], supporting the concept that polyene and triazole antifungal agents that target this pathway should be therapeutically effective for both aspergillosis and mucormycosis. However, nearly 50% of the genes involved in ergosterol biosynthesis in R. oryzae, including 14α-demethylase (ERG11), the principal target of triazoles such as posaconazole, are present in multiple copies with diverged or duplicated protein sequences [12]. This redundancy and variability in the target of triazole antifungals may contribute to inherently lower potency and diminished fungicidal effects of triazoles against Mucorales, relative to Aspergillus [13]. In patients, the problems of reduced potency are compounded by pharmacokinetic variability inherent to broad-spectrum triazoles such as posaconazole, where nearly one-third of patients receiving the current oral formulation have low or even undetectable (<0.5 mg/L) plasma concentrations [37, 38].
Given this background, our demonstration of synergy between a calcineurin inhibitor and posaconazole against R. oryzae raises a number of intriguing questions. A first question is whether this synergy is clinically relevant or expected to occur with clinically used doses of tacrolimus and posaconazole. Although we did not specifically analyze drug concentrations of either agent in our Drosophila or cutaneous models of mucormycosis, the doses of tacrolimus and posaconazole selected for testing in mice have been previously reported to achieve plasma drug exposures consistent with dosing regimens in humans [31, 39, 40]. Similarly, our in vitro analysis suggests synergistic interactions between posaconazole and tacrolimus over a wide range of achievable concentrations in humans, with a 2-fold enhancement of posaconazole potency at whole-blood concentrations of tacrolimus typically encountered in patients (0.007–0.015 mg/mL) (Figure 1A) and at plasma concentrations of posaconazole that have been reported to be borderline therapeutic for aspergillosis (0.5–1 mg/L) [32, 38]. This finding is also consistent with data from a case series involving solid-organ transplant recipients that reported improved response to antifungal therapy for Cryptococcus when patients were receiving calcineurin inhibitors [41]. However, our data do not rule out the possibility that the enhanced activity of the combination could still be affected by an unexpected pharmacokinetic drug-drug interactions in vivo (ie, posaconazole increasing the concentrations of tacrolimus, or vice versa).
A second question is whether synergism between posaconazole and tacrolimus could be consistently observed across a wide range of infections, underlying immunosuppression, or for different Mucorales. Although we observed evidence of enhanced posaconazole potency in vivo in 2 phylogenetically different backgrounds, it would be important to formally test the activity of tacrolimus-posaconazole combinations in neutropenic and corticosteroid-immunosuppressed pulmonary infection models, as well as in a systemic model of infection in diabetic ketoacidotic mice, as described by Ibrahim et al [42]. We found evidence of posaconazole-tacrolimus synergism in vitro in 4 clinical isolates of R. oryzae screened from our institution, but others have reported that synergy is not uniformly observed in vitro for all Mucorales species or isolates [14, 15]. Cunninghamella bertholletiae, in particular, should be the focus of future studies, given the higher mortality rates and the high degree of resistance that have been reported with this species [3, 43].
A final question is whether the narrow therapeutic index of current calcineurin inhibitors really supports their consideration as antifungal agents. Although some toxic and immunosuppressive effects could certainly be reduced with limited treatment durations and therapeutic drug monitoring, the clinical use of a calcineurin-triazole combination for mucormycosis ultimately will require a novel agent that selectively targets fungal calcineurin pathways without collateral effects in human cells [34].
In conclusion, a combination of 2 common oral agents used in the transplant patients, tacrolimus and posaconazole, displayed synergistic interactions in vitro against clinical isolates of R. oryzae and evidence of enhanced potency in 2 in vivo models of mucormycosis. Our findings suggest this strategy may have therapeutic benefits in humans.
Notes
Acknowledgments. D. P. K. acknowledges the Frances King Black Endowed Professorship for Cancer Research.
Financial Support. This work was supported by the National Institutes of Health, through the M. D. Anderson Cancer Center (grant CA016672).
Potential conflicts of interest. D. P. K. has received research support and honoraria from Pfizer, Astellas Pharma US, and Merck. R. B.-A. has received consulting fees from Pfizer. R. E. L. has received research support from Merck and serves on the advisory boards for Merck and Gilead. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Bitar D. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15:1395–1401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–50. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pongas GN, Lewis RE, Samonis G, Kontoyiannis DP. Voriconazole-associated zygomycosis: a significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin Microbiol Infect. 2009;15:93–7. doi: 10.1111/j.1469-0691.2009.02988.x. [DOI] [PubMed] [Google Scholar]
- 6.Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J, Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48:1743–51. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond SP, Baden LR, Marty FM. Mortality in hematologic malignancy and hematopoietic stem cell transplant patients with mucormycosis, 2001 to 2009. Antimicrob Agents Chemother. 2011;55:5018–21. doi: 10.1128/AAC.00536-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahan BD. Timeline: Individuality: the barrier to optimal immunosuppression. Nat Rev Immunol. 2003;3:831–8. doi: 10.1038/nri1204. [DOI] [PubMed] [Google Scholar]
- 9.Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5:e1000471. doi: 10.1371/journal.ppat.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbach WJ, Cramer RA, Perfect BZ, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryotic Cell. 2006;5:1091–103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reedy JL, Filler SG, Heitman J. Elucidating the Candida albicans calcineurin signaling cascade controlling stress response and virulence. Fungal Genet Biol. 2010;47:107–16. doi: 10.1016/j.fgb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L-J, Ibrahim AS, Skory C, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis RE, Lortholary O, Spellberg B, Roilides E, Kontoyiannis DP, Walsh TJ. How does antifungal pharmacology differ for mucormycosis versus aspergillosis? Clin Infect Dis. 2012;54(Suppl 1):S67–72. doi: 10.1093/cid/cir884. [DOI] [PubMed] [Google Scholar]
- 14.Dannaoui E, Afeltra J, Meis JFGM, Verweij PE Eurofung Network. In vitro susceptibilities of zygomycetes to combinations of antimicrobial agents. Antimicrob Agents Chemother. 2002;46:2708–11. doi: 10.1128/AAC.46.8.2708-2711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narreddy S, Manavathu E, Chandrasekar PH, Alangaden GJ, Revankar SG. In vitro interaction of posaconazole with calcineurin inhibitors and sirolimus against zygomycetes. J Antimicrob Chemother. 2010;65:701–3. doi: 10.1093/jac/dkq020. [DOI] [PubMed] [Google Scholar]
- 16.Kontoyiannis DP, Lewis RE, Osherov N, Albert ND, May GS. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J Antimicrob Chemother. 2003;51:313–6. doi: 10.1093/jac/dkg090. [DOI] [PubMed] [Google Scholar]
- 17.Bastidas RJ, Shertz CA, Lee SC, Heitman J, Cardenas ME. Rapamycin exerts antifungal activity in vitro and in vivo against Mucor circinelloides via FKBP12-dependent inhibition of Tor. Eukaryot Cell. 2012;11:270–81. doi: 10.1128/EC.05284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SC-A, Lewis RE, Kontoyiannis DP. Direct effects of non-antifungal agents used in cancer chemotherapy and organ transplantation on the development and virulence of Candida and Aspergillus species. Virulence. 2011;2:280–95. doi: 10.4161/viru.2.4.16764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 20.Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis. 2009;199:1399–1406. doi: 10.1086/597615. [DOI] [PubMed] [Google Scholar]
- 21.CLSI. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 2008. pp. 1–52. Approved Standard. 2nd ed. Document M38-A2 Wayne, PA: CLSI. [Google Scholar]
- 22.Espinel-Ingroff A, Fothergill A, Peter J, Rinaldi MG, Walsh TJ. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J Clin Microbiol. 2002;40:3204–8. doi: 10.1128/JCM.40.9.3204-3208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinel-Ingroff A, Chaturvedi V, Fothergill A, Rinaldi MG. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J Clin Microbiol. 2002;40:3776–81. doi: 10.1128/JCM.40.10.3776-3781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamilos G, Lewis RE, Kontoyiannis DP. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob Agents Chemother. 2006;50:96–103. doi: 10.1128/AAC.50.1.96-103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 26.Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 27.Bowman JC, Hicks PS, Kurtz MB, et al. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother. 2002;46:3001–12. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann PF, White LO. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect Immun. 1975;12:987–92. doi: 10.1128/iai.12.5.987-992.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamilos G, Lionakis MS, Lewis RE, et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193:1014–22. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ami R, Lewis RE, Leventakos K, Latgé J-P, Kontoyiannis DP. Cutaneous model of invasive aspergillosis. Antimicrob Agents Chemother. 2010;54:1848–54. doi: 10.1128/AAC.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard SJ, Lestner JM, Sharp A, et al. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J Infect Dis. 2011;203:1324–32. doi: 10.1093/infdis/jir023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2008;53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heitman J. Cell biology: a fungal Achilles’ heel. Science. 2005;309:2175–6. doi: 10.1126/science.1119321. [DOI] [PubMed] [Google Scholar]
- 34.Cowen LE, Singh SD, Kohler JR, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A. 2009;106:2818–23. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell. 2008;7:747–64. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaFayette SL, Collins C, Zaas AK, et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 2010;6:e1001069. doi: 10.1371/journal.ppat.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53:958–66. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang SH, Colangelo PM, Gobburu JVS. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther. 2010;88:115–9. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 39.High KP, Washburn RG. Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin) J Infect Dis. 1997;175:222–5. doi: 10.1093/infdis/175.1.222. [DOI] [PubMed] [Google Scholar]
- 40.Moffatt SD, McAlister V, Calne RY, Metcalfe SM. Comparative efficacy of liposomal FK 506 with FK 506 (tacrolimus) with and without anti-CD4/CD8 monoclonal antibodies. Transplant Proc. 1999;31:2754. doi: 10.1016/s0041-1345(99)00553-9. [DOI] [PubMed] [Google Scholar]
- 41.Singh N, Alexander BD, Lortholary O, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756–64. doi: 10.1086/511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibrahim AS, Gebremariam T, Fu Y, Edwards JE, Spellberg B. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob Agents Chemother. 2008;52:1556–8. doi: 10.1128/AAC.01458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almyroudis NG, Sutton DA, Fothergill AW, Rinaldi MG, Kusne S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob Agents Chemother. 2007;51:2587–90. doi: 10.1128/AAC.00452-07. [DOI] [PMC free article] [PubMed] [Google Scholar]