Abstract
Objective
Chronic obstructive pulmonary disease (COPD) represents a burden on patients and health systems. Roflumilast, an oral, selective phosphodiesterase-4-inhibitor reduces exacerbations and improves lung function in severe/very severe COPD patients with a history of exacerbations. This study aimed to estimate the lifetime cost and outcomes of roflumilast added-on to commonly used COPD regimens in Switzerland.
Methods
A Markov cohort model was developed to simulate COPD progression in patients with disease states of severe, very severe COPD, and death. The exacerbation rate was assumed to be two per year in severe COPD. COPD progression rates were drawn from the published literature. Efficacy was expressed as relative ratios of exacerbation rates associated with roflumilast, derived from a mixed-treatment comparison. A cost-effectiveness analysis was conducted for roflumilast added to long-acting muscarinic antagonists (LAMA), long-acting β2-agonist/ inhaled corticosteroids (LABA/ICS), and LAMA + LABA/ICS. The analysis was conducted from the Swiss payer perspective, with costs and outcomes discounted at 2.5% annually. Parameter uncertainties were explored in one-way and probabilistic sensitivity analyses.
Results
In each of the comparator regimens mean life expectancy was 9.28 years and quality-adjusted life years (QALYs) gained were 6.19. Mean estimated lifetime costs per patient in the comparator arms were CHF 83,364 (LAMA), CHF 88,161 (LABA/ICS), and CHF 95,564 (LAMA + LABA/ICS) respectively. Adding roflumilast resulted in a mean cost per patient per lifetime of CHF 86,754 (LAMA + roflumilast), CHF 91,470 (LABA/ICS + roflumilast), and CHF 99,364 (LAMA + LABA/ICS + roflumilast), respectively. Life-expectancy and quality-adjusted life-expectancy were 9.63 years and 6.47 QALYs (LAMA + roflumilast), 9.64 years and 6.48 QALYs (LABA/ICS + roflumilast), and 9.63 years and 6.47 QALYs (LAMA + LABA/ ICS + roflumilast). Incremental cost-effectiveness ratios were CHF 12,313, CHF 11,456, and CHF 13,671 per QALY when roflumilast was added to the three regimens.
Conclusion
Treatment with roflumilast is estimated to reduce the health and economic burden of COPD exacerbations and represent a cost-effective treatment option for patients with frequent exacerbations in Switzerland.
Keywords: COPD, treatment, exacerbations, economic, cost-effectiveness, modeling
Background and objective
Chronic obstructive pulmonary disease (COPD)
COPD is a chronic disease characterized by airflow limitation that is only partially reversible and progresses over time.1 It is a major cause of disability and death worldwide. An international study, The Burden of Obstructive Lung Disease Program (BOLD) showed “higher levels and more advanced staging of spirometrically confrmed COPD than have typically been reported.”2 The same study also showed a fairly high prevalence of stage II or more COPD in individuals who have never smoked.
The overall prevalence of COPD for Global Initiative for Chronic Obstructive Lung Disease (GOLD)3 stage II or higher reported in this study was 10.1% in adults aged 40 years and older. Symptoms including activity limitation and exacerbations place a heavy burden on patients and impair their quality of life. Spirometry is currently used as the main method to confrm the diagnosis of COPD and to ascertain its severity by measuring post bronchodilator forced expiratory volume in 1 second (FEV1). COPD exacerbations represent a major concern as they are linked with disease severity as well as disease progression and therefore represent a substantial burden on health care systems.4 The objective of COPD management, therefore, is to reduce the frequency of exacerbations and to limit disease progression. The importance of not only treating symptoms but of specifically reducing the risk of exacerbations has been recently emphasized in the 2011 update of the GOLD document, Global Strategy for the Diagnosis, Management, and Prevention of COPD.3
Treatment regimens
Pharmacological management of COPD includes a stepwise escalation in treatment according to disease severity and symptoms. In Switzerland common therapy regimens include long-acting muscarinic antagonists (LAMA), and long-acting β2-agonist/inhaled corticosteroids (LABA/ICS) and LAMA + LABA/ICS administered concomitantly. None of the treatments for COPD have been shown conclusively to slow (or reverse) disease progression (ie, improve the long-term decline in lung function). Also until now, no therapy has been shown to treat the underlying inflammation found in COPD.
Roflumilast
Roflumilast is an oral, once-daily selective phosphodi-esterase-4 inhibitor with a broad range of action against inflammatory cells playing a major role in COPD. It was approved for Switzerland in November 2011 for use as concomitant maintenance treatment of severe COPD as a supplementary therapy to bronchodilators in patients with frequent exacerbations in the past.
As demonstrated in clinical trials,5,6 roflumilast reduced the rate of exacerbations in patients with severe airflow obstruction and a history of frequent exacerbations, whose COPD was associated with chronic bronchitis. Daxas® ([roflumilast] Takeda Pharma AG, Pfäffikon, Switzerland) was included in the Swiss positive list for reimbursement in February 2012.7 The objective of this analysis is to estimate the lifetime cost and outcomes and the cost-effectiveness of roflumilast as a supplementary therapy to LAMA, LABA/ ICS, and LAMA + LABA/ICS from a health care payer perspective in Switzerland.
Materials and methods
Economic model
A state-transition, cohort-based Markov model was constructed to estimate the cost and health outcomes in a cohort of patients with COPD. The key features of the model were similar to that reported in a study published earlier8 and included: (1) a lifetime simulation of the progression of COPD based on FEV1; (2) simulation of direct health care costs and health outcomes; (3) treatment effect represented by a reduction of exacerbations; and (4) modeling the impact of moderate (community-treated) and severe (hospital-treated) exacerbations on patients’ health-related quality of life, and the consequent reduction of in-hospital mortality due to severe exacerbations. In addition, the model has the ability to simulate lung function improvement, however in the base case no impact of treatment on lung function was assumed.
The structure of the Markov cohort model included three states: severe COPD (FEV1 30%–49%), very severe COPD (FEV1 30%) according to the classification of COPD severity by GOLD criteria,3 and death (Figure 1). In a recent revision of the GOLD guidelines, frequent exacerbations were added as a determinant of severity of disease. In this analysis, the rate of exacerbations in the comparator patient groups was assumed to be two exacerbations annually, reflecting the definition in the revised GOLD guidelines.
Figure 1.
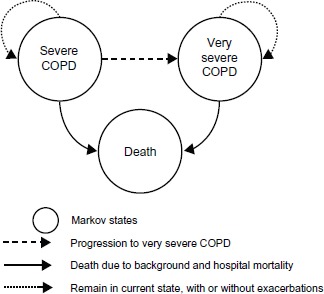
COPD cost-effectiveness model structure.
Abbreviation: COPD, chronic obstructive pulmonary disease.
The framework of the analysis and the economic model are based on the best practice accepted in the COPD therapy areas, and results of the economic analysis are also in line with the economic evaluation for roflumilast reported earlier.8
Patient cohort
The modeled cohort was characterized by a mean age at the start of the simulation, COPD severity (mean post-bronchodilator FEV1), proportion of males, patient height, and the combined rates of moderate and severe exacerbations in patients on comparator regimens (Table 1). Proportions of severe exacerbations in patients with severe and very severe COPD were based on the LABA subgroup in studies M2-124 and M2-125;5,6 the hospital case fatality rate due to severe exacerbations was drawn from a UK COPD audit report,9 and was assumed to be similar in Switzerland.
Table 1.
Demographic characteristic and outcomes in COPD cohort
| Average age at start, yearsa | 64 |
| Male/female ratioa | 0.75/0.25 |
| Height (males), cma | 171.3 |
| Height (females), cma | 161.8 |
| COPD severity at start | 100% severe (assumption) |
| Average FEV1% at startb | 40 |
| The annual rate of background exacerbations in the severe COPD state in any of the comparatorsc | 2.0 |
| The annual rate of background exacerbations | 2.436 = 2.0 × |
| in the very severe COPD stated | (1.776/1.458) |
| Proportion of hospital-treated exacerbations | 0.155 |
| among all exacerbations in severe COPDa | (95% C1 0.133–0.177) |
| Proportion of hospital-treated exacerbations | 0.244 |
| among all exacerbations in very severe COPDa | (95% C1 0.208–0.280) |
| SMR for background death in severe COPD13 | 1.5 |
| SMR for background death in very severe COPD13 | 2.0 |
| Hospital CFR, %9 | 7.7 |
Notes: aPooled analysis from studies M2-124 and M2-125 for the LABA subgroup;6
median of the FEV1% interval for severe COPD;
an assumption, to indicate the group of patients with a history of frequent exacerbations who continue to exacerbate in clinical practice (95% CI from studies M2-124 and M2-125 and adjusted for the rate of exacerbations of 2.0 used in the model);
derived from the annual background rate of exacerbation in the severe COPD state of 2.0 and the ratio of the rates of exacerbations in the very severe COPD and the severe COPD states from studies M2-124 and M2-125.
Abbreviations: COPD, chronic obstructive pulmonary disease; CI, confidence interval; FEV1, post-bronchodilator forced expiratory volume achieved in 1 second, as a percentage predicted of that for the general population; SMR, standardized mortality ratio; CFR, case fatality rate (the percent of patients who died during a hospital-treated exacerbation); LABA, long-acting β2-agonist.
COPD progression
Progression of patients with severe COPD to very severe COPD depends on the starting FEV1 value and the estimated annual lung volume decline in COPD patients. FEV1 naturally declines with age in the general healthy population,10 but it declines more rapidly in patients with COPD (estimated at 52 mL a year).11 Predictive equations for lung function for males and females in a general healthy population were taken from Crapo et al.10 This allowed the estimation of the average time, T, required for lung function in the COPD cohort to reach the FEV1 threshold for very severe COPD (30% of post-bronchodilator FEV1 in a healthy population, according to the GOLD classification).4 Subsequently, the monthly probability of progression from severe to very severe COPD was estimated as the reciprocal of the average time to progression, 1/T.
Exacerbations
Exacerbations in the model are either community-treated (moderate) or hospital-treated (severe); each is associated with costs and health consequences estimated as quality-adjusted life years (QALYs) lost per exacerbation event. The annual rates of exacerbations in each of the comparator treatments (ie, LAMA, LABA/ICS, and LAMA + LABA/ICS) were assumed to be equal to two in the severe COPD state representing patients with frequent exacerbations. Exacerbation rates in very severe COPD were estimated using the ratio of exacerbation frequency in patients with very severe COPD to that of patients with severe COPD obtained from a pooled analysis of data from the M2-124 and M2-125 studies (ie, 1.776/1.458 annually).
Therefore, using an assumption of two exacerbations per year per patient in the severe COPD state, the estimated rate of exacerbations in the comparator arms in the very severe COPD state would be 2.44 per year per patient.
A fixed proportion of all exacerbations in patients with severe and very severe COPD are hospital-treated, and the proportions applied in the model are drawn from the pooled analysis of the LABA subgroup in M2-124 and M2-125: 0.16 [95% confidence interval (CI): 0.133–0.178] in the severe COPD state, and 0.24 (95% CI: 0.208–0.280) in the very severe COPD state (Table 1).
Efficacy of roflumilast
The treatment effect of roflumilast in the analysis is determined using the relative ratios of exacerbation rates (RRRs). The RRRs for treatment regimens with roflumilast as an add-on, presented in Table 2, were calculated based on the results of a mixed treatment comparison (MTC) study,12 and used in a similar manner to a recently-published analysis that conducted a fully incremental economic analysis comparing various treatment regimens used in the management of COPD in the UK.8 Efficacy in terms of lung function improvement was not reported in the MTC, as the primary outcome of the study was event rates of exacerbations.
Table 2.
Treatment efficacy – reduction of exacerbations
| Treatment | Reference regimen | RRRa | 95% CIa |
|---|---|---|---|
| LAMA + roflumilast | LAMA | 0.843 | 0.61–1.14 |
| LABA/ICS + roflumilast | LABA/ICS | 0.835 | 0.74–0.95b |
| LAMA + LABA/ICS + | LAMA + LABA/ | 0.841 | 0.74–0.95b |
| roflumilast | ICS |
Notes:aValues are based on Mills et al;12
reduction of exacerbation rates statistically significant.
Abbreviations: RRR, relative ratios of exacerbation rates; 95% CI, 95% confidence interval; LAMA, long-acting muscarinic antagonists; LABA, long-acting beta-agonists; ICS, inhaled corticosteroids.
Mortality
Mortality in the modeled COPD cohort occurs both as a result of case fatality during a hospital-treated exacerbation and as background mortality.
Hospital mortality
The hospital case fatality rate due to a hospital-treated exacerbation was taken from the 2008 UK National COPD Audit9 where hospital mortality was reported at 7.7% for a sample of 9716 admissions for exacerbations.
Background mortality and standardized mortality ratios (SMRs)
The background mortality is expressed via the age- and sex-specific risk of death in the general population and the SMR for background mortality that excludes hospital death, in the severe COPD and very severe COPD states. The background mortality and the hospital mortality combined represent the true all-cause mortality in severe and very severe COPD. The SMRs for COPD populations are not well known, and in the model the SMRs for severe and very severe COPD are assumed to be 1.5 and 2.0 respectively.13 As the SMRs are uncertain parameters, their impact on the cost-effectiveness of roflumilast was tested in a sensitivity analysis.
Health-related utilities
Two types of health-related utility measurement are employed in the model (Table 3). The severe COPD and very severe COPD states are associated with the health-related utility values of 0.751 (95% CI:0.738–0.765) and 0.657 (95% CI:0.635–0.678), respectively.5 The loss of utility due to hospital-treated exacerbations and community-treated exacerbations translates into a loss in QALYs. The loss of QALYs associated with an exacerbation was estimated based on the absolute reduction of utility of 0.01 (standard error [SE] = 0.007) and 0.042 (SE = 0.009) over a 1-year period for a community-treated exacerbation and a hospital-treated exacerbation respectively, as reported in Rutten-van Mölken et al.14 In the model these values were applied to accrue the loss of QALYs within one model cycle when an exacerbation occurs, ie, monthly health utility reductions of 0.12 and 0.50 for moderate and severe exacerbations, respectively (see notes to Table 3).
Table 3.
Health-related utilities and utility decrements
| Parameter | Value | 95% CI/SE |
|---|---|---|
| Utility in severe COPDa | 0.751 | 0.738–0.765 |
| Utility in very severe COPDa | 0.657 | 0.635–0.678 |
| Utility decrement (annual) associated | 0.010 | SE = 0.007 |
| with community-treated exacerbationb | ||
| Utility decrement (annual) associated | 0.042 | SE = 0.009 |
| with hospital-treated exacerbationsb |
Notes:aData on file, Takeda Pharma AG, Pfäffikon, Switzerland, 2010;5
utility decrements are based on Rutten-van Mölken et al14 and the values represent utility loss over 1 year; the loss of utility attributed to 1-month model cycle length is estimated as: 0.01 × 12 = 0.12 and 0.042 × 12 = 0.504.
Abbreviations: 95% CI, 95% confidence interval; COPD, chronic obstructive pulmonary disease; SE, standard error.
Adverse events
The key roflumilast studies M2-124 and M2-1255,6 suggest that the majority of adverse events (AEs) in patients treated with roflumilast were mild-to-moderate, transient, occurred within the first month from the beginning of treatment, and would not have substantial impact on cost-effectiveness. For the treatment combinations used in this economic model there is no comparable data on AEs and no consideration of AEs were made.
Resource use and costs
Costs were estimated from a payer perspective in Switzerland. Total costs in the analysis included the on-going maintenance cost in severe and very severe COPD, the cost of COPD regimens, and the resource use estimates and cost of treatment of moderate and severe exacerbations, as shown in Table 4.
Table 4.
Key model inputs: resource use and unit costs
| Resource type | Cost, CHF | Proportion of patients, or frequency of use | Subtotal/total, CHFe |
|---|---|---|---|
| Hospital-treated exacerbation | |||
| Intensive care unit | 19,496a | 11%a | 2164 |
| Normal ward | 13,848a | 78%a | 10,774 |
| Ambulance transport | 804a | 25%a | 201 |
| Cost per hospital-treated exacerbation | 13,139 | ||
| Community-treated (moderate) exacerbation | |||
| GP visit (includes treatment) | 121.34b | 2/3 (67%)c | 80.90 |
| Accident and emergency (not leading to admission) | 27.41b | 1/3 (33%)c | 9.14 |
| Prednisolone 30 mg, 7-day course | 0.88c | 50%c | 3.08 |
| Prednisolone 30 mg, 14-day course | 0.88c | 50%c | 6.16 |
| Co-amoxicillin, 3 per day, 5-day course | 1.90 × 3 × 5c | 50%c | 14.27 |
| Co-amoxicillin, 3 per day, 10-day course | 1.90 × 3 × 10c | 50%c | 28.54 |
| Cost per community-treated exacerbation | 142.08 | ||
| Maintenance in severe COPD, monthly | |||
| Outpatient visit to respiratory physician | 18.10b | Twice a yearc | 3.02 |
| Spirometry | 97.40b | Twice a yearc | 16.23 |
| Influenza vaccination, annual | 17.95c | 75%c | 1.12 |
| Oxygen therapy | 35.49c | 14.6 per yearc | 43.8 |
| Maintenance in severe COPD, monthly | 63.55 | ||
| Maintenance in very severe COPD, monthly | |||
| Outpatient visit to respiratory physician | 18.10b | Four times a yearc | 6.03 |
| Spirometry | 97.40b | Four times a yearc | 32.47 |
| Influenza vaccination, annual | 17.95c | 75%c | 1.12 |
| Oxygen therapy | 35.49c | 73 a yearc | 215.90 |
| Maintenance in very severe COPD, monthly | 255.52 | ||
| COPD treatments | (CHF, monthly) | ||
| Roflumilast (Daxas®) 500 mcg, 30 tabs, | 78.55 per packc | 1 tab daily | 79.64 |
| LAMA, Tiotropium, Spiriva® Inhal Kaps 18 mcg 90 | 196.75 per packc | 18 mcg daily | 66.46 |
| LABA/ICS, budesonide + formoterol, Symbicort® 400/12, Turbuhaler 60 puffs | 93.5 per packc | 2 puffs daily | 94.80 |
| LABA/ICS, fluticasone + salmeterol, Seretide® 500 Diskus 60 doses | 130.2 per packc | 2 puffs daily | 132.01 |
| LABA/ICS, weighted average by market share | 109.59 | ||
| LAMA+LABA/ICS | (CHF 66.46 + CHF 132. | 01)c | 176.09 |
Notes:aCost values were drawn from Schramm et al18 and inflated to 2011 values;
cost values were drawn from TARMED Suisse, version 02.04.2010:01.07.01 (July 2012);21
resource use data were drawn from Oostenbrink et al;19
costs of pharmaceuticals obtained from the Medicines Compendium of Switzerland (July 2012);20
subtotals/totals calculated from cost and proportion of patients or frequency of use. Values in bold are the subtotals of the preceding values.
Abbreviations: GP, general practitioner; COPD, chronic obstructive pulmonary disease; LAMA, long-acting muscarinic antagonists; LABA, long-acting beta-agonists; ICS, inhaled corticosteroids.
Results
Base case
Total costs, life years, and QALYs were estimated over a lifetime horizon. Table 5 shows the results for all three treatment comparator pairs. The results show similar patterns for each treatment comparator pair whereby the addition of roflumilast to each of the treatments results in a total lifetime incremental cost of CHF 3390, CHF 3308, and CHF 3799 and a QALY gain of 0.275, 0.289, and 0.278 in LAMA + roflumilast versus LAMA, LABA/ICS + roflumilast versus LABA/ICS, and LAMA + LABA/ICS + roflumilast versus LABA/ICS, respectively.
Table 5.
Cost-effectiveness results and cost breakdown, base case
| LAMA + roflumilast | LAMA | Incremental | LABA/ICS + roflumilast | LABA/ICS | Incremental | LAMA + LABA/ ICS + roflumilast | LAMA + LABA/ICS | Incremental | |
|---|---|---|---|---|---|---|---|---|---|
| Total cost, CHF | 86,754 | 83,364 | 3390 | 91,470 | 88,161 | 3308 | 99,364 | 95,564 | 3799 |
| Cost of maintenancea, CHF | 35,857 | 25,481 | 10,376 | 40,917 | 30,279 | 10,638 | 48,533 | 37,682 | 10,851 |
| Cost of community-treated exacerbations, CHF | 2039 | 2331 | −292b | 2025 | 2331 | −306b | 2036 | 2331 | −295b |
| Cost of hospital-treated exacerbations, CHF | 48,858 | 55,552 | −6694b | 48,528 | 55,552 | −7024b | 48,795 | 55,552 | −6757b |
| Life years (LY) | 9.625 | 9.278 | 0.347 | 9.642 | 9.278 | 0.364 | 9.628 | 9.278 | 0.351 |
| Quality-adjusted life years (QALY) | 6.466 | 6.191 | 0.275 | 6.479 | 6.191 | 0.289 | 6.468 | 6.191 | 0.278 |
| ICER, CHF per QALY | 12,313 | 1 1,456 | 13,671 | ||||||
| ICER, CHF per LY | 9757 | 9078 | 10,833 |
Notes:aCost of maintenance includes the monthly medical services, and the cost of COPD drugs;
cost offset (reduction in the roflumilast arm).
Abbreviations: LAMA, long-acting muscarinic antagonists; LABA, long-acting beta-agonists; ICS, inhaled corticosteroids; ICER, incremental cost-effectiveness ratio.
The additional costs and additional health gain result in incremental cost-effectiveness ratios (ICERs) of CHF 12,313, CHF 11,456, and CHF 13,671 per QALY gained (Table 5). The cost breakdown in Table 5 suggests that the additional cost in the regimens with roflumilast added-on come from the cost of maintenance (which includes the cost of COPD drugs as well as the cost of ongoing maintenance), and are equal to CHF 10,376, CHF 10,638, and CHF 10,851, which is explained by roflumilast add-on therapy. These incremental costs are partly offset by cost savings due to the reduction of moderate exacerbations and most importantly severe exacerbations (see notes to Table 5).
Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analysis were performed to identify the key determinants of cost-effectiveness in the Swiss setting and to assess the robustness of the model through testing the combined impact of all uncertainties in model input parameters on cost-effectiveness.
One-way sensitivity analysis
One-way sensitivity analyses for each treatment and comparator pairs are presented in Table 6 (LABA/ICS + roflumilast vs LABA/ICS), Table 7 (LAMA + LABA/ICS + rofumilast vs LAMA + LABA/ICS), and Table 8 (LAMA + roflumilast vs LAMA).
Table 6.
One-way sensitivity analysis: LABA/ICS + roflumilast vs LABA/ICS
| Parameter | Base case and range (low estimate; base case; high estimate) | ICER at low parameter estimate | ICER at high parameter estimate, CHF per QALY gained | Variation in ICER between low and high estimates |
|---|---|---|---|---|
| Relative ratios of exacerbation rates | (0.74; 0.835; 0.95)a | Roflumilast dominant | 84,682 | N/Ab |
| Hospital case fatality rate, % | (0; 7.7; 15) | Roflumilast dominant | 13,732 | N/Ab |
| Cost of hospital-treated exacerbation, CHF | (10,511; 13,139; 15,767) | 16,321 | 6591 | 9730 |
| Annual discount rate, cost | (0%; 2.5%; 5%) | 15,109 | 8973 | 6136 |
| Annual discount rate, QALY | (0%; 2.5%; 5%) | 8765 | 14,563 | 5798 |
| Background rate of exacerbations in very severe COPD, per patient per year | (1.95; 2.436; 2.92) | 14,409 | 9411 | 4998 |
| Background rate of exacerbations in severe COPD, per patient per year | (1.6; 2.0; 2.4) | 13,735 | 9586 | 4149 |
| Proportion of hospital treated exacerbations among all exacerbations in very severe COPD | (0.208; 0.244; 0.280) | 13,312 | 10,016 | 3296 |
| Proportion of hospital treated exacerbations among all exacerbations in severe COPD | (0.133; 0.155; 0.177) | 12,828 | 10,246 | 2582 |
Notes: Base case ICER for LABA/ICS + roflumilast vs LABA/ICS; CHF 11,456 per QALY gained.
Exacerbation rate reduction statistically significant;
in sensitivity analyses where cost-effectiveness changes to dominance, range of ICERs is not applicable.
Abbreviations: LABA, long-acting beta-agonists; ICS, inhaled corticosteroids; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years; COPD, chronic obstructive pulmonary disease.
Table 7.
One-way sensitivity analysis: LAMA + LABA/ICS + roflumilast vs LAMA + LABA/ICS
| Parameter | Base case and range (low estimate; base case; high estimate) | ICER at low parameter estimate | ICER at high parameter estimate, CHF per QALY gained | Variation in ICER between low and high estimates |
|---|---|---|---|---|
| Relative ratios of exacerbation rates | (0.74; 0.841; 0.95)a | 637 | 85,689 | 85,052 |
| Hospital case fatality rate, % | (0; 7.7; 15) | Roflumilast dominant | 15,621 | N/Ab |
| Cost of hospital-treated exacerbation, CHF | (10,511; 13,139; 15,767) | 18,460 | 8808 | 9652 |
| Annual discount rate, cost | (0%; 2.5%; 5%) | 17,890 | 10,787 | 7103 |
| Annual discount rate, QALY | (0%; 2.5%; 5%) | 10,461 | 17,377 | 6916 |
| Background rate of exacerbations in very severe COPD, per patient per year | (1.95; 2.436; 2.92) | 16,725 | 11,548 | 5177 |
| Background rate of exacerbations in severe COPD, per patient per year | (1.6; 2.0; 2.4) | 16,057 | 11,713 | 4344 |
| Proportion of hospital treated exacerbations among all exacerbations in very severe COPD | (0.208; 0.244; 0.280) | 15,586 | 12,184 | 3402 |
| Proportion of hospital treated exacerbations among all exacerbations in severe COPD | (0.133; 0.155; 0.177) | 15,101 | 12,411 | 2690 |
Notes: Base case ICER for LAMA + LABA/ICS + roflumilast vs LAMA + LABA/ICS: CHF 13,671 per QALY gained.
Exacerbation rate reduction statistically significant;
in sensitivity analyses where cost-effectiveness changes to dominance, range of ICERs is not applicable.
Abbreviations: LAMA, long-acting muscarinic antagonists; LABA, long-acting beta-agonists; ICS, inhaled corticosteroids; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years; COPD, chronic obstructive pulmonary disease.
Table 8.
One-way sensitivity analysis: LAMA + roflumilast vs LAMA
| Parameter | Base case and range (low estimate; base case; high estimate) | ICER at low parameter estimate | ICER at high parameter estimate, CHF per QALY gained | Variation in ICER between low and high estimates |
|---|---|---|---|---|
| Relative ratios of exacerbation rates | (0.61; 0.843; 1.14)a | Roflumilast dominant | Roflumilast + LAMA dominated | N/Ab |
| Hospital case fatality rate, % | (0; 7.7; 15) | Roflumilast dominant | 14,029 | N/Ab |
| Cost of hospital-treated exacerbation, CHF | (10,511; 13,139; 15,767) | 17,176 | 7451 | 9725 |
| Annual discount rate, cost | (0%; 2.5%; 5%) | 16,030 | 9771 | 6259 |
| Annual discount rate, QALY | (0%; 2.5%; 5%) | 9422 | 15,651 | 6229 |
| Background rate of exacerbations in very severe COPD, per patient per year | (1.95; 2.436; 2.92) | 15,402 | 10,164 | 5238 |
| Background rate of exacerbations in severe COPD, per patient per year | (1.6; 2.0; 2.4) | 14,721 | 10,337 | 4384 |
| Proportion of hospital treated exacerbations among all exacerbations in very severe COPD | (0.208; 0.244; 0.280) | 14,263 | 10,798 | 3465 |
| Proportion of hospital treated exacerbations among all exacerbations in severe COPD | (0.133; 0.155; 0.177) | 13,768 | 11,032 | 2736 |
Notes: Base case ICER for roflumilast + LAMA vs LAMA: CHF 12,313 per QALY gained.
Exacerbation rate reduction not statistically significant;
in sensitivity analyses where cost-effectiveness changes to dominance, range of ICERs is not applicable.
Abbreviations: LAMA, long-acting muscarinic antagonists; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years; COPD, chronic obstructive pulmonary disease.
The low and high estimates for the parameters used in the one-way sensitivity analysis were based where possible on the respective 95% CIs. The parameters with the largest impact on the ICER are treatment efficacy (RRR) and hospital case fatality rate. At the low estimates of each of these parameters roflumilast appears either dominant or highly cost-effective. On the other hand at the upper limits of the 95% CI for RRR and for hospital case fatality rate, the values of the ICERs increase. The cost of a hospital-treated exacerbation, background exacerbation rates in the comparator regimens, and the proportion of hospital treated exacerbations have a high impact on the ICER. On the other hand parameters such as the cost of underlying/background COPD therapies, or SMRs have a low impact on the ICER (Tables 6–8). The high impact on the ICER of discount rates on the cost and outcomes and the impact of the analysis time horizon, result from the survival benefit associated with the reduction of hospital exacerbations, and the subsequent decrease in hospital mortality.
Probabilistic sensitivity analysis
In a probabilistic sensitivity analysis relevant distributions were applied to all input parameters, based where pos-sible on their estimated 95% CIs. Although in Switzerland there is no established willingness-to-pay (WTP) threshold the commonly quoted WTP value is €60,000 per QALY (approximately CHF 72,000 at the current exchange rate).15,16 These also broadly correspond to the cost-effectiveness criteria recommended by the World Health Organization (1–3 × gross domestic product per capita).17 The probabilistic sensitivity analysis suggested that the likelihood of rofumilast being cost-effective for each of the three treatment-comparator pairs is 79%, 96%, and 96% at the WTP of CHF 70,000 per QALY when roflumilast is added to LAMA, LABA/ICS, or LAMA + LABA/ICS, respectively.
Discussion
The value of roflumilast as a supplementary regimen to the commonly used COPD treatments comes from the reduction of exacerbation rates, thus reducing the burden of exacerbations on COPD patients and health care providers. The additional cost of the treatment regimens with rofumilast is partly offset by the reduction of exacerbations. The economic effect of the reduction of exacerbation is particularly prominent in patients who experience frequent exacerbations despite current treatments and are mainly represented by patients with severe and very severe COPD. This assumption in the analysis is particularly relevant in light of the recent revision of the GOLD guidelines for the prevention, management, and treatment of COPD.3
The limitations of the analysis mainly relate to (1) the uncertainty in the published MTC data on the efficacy of roflumilast, and (2) the lack of published data for the Swiss setting on such parameters as hospital case fatality rates. The uncertainty of the efficacy inputs result from the absence of studies directly comparing health outcomes for the treatment and comparators of interest and the need to use a mixed treatment comparison study to derive the required parameters. In the model it was also assumed that the reduction in the rate of exacerbations occurs over the entire duration of the simulation and that the reduction of all exacerbation rates results in a proportional reduction of severe (hospital-treated) exacerbations and subsequently, reduction of the hospital case fatality rate. In the absence of published data in Switzerland on hospital mortality due to severe COPD exacerbations, we used the results of a UK COPD audit.9 As all of the above limitations of the study are related to the lack of, or uncertainty of input parameters, further country-specific research is required to narrow the uncertainties in future analyses.
Observational studies (ie, real-world evidence) would be a valuable source of such data. Despite the above limitations, this analysis represents a robust estimate of the cost- effectiveness of roflumilast in the Swiss setting using the best available evidence.
The most recently updated GOLD guidelines3 describe the importance of the frequency and severity of symptoms and exacerbations in the diagnosis and progression of disease severity. In the management of stable COPD, the goals for treatment include the reduction of symptoms (symptom relief, improvement in health status, and exercise tolerance) and the reduction of risk, through the prevention of disease progression, prevention and treatment of exacerbations, and the consequential reduction in mortality. Roflumilast (Daxas®) was approved for reimbursement in Switzerland in February 20127 for severe and very severe COPD patients with frequent exacerbations in the past and being pretreated with inadequate bronchodilator therapy. Roflumilast is included in the revised GOLD guidelines3 in the list of medications commonly used to treat COPD, and is recommended for use in reducing exacerbations for patients with chronic bronchitis, severe and very severe COPD, and with a history of frequent exacerbations.
The cost-effectiveness analysis presented in this paper suggests that roflumilast can be a cost-effective treatment option for COPD patients with frequent exacerbations in Switzerland.
Considering the results of this analysis in the context of the revised GOLD guidelines presents an opportunity for clinicians to decrease the number of exacerbations experienced by COPD patients, in a cost-effective manner. Additionally, decreasing the number of hospitalized exacerbations would also be sensible with respect to the budgetary efficiency of the Swiss health care system.
Conclusion
In Switzerland, treatment with roflumilast in combination with commonly used regimens such as bronchodilators with or without inhaled corticosteroids is estimated to reduce the health and economic burden of COPD exacerbations and represents a cost-effective treatment option for patients with frequent exacerbations.
Acknowledgments and disclosure
This study was sponsored by Takeda Pharma AG, Switzerland. All the authors participated in the study design, data analysis, interpretation of the results, writing and reviewing of the manuscript, and the decision to submit the manuscript for publication. Yevgeniy Samyshkin and Matthew Radford are employees of IMS Health, which was commissioned by Takeda Pharma AG, Switzerland to conduct this study and write the manuscript. Michael Schlunegger, Susan Haefliger, and Sabine Ledderhose are employees of Takeda Pharma AG, Switzerland. The authors report no other conflicts of interest in this work.
References
- 1.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Defnition, epidemiology and natural history of COPD. Eur Respir J. 2007;30(5):993–1013. doi: 10.1183/09031936.00082507. [DOI] [PubMed] [Google Scholar]
- 2.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Revised 2011 Global Initiative for Chronic Obstructive Lung Disease, Inc; 2011Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed August 20, 2012 [Google Scholar]
- 4.Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax. 2006;61(3):250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calverley PM, Rabe K F, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 6.Bateman ED, Rabe KF, Calverley PM, et al. Roflumilast with longacting β2-agonists for COPD: infuence of exacerbation history. Eur Respir J. 2011;38(3):553–560. doi: 10.1183/09031936.00178710. [DOI] [PubMed] [Google Scholar]
- 7.Bundesamt für Gesundheit Bulletin 6/12 Bern: Bundesamt für Gesundheit BAG; 2012Available from: http://www.bag.admin.ch/dokumentation/publikationen/01435/11505/12789/index.html?lang=de&sort=ta&superflex=0_0&filter_dms_thema=10&filter_dms_fx=327&flter_dms_jahre=Accessed November 11, 2012 [Google Scholar]
- 8.Hertel N, Kotchie RW, Samyshkin Y, Radford M, Humphreys S, Jameson K. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis. 2012;7:183–199. doi: 10.2147/COPD.S29820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royal College of Physicians Report of the National Chronic Obstructive Pulmonary Disease Audit 2008: Clinical Audit of COPD Exacerbations Admitted to Acute NHS Units Across the UK 2008 London: Royal College of Physicians; 2008Available from: http://www.rcplondon.ac.uk/sites/default/files/report-of-the-national-copd-audit-2008-clinical-audit-of-copd-exacerbations-admitted-to-acute-nhs-units-across-the-uk.pdfAccessed August 15, 2012 [Google Scholar]
- 10.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 11.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381–390. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 12.Mills EJ, Druyts E, Ghement I, Puhan MA. Pharmacotherapies for chronic obstructive pulmonary disease: a multiple treatment comparison meta-analysis. Clin Epidemiol. 2011;3:107–129. doi: 10.2147/CLEP.S16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer M, Briggs AH, Grossman R F, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–637. doi: 10.2165/00019053-200523060-00008. [DOI] [PubMed] [Google Scholar]
- 14.Rutten-van Mölken M P, Hoogendoorn M, Lamers LM. Holistic preferences for 1-year health profiles describing fuctuations in health: the case of chronic obstructive pulmonary disease. Pharmacoeconomics. 2009;27(6):465–477. doi: 10.2165/00019053-200927060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Matter-Walstra K W, Dedes KJ, Schwenkglenks M, Brauchli P, Szucs TD, Pestalozzi BC. Trastuzumab beyond progression: a cost-utility analysis. Ann Oncol. 2010;21(11):2161–2168. doi: 10.1093/annonc/mdq250. [DOI] [PubMed] [Google Scholar]
- 16.Matter-Walstra K, Joerger M, Kühnel U, Szucs T, Pestalozzi B, Schwenkglenks M. Cost-effectiveness of maintenance pemetrexed in patients with advanced nonsquamous-cell lung cancer from the perspective of the Swiss health care system. Value Health. 2012;15(1):65–71. doi: 10.1016/j.jval.2011.08.1737. [DOI] [PubMed] [Google Scholar]
- 17.WHO. WHO-CHOICE [webpage on the Internet] CHOosing Interventions that are Cost Effective (WHO-CHOICE) Geneva: World Health Organization; 2012. Available from: http://www.who.int/choice/enAccessed July 2, 2012 [Google Scholar]
- 18.Schramm W, Haake D, Brandt A. Die Wirtschaftlichkeit von Tiotropium bei chronisch obstruktiver Lungenerkrankung [Economic value of tiotropium in the treatment of chronic obstructive pulmonary disease] Praxis (Bern 1994) 2005;94(46):1803–1810. doi: 10.1024/0369-8394.94.46.1803. German. [DOI] [PubMed] [Google Scholar]
- 19.Oostenbrink JB, Rutten-van Mölken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health. 2005;8(1):32–46. doi: 10.1111/j.1524-4733.2005.03086.x. [DOI] [PubMed] [Google Scholar]
- 20.compendiumch [webpage on the Internet] medicines Compendium of Switzerland® Basel: Documed Ltd; 2012Available from: http://www.kompendium.chAccessed July 2, 2012German, French, English. [Google Scholar]
- 21.TARMED Suisse [homepage on the Internet] Bern: TARMED Suisse; 2011Available from: http://www.tarmedsuisse.ch/Accessed July 2, 2012 [Google Scholar]


