Abstract
Background
Long-term prefrontal cortex and hippocampus-based cognitive deficits are the sequelae of perinatal iron deficiency, despite iron supplementation starting in the newborn period. Whether high dose iron supplementation prevents these deficits is not known.
Methods
Perinatal iron deficiency was induced in rat pups using low-iron (3 mg/kg diet) diet during gestation until postnatal day (P) 8. Iron was supplemented using standard (40 mg/kg diet) or 10-fold higher (400 mg/kg diet) iron-containing diet until P21. Prefrontal cortex and hippocampal neurochemistry was determined using in vivo 1H nuclear magnetic resonance spectroscopy at 9.4 tesla on P90.
Results
Both iron supplementation doses corrected anemia and brain iron deficiency by P21. The neurochemical profile of the prefrontal cortex in both supplementation groups was comparable to the control group. In the hippocampus, standard-dose iron supplementation resulted in lower N-acetylaspartate and phosphoethanolamine, and higher N-acetylaspartylglutamate and glycerophosphocholine + phosphocholine concentrations. High-dose iron supplementation resulted in lower phosphoethanolamine and higher glycerophosphocholine + phosphocholine concentrations.
Conclusions
The iron supplementation dose for perinatal iron deficiency differentially alters the neurochemical profile of the prefrontal cortex and hippocampus in adulthood. The neurochemical changes suggest altered glutamatergic neurotransmission, hypomyelination and abnormal phospholipid metabolism in the formerly iron-deficient hippocampus.
Introduction
Iron deficiency during gestation and early lactation (perinatal iron deficiency) is common in humans and is associated with cognitive deficits that persist into adulthood (1, 2). Abnormalities in recognition memory, attention, planning ability and inhibitory control, increased anxiety and depression, hesitancy and wariness in novel situations are the long-term sequelae of early life iron deficiency (3, 4). The nature of the cognitive and behavioral deficits suggests the involvement of prefrontal cortex (PFC) and hippocampus. Both brain regions are rapidly developing during the perinatal period, although hippocampal development precedes that of PFC (5). Animal studies demonstrate that iron deficiency has profound effect on energy metabolism, myelination and monoamine (primarily dopamine) metabolism (6–10). Perinatal iron deficiency may independently perturb each of these processes. Moreover, these processes work together in integrated, interacting circuits. Both the independent and synergistic effects likely lead to the complex long-term cognitive and behavioral impairments following early life iron deficiency.
The persistent cognitive and neurometabolic effects of perinatal iron deficiency may be due to incomplete iron repletion prior to the completion of the rapid phase of brain development (11, 12). Thus, a more rapid correction of brain iron deficiency, for example, by using a higher than the standard dose of iron may prevent or lessen the cognitive deficits. Conversely, high dose iron presents the potential for excess iron transport and iron-induced neurotoxicity, particularly when iron supplementation is initiated during the neonatal period when iron transport is avid (13, 14). The risk of toxicity from excess iron may be further exacerbated in the setting of perinatal iron deficiency due to the earlier appearance and increased expression of iron transport proteins and receptors in specific brain regions, including the PFC and hippocampus (15).
The effects of different doses of iron supplementation for perinatal iron deficiency on long-term brain metabolism have not been studied. The objective of the present study was to compare the effects of two different doses of postnatal iron supplementation – standard dosing and 10-fold higher dosing – on the neurochemical profiles of the PFC and hippocampus in adult rats that were perinatally iron-deficient. These brain regions have substantially different developmental trajectories; however, both are affected in perinatal iron deficiency (16–18). We used high-field in vivo 1H nuclear magnetic resonance (NMR) spectroscopy to determine the regional neurochemical profiles, consisting of metabolites indexing neuronal and glial integrity, phospholipid and energy metabolism, neurotransmission, osmoregulation and antioxidants, and related the neurochemical changes to concurrent alterations in the expression of a targeted group of genes.
Results
Effect of Dietary Iron on Milk Iron Concentration
In order to estimate daily iron intake by the pups, milk samples were collected from dams maintained on different dietary iron concentrations, ranging from 3 mg/kg diet to 1200 mg/kg diet. The milk iron concentration increased steadily with increasing iron concentration in maternal diet until 400 mg/kg (Table 1). There was no further increase in milk iron concentration with 1200 mg/kg iron-diet. Based on the average daily milk consumption by pups at corresponding ages (19), these data translate to a daily iron delivery of 1 mg/kg body weight during the period of iron deficiency, 2–3 mg/kg body weight during supplementation with standard dose iron (40 mg/kg diet) and 6 mg/kg body weight during supplementation with high dose iron (400 mg/kg diet) in maternal diet.
Table 1.
Effect of maternal dietary iron on milk iron concentration
| Iron concentration in the diet (mg/kg) | Iron concentration in the milk (μg/l) |
|---|---|
| 3 | 1397±77 |
| 80 | 3752±204 |
| 400 | 8736±224 |
| 1200 | 7381±885 |
Values are mean±SEM, n = 4–6.
Effect of Iron Supplementation Dose on Hematocrit and Tissue Iron Status in PFC and Hippocampus
The hematocrit and tissue iron concentration in the PFC and hippocampus were determined on postnatal day (P) 21 and P90 in the Control group and the two formerly iron-deficient (FID) groups that were supplemented with either standard dose iron (40 mg/kg diet; FID-40 group) or high dose iron (400 mg/kg diet; FID-400 group) in maternal diet from P8 to P21 (Figure 1). The hematocrit and tissue iron concentrations in the two FID groups were comparable to the Control group on both days (Table 2). However, compared with the FID-40 group, the hematocrit on P21 was higher and hippocampal iron concentration on P90 was lower, in the FID-400 group (p<0.05, Table 1). Transferrin receptor 1 mRNA (Tfrc) expression in the PFC and hippocampus was determined on P90 to rule out latent tissue iron deficiency. In both FID groups, Tfrc expressions in the two brain regions were comparable to the Control group (Table 2).
Figure 1.
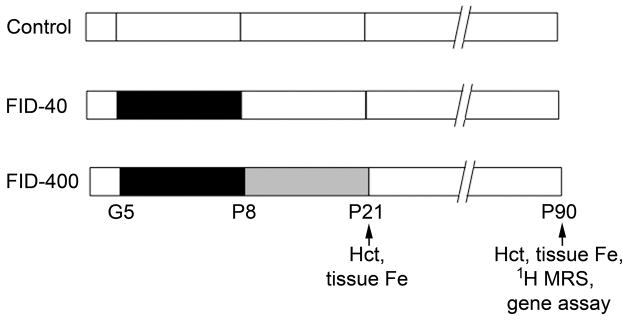
Study design. The control group (Control) and the two formerly iron-deficient (FID) groups were generated by maintaining the dams during gestation (G) and postnatal (P) periods, and the pups after weaning on diets containing the following iron concentrations: 40 mg/kg diet (white), 3 mg/kg diet (black) and 400 mg/kg diet (gray). The numbers refer to gestational or postnatal age in days. Fe, iron; Hct, hematocrit; MRS, magnetic resonance spectroscopy.
Table 2.
Effect of the iron supplementation dose for perinatal iron deficiency on hematocrit, tissue iron concentration and transferrin receptor mRNA expression in the prefrontal cortex and hippocampus of 21-day-old and adult rats
| Parameter | Age/Region | Control Group | FID-40 Group | FID-400 Group |
|---|---|---|---|---|
| Hematocrit (%) | P21 | 39.2±0.6 | 36.0±1.8 | 41.7±1.0* |
| P90 | 49.3±0.3 | 49.3±0.9 | 47.9±0.7 | |
| Tissue iron conc. (μg/g) on P21 | PFC | 16.6±1.4 | 16.2±1.5 | 13.4±0.9 |
| Hippocampus | 15.2±1.4 | 14.8±1.1 | 15.3±1.3 | |
| Tissue iron conc. (μg/g) on P90 | PFC | 18.1±1.2 | 18.7±1.3 | 16.1±0.7 |
| Hippocampus | 18.0±1.1 | 21.1±1.2 | 15.5±1.0* | |
| Tfrc expression on P90 | PFC | 1.00±0.12 | 1.17±0.11 | 1.26±0.10 |
| Hippocampus | 1.00±0.09 | 1.13±0.07 | 1.19±0.14 |
Values are mean±SEM (n = 6–8 per group). Iron concentrations are expressed as μg/g wet tissue weight. Transcript data are normalized to the Control group for each brain region.
Different from FID-40 group (p<0.05); the rest are NS. FID-40 Group, formerly iron-deficient, supplemented with 40 mg/kg iron diet from postnatal day 8; FID-400 Group, formerly iron-deficient, supplemented with 400 mg/kg iron diet from postnatal day 8; P, postnatal day; PFC, prefrontal cortex; Tfrc, transferrin receptor 1 gene.
Effect of Iron Supplementation Dose on Neurochemical Profiles of PFC and Hippocampus
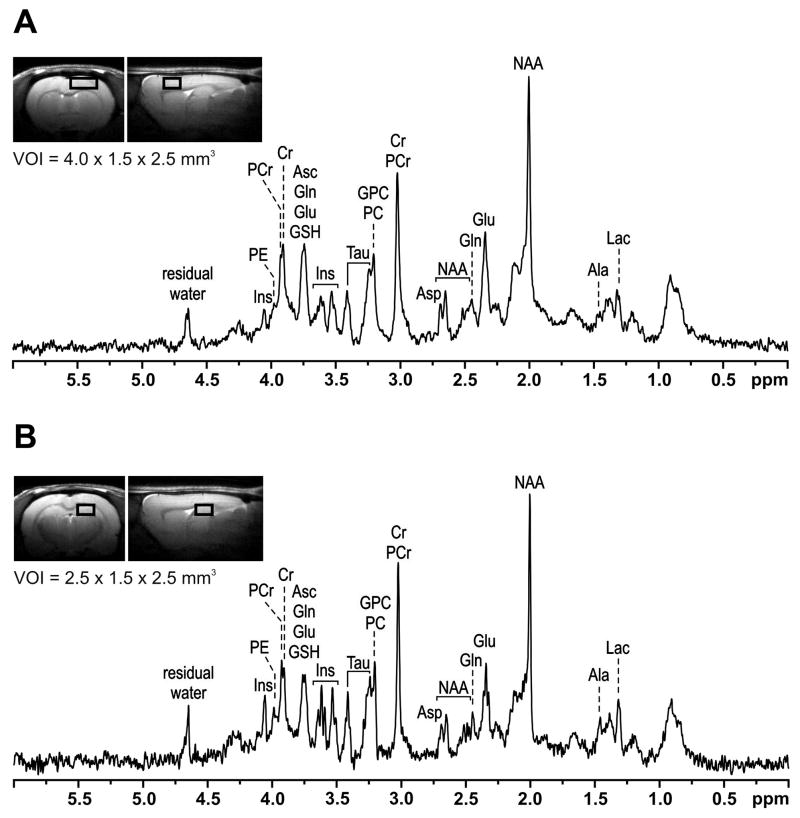
The effects of iron supplementation dose on neurochemical profiles of the brain regions were determined using high-field in vivo 1H NMR spectroscopy on P90. 1H NMR spectra from the PFC and hippocampus from a rat in the Control group are shown in Figure 2. Hippocampal spectra from two rats, one from each FID group, were excluded due to motion artifacts. Seventeen metabolites were consistently quantified from the remaining NMR spectra. There were no differences between male and female rats in the neurochemical profiles of both brain regions. Therefore, spectra from both sexes were combined.
Figure 2.
In vivo 1H NMR spectra from the prefrontal cortex (A) and hippocampus (B) of a postnatal day 90 rat in the Control group. The MRI shows the volume of interest (VOI) used for acquiring the spectra. Ala, alanine; Asc, ascorbate; Asp, aspartate; Cr, creatine; Glu, glutamate; Gln, glutamine; GSH, glutathione; GPC, glycerophosphocholine; Lac, lactate; Ins, myo-inositol; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PC, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Tau, taurine.
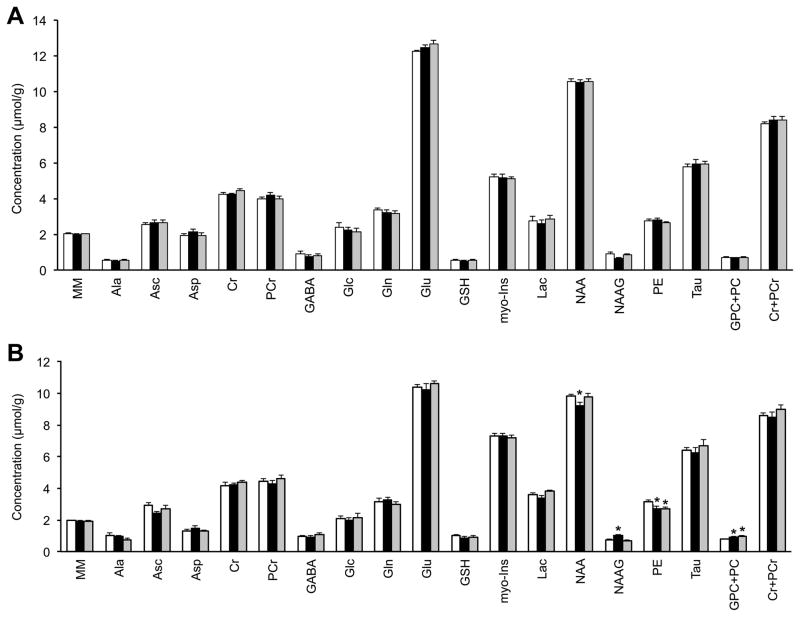
Effect of iron supplementation dose on the regional neurochemical profiles
There were no differences between the Control group and the two FID groups in any of the 17 metabolites in the PFC (Figure 3A). In contrast, the two iron supplementation doses had differential effects on hippocampal neurochemistry (Figure 3B). Compared with the Control group, there was 6% decrease in NAA, 37% increase in NAAG, 15% decrease in PE and 17% increase in GPC+PC concentrations in the FID-40 group (p<0.05, Figure 3B). In the FID-400 group, NAA and NAAG concentrations were comparable to the Control group, but there was 15% decrease in PE and 27% increase in GPC+PC concentrations (p<0.05, Figure 3B).
Figure 3.
The neurochemical profiles of the prefrontal cortex (A) and hippocampus (B) in the Control (white), FID-40 (black) and FID-400 (gray) groups. Values are mean±SEM, n = 7–8; *p <0.05 vs. Control group. FID-40, formerly iron-deficient, supplemented with 40 mg/kg iron diet from postnatal day 8; FID-400, formerly iron-deficient, supplemented with 400 mg/kg iron diet from postnatal day 8; Ala, alanine; Asc, ascorbate; Asp, aspartate; Cr, creatine; Glc, glucose; Gln, glutamine; Glu, glutamate; GSH, glutathione; GPC, glycerophosphocholine; Lac, lactate; Ins, myo-inositol; MM, macromolecules; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PC, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Tau, taurine.
Effect of Iron Supplementation Dose on Gene Expression in PFC and Hippocampus
The mRNA expression of a targeted group of genes was determined on P90 to relate neurochemistry results to specific neurodevelopmental processes. Similar to neurochemistry results, myelin basic protein (Mbp), profilin 1 (Pfn1) and calcium/calmodulin-dependent protein kinase II α (CaMKIIα, Camk2a) mRNA expressions in PFC were comparable to the Control group in both FID groups (Table 3). In the hippocampus, Mbp and Pfn1 expressions were increased in both FID groups (Control group <FID-40 group <FID-400 group, p<0.05). Camk2a was increased only in the FID-400 group (p<0.05; Table 3).
Table 3.
Effect of the iron supplementation dose for perinatal iron deficiency on mRNA expression of a target group of genes in the prefrontal cortex and hippocampus in adult rats
| Gene | Prefrontal Cortex | Hippocampus | ||||
|---|---|---|---|---|---|---|
| Control Group | FID-40 Group | FID-400 Group | Control Group | FID-40 Group | FID-400 Group | |
| Mbp | 1.00±0.10 | 1.08±0.05 | 1.36±0.20 | 1.00±0.10 | 1.22±0.06* | 1.75±0.19*,** |
| Camk2a | 1.00±0.24 | 1.03±0.50 | 1.39±0.29 | 1.00±0.10 | 1.19±0.08 | 1.32±0.05* |
| Pfn1 | 1.00±0.11 | 1.08±0.07 | 1.25±0.20 | 1.00±0.08 | 1.28±0.05* | 1.54±0.03*,** |
Values are mean±SEM normalized to the Control group (n = 6–8 per group). There was a group effect for all genes in the hippocampus, p<0.04 (ANOVA), but not in the prefrontal cortex.
Different from the Control group, p<0.05; and
different from FID-40 group, p<0.05 (Bonferroni-adjusted unpaired t tests). FID-40 Group, formerly iron-deficient, supplemented with 40 mg/kg iron diet from postnatal day 8; FID-400 Group, formerly iron-deficient, supplemented with 400 mg/kg iron diet from postnatal day 8; Mbp, myelin basic protein; Camk2a, calcium/calmodulin-dependent protein kinase II α; Pfn1, profilin 1.
Discussion
This study demonstrates that the iron supplementation dose for perinatal iron deficiency has a region-specific long-term effect on the neurochemical profile and gene expression in the PFC and hippocampus of adult rats. Both doses of iron supplementation, beginning in the neonatal period, corrected anemia and normalized tissue iron concentrations in both brain regions. Whereas the neurochemical profile of the PFC in both FID groups was comparable to the Control group, the hippocampal neurochemical profile was abnormal in adulthood. These region-specific neurochemical effects, which index glutamatergic neurotransmission, myelination and phospholipid metabolism, may have a role in the hippocampus-mediated cognitive deficits that persist in adulthood following early life iron deficiency. Furthermore, these region-specific changes may unbalance the inputs and outputs of these two circuits on other critical brain systems (e.g., ventral tegmentum) that are necessary for normal behavioral function (2, 20).
The concentration of 10 of the 17 metabolites differed by 10% to 40% in the PFC and hippocampus in the Control group. Similar interregional metabolite concentration differences were also present in the two FID groups with few exceptions. Such large, disparate differences in individual metabolites suggest that the data reflect region-specific effects and rule out technical factors, such as partial volume effect for the results. These data likely stem from the differences in metabolic demands and processes inherent to each brain region (5) and suggest that the effect of perinatal iron deficiency on a brain region depends on its developmental stage and metabolic demand at the time of iron deficiency.
The neurochemical profile of the PFC in both FID groups was comparable to the Control group. Based on previously demonstrated impaired energy metabolism in this region during perinatal iron deficiency (16) and persistent PFC-mediated behavioral deficits in iron-deficient human infants at adulthood (1), we expected neurochemical changes in PFC. One possible explanation for our results is that both iron supplementation doses corrected tissue iron deficiency in the PFC by P21, i.e., before the onset of its peak developmental period (5) and may have protected the region from the deleterious effects of perinatal iron deficiency. Consistent with this possibility, PFC-mediated behaviors are preserved in adult rats that were perinatally iron-deficient and received iron supplementation from P8 (17).
Unlike the PFC, hippocampal neurochemistry was abnormal in both FID groups, in spite of normalization of tissue iron concentration by P21. Latent or new-onset hippocampal iron deficiency is unlikely to explain these results, since tissue iron concentration and Tfrc expression in this region were normal at P90. While the magnitude of the neurochemical changes (6%–37%) in the FID hippocampus may appear modest, it is comparable to those reported during the period of iron deficiency and following iron supplementation in a slightly more severe perinatal iron deficiency model (10, 11) and is associated with cognitive deficits in humans (21, 22). Unlike the PFC, the period of brain iron deficiency and anemia in our model coincides with the period of peak hippocampal development (5) and may have perturbed its development. The iron supplementation doses also appear to have a more variable effect. A two to three-fold higher daily iron intake was ineffective in achieving normal hippocampal neurochemistry. Collectively, these data suggest that the hippocampus is more sensitive to perinatal iron deficiency and may not benefit from iron repletion paradigms starting in the newborn period. It is not known whether starting iron supplementation earlier than P8 or continuing it beyond P90 would have normalized hippocampal neurochemical profile. A previous study demonstrated that iron supplementation from P4 normalizes monoamine metabolism and behavior in a slightly less severe model of perinatal iron deficiency (12).
The abnormal aspects of hippocampal neurochemistry are informative in terms of the processes they index. Decreased NAA and increased NAAG in the context of iron deficiency suggests altered glutamatergic turnover (23). Our previous studies have demonstrated suppressed glutamate-glutamine cycling and NMDA receptor expression in the hippocampus during perinatal iron deficiency (10, 24). NAAG modulates NMDA receptor function and suppresses long-term potentiation (LTP) in the hippocampus (25), a finding consistent with reduced LTP at adulthood following perinatal iron deficiency (26). The NAA and NAAG changes in the FID-40 hippocampus are potentially correctable with higher doses of iron supplementation, since these changes were absent in the FID-400 hippocampus. Camk2a upregulation, which is necessary for induction of LTP (27) in the FID-400 group, but not in the FID-40 group, supports this interpretation.
In addition to its direct effect on hippocampus-specific cognitive function, altered glutamatergic neurotransmission may indirectly influence the overall memory system in perinatal iron deficiency. The interconnections between the hippocampus, PFC and ventral tegmentum, known as the ventral tegmental area loop, are regulated by the glutamatergic, GABA-ergic and dopaminergic neurotransmitter systems. The loop is important for long-term memory and cognitive flexibility (20). The ventral tegmentum integrates glutamatergic inputs from the hippocampus and PFC and subsequently sends output to these structures via dopaminergic neurons, in turn modifying their activity. Importantly, early life iron deficiency profoundly alters dopaminergic metabolism in all three of these structures in adulthood (28). Combined with the altered glutamatergic changes demonstrated in the present study, these neurotransmitter changes likely cause long-term impairments in cognitive performance and behavior (3, 7, 28).
Altered PE and GPC+PC, which index phospholipid metabolism in the hippocampus has been previously demonstrated during the period of perinatal iron deficiency (10). Similar results in both FID groups in the present study suggest that perinatal iron deficiency irreversibly alters phospholipid metabolism in the hippocampus and that this effect is not amenable to correction with iron supplementation even at higher doses. Decreased PE suggests hypomyelination or demyelination (9, 29). A corresponding increase in Mbp likely represents a compensatory response towards remyelination, similar to other demyelinating neuropathologies (30, 31). Although the magnitude of PE decrease was similar in the two FID groups, a dose-response effect was present in the magnitude of Mbp expression (Control group <FID-40 group <FID-400 group), suggesting the potential for greater compensatory remyelination with higher dose of iron supplementation.
Increased GPC+PC also indicates altered phospholipid metabolism due to previous iron deficiency in the two FID groups. Increased GPC and/or PC could be responsible for the increased GPC+PC, since the close spectral similarities of the two compounds preclude their differentiation using in vivo NMR spectroscopy. However, increased Pfn1 expression, which regulates neurite formation (32), suggests increased PC more than GPC likely caused increased GPC+PC in both FID groups. In this regard, we have previously demonstrated increased, but abnormal appearing neurites in the FID hippocampus (24, 33). Our recent study in FID monkeys also demonstrates persistent changes in cerebrospinal fluid protein profiles that are indicative of abnormal neurite formation (unpublished observations). Unlike PE, a dose-response effect was present for GPC+PC and Pfn1 expression (Control group <FID-40 group <FID-400 group). However, greater PC concentration may not portend a beneficial effect with higher doses of iron. Indeed, increased PC and neurite formation is a hallmark of neurodegenerative disorders, such as early stage Alzheimer’s disease (AD) (34). In this context, we have previously demonstrated premature upregulation of AD-related genes in the hippocampus during perinatal iron deficiency (35). Considering the potential role of iron dyshomeostasis in AD (36), we posit that increased GPC+PC may indicate a greater risk of AD in the FID-400 group. Future studies are necessary to corroborate this hypothesis.
In summary, the present study supports the presence of long-term region-specific adverse effects of perinatal iron deficiency on the developing brain in spite of iron supplementation. The dietary protocols resulted in a daily iron intake of 2 to 6 mg/kg body weight and rapidly corrected anemia and brain iron deficiency, but were unable to achieve neurochemical normalcy in the hippocampus at adulthood. These data may have relevance to iron-deficient human infants and toddlers. The iron supplementation dose in the present study falls within the range used for treating these children (37). Although the data may not be directly translatable to humans, the inability to achieve neurochemical normalcy even with high doses of iron suggests the importance of additional research on treatment strategies for ensuring normal neurodevelopment in these infants.
Methods
Animals and Dietary Treatment
All procedures conformed to guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committees at University of Michigan and University of Minnesota. Male and female Sprague-Dawley rats were used. The overall study design is shown in Figure 1. Custom research diets were purchased (Harlan Laboratories, Madison, WI, USA). Pregnant dams were maintained on a low iron diet (TD80396; iron concentration, 3 mg/kg diet) from gestational day 5 to P7 to induce anemia and brain iron deficiency in the pups. On P8, pups were treated by cross fostering to dams maintained on diets containing one of two iron concentrations, 40 mg/kg diet (TD89300) or 400 mg/kg diet (TD02545) until weaning on P21 to create FID-40 group and FID-400 group, respectively. Pups born to dams maintained on the 40 mg/kg iron-containing diet throughout gestation and postnatal periods formed the always iron-sufficient control group (Control group). All groups were maintained on the 40 mg iron/kg diet from P21. Each group was consisted of pups from at least two litters.
Determination of Milk and Tissue Iron Concentration
Collection of milk
Milk samples were collected on P9, P12 and P15 from a separate group of dams maintained on diet containing one of the following iron concentrations: 3 mg/kg, 80 mg/kg, 400 mg/kg and 1200 mg/kg. Under 3% isoflurane anesthesia, the abdomen was cleaned with nanopure water followed by ethanol. Milk was collected by manual expression after injecting oxytocin (20 U/ml solution; 300 μl, s.c.) to stimulate milk production. The volume of collected milk was recorded and samples were stored at −20°C. The entire procedure took less than 20 min.
Collection of brain tissue
Brain tissue was collected from a separate group of rats on P21 and P90 after in situ perfusion with PBS. The PFC and the hippocampus were dissected on ice and kept frozen at −80°C until analysis.
Determination of iron concentration
Milk and the brain regions were thawed on ice and wet digested using published protocols (38). The iron concentration was determined using atomic absorption spectrometry (Perkin-Elmer AAnalyst 600, Perkin-Elmer, Norwalk, CT). Standards were prepared by diluting iron standards (PE#N9300126; Perkin-Elmer) in 0.2% ultra-pure nitric acid. Blanks were prepared by digesting and diluting reagents to control for possible contamination. All standard curves exceeded r > 0.99.
In vivo 1H NMR Spectroscopy
In vivo 1H NMR spectroscopy was performed on P90 in spontaneously breathing rats under inhalational anesthesia (isoflurane, 3% for induction and 1–2% for maintenance in an 1:1 mixture of O2 and N2O). Measurements were performed using a horizontal bore 9.4 tesla/31 cm magnet (Varian/Magnex Scientific; Oxford, UK) interfaced to a Varian INOVA/Direct Drive consoles (Varian, Inc.; Palo Alto, CA) using previously published protocols (2, 10). The volume of interest was approximately 15 μl (4.0 ×1.5 ×2.5 mm3) for the PFC and 9 μl (2.5 ×1.5 ×2.5 mm3) for the hippocampus and was based on multi-slice fast spin-echo MRI (echo train length = 8, echo spacing = 12 ms, echo time = 48 ms, field of view = 3 cm ×3 cm, matrix = 256 ×256, slice thickness = 1 mm). The order of data acquisition from the two regions was randomly chosen in each rat. The study of a single rat did not exceed 100 min.
Quantification of neurochemicals
In vivo 1H NMR spectra were analyzed with the spectrum of fast relaxing macromolecules included in the basis set as previously described (2, 10). Unsuppressed water signal was used as internal reference assuming 80% brain water content. Experiments with poor signal-to-noise ratio (Cramer Rao lower bounds >30%) were excluded from analysis. The following metabolites were included in the final analysis: alanine (Ala), ascorbate (Asc), aspartate (Asp), creatine (Cr), phosphocreatine (PCr), GABA, glucose (Glc), glutamate (Glu), glutamine (Gln), glutathione (GSH), lactate (Lac), myo-inositol (Ins), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE) and taurine (Tau). In addition, the sum of glycerophosphocholine (GPC) and phosphocholine (PC) was determined, since it was not possible to differentiate the two metabolites due to their close spectral similarity. Thus, the neurochemical profile consisted of 17 metabolites.
Quantitative RT-PCR
Rats were killed at the conclusion of NMR spectroscopy using an overdose of pentobarbital (120 mg/kg i.p.). The brain was removed, the PFC and hippocampi were dissected out, flash frozen and stored at −80°C. The mRNA expression was measured using published methods (39) and readymade primer/probes (TaqMan Gene Expression Assays; Life Technologies Corporation, Carlsbad, CA; Supplemental Table 1, online). Samples were assayed in duplicate and normalized against ribosomal protein S18.
Statistical Analysis
The effects of iron supplementation dose on regional neurochemistry and mRNA expression were determined using ANOVA and Bonferroni-corrected t tests. Data are presented as mean±SEM. Significance was set at p<0.05.
Supplementary Material
Acknowledgments
Statement of Financial Support: This work was supported by a grant (P01 HD039386, PI: Lozoff B) from the National Institutes of Health. The Center for MR Research is supported by National Center for Research Resource grant (P41 RR008079), Neuroscience Center Core Blueprint Award (P30 NS057091) and the W.M. Keck Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors acknowledge the technical assistance of Liam Callahan, Katie Thibert and Kim-Thao Le.
Footnotes
Category of Study: Basic Science
Disclosure: Presented as abstracts during the annual meetings of 1) Pediatric Academic Societies (Rao R, Tkac I, Czerniak K, Hurst A, Felt B, Georgieff M. Long-term effects of perinatal iron deficiency and its treatment on the neurochemical profile of the hippocampus and frontal cortex in adult rats) on May 2, 2011 at Denver, CO, USA, and 2) International Society of Magnetic Resonance in Medicine (Tkac I, Czerniak K, Hurst A, Felt B, Georgieff MK, Rao R. Long-term dosage effects in iron repletion treatment on neurochemical profiles and gene expression in rat model of neonatal iron deficiency) on May 8, 2012 at Melbourne, Australia.
References
- 1.Lukowski AF, Koss M, Burden MJ, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt AT, Alvarez GC, Grove WM, Rao R, Georgieff MK. Early iron deficiency enhances stimulus-response learning of adult rats in the context of competing spatial information. Dev Cogn Neurosci. 2012;2:174–80. doi: 10.1016/j.dcn.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr. 2011;141:740S–6S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69 (Suppl 1):S43–8. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallman PR, Siimes MA, Manies EC. Brain iron: persistent deficiency following short-term iron deprivation in the young rat. Br J Haematol. 1975;31:209–15. doi: 10.1111/j.1365-2141.1975.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 7.Youdim MB, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cell Mol Biol. 2000;46:491–500. [PubMed] [Google Scholar]
- 8.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;170:224–32. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Wu LL, Zhang L, Shao J, Qin YF, Yang RW, Zhao ZY. Effect of perinatal iron deficiency on myelination and associated behaviors in rat pups. Behav Brain Res. 2008;188:263–70. doi: 10.1016/j.bbr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 11.Rao R, Tkac I, Schmidt AT, Georgieff MK. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutr Neurosci. 2011;14:59–65. doi: 10.1179/1476830511Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J Nutr. 2007;137:1176–82. doi: 10.1093/jn/137.5.1176. [DOI] [PubMed] [Google Scholar]
- 13.Schroder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T. Memory deficits in adult rats following postnatal iron administration. Behav Brain Res. 2001;124:77–85. doi: 10.1016/s0166-4328(01)00236-4. [DOI] [PubMed] [Google Scholar]
- 14.Kaur D, Peng J, Chinta SJ, et al. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging. 2007;28:907–13. doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Siddappa AJ, Rao RB, Wobken JD, et al. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res. 2003;53:800–7. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- 16.deUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt AT, Ladwig EK, Wobken JD, Grove WM, Georgieff MK. Delayed alternation performance in rats following recovery from early iron deficiency. Physiol Behav. 2010;101:503–8. doi: 10.1016/j.physbeh.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEchron MD, Alexander DN, Gilmartin MR, Paronish MD. Perinatal nutritional iron deficiency impairs hippocampus-dependent trace eyeblink conditioning in rats. Develop Neurosci. 2008;30:243–54. doi: 10.1159/000110502. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor DL, Picciano MF, Sherman AR. Impact of maternal iron deficiency on quality and quantity of milk ingested by neonatal rats. Br J Nutr. 1988;60:477–85. doi: 10.1079/bjn19880120. [DOI] [PubMed] [Google Scholar]
- 20.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2005;384:23–8. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007;28:1330–9. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ill AM, Mitchell TR, Neely EB, Connor JR. Metabolic analysis of mouse brains that have compromised iron storage. Metab Brain Dis. 2006;21:77–87. doi: 10.1007/s11011-006-9022-5. [DOI] [PubMed] [Google Scholar]
- 24.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 25.Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100:346–57. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- 26.Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- 27.Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 28.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettegrew JW, Panchalingam K, Withers G, McKeag D, Strychor S. Changes in brain energy and phospholipid metabolism during development and aging in the Fischer 344 rat. J Neuropathol Exp Neurol. 1990;49:237–49. doi: 10.1097/00005072-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Yao DL, Liu X, Hudson LD, Webster HD. Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1995;92:6190–4. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristensson K, Holmes KV, Duchala CS, Zeller NK, Lazzarini RA, Dubois-Dalcq M. Increased levels of myelin basic protein transcripts in virus-induced demyelination. Nature. 1986;322:544–7. doi: 10.1038/322544a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birbach A. Profilin, a multi-modal regulator of neuronal plasticity. Bioessays. 2008;30:994–1002. doi: 10.1002/bies.20822. [DOI] [PubMed] [Google Scholar]
- 33.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–48. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettegrew JW, Klunk WE, Panchalingam K, McClure RJ, Stanley JA. Magnetic resonance spectroscopic changes in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:282–306. doi: 10.1111/j.1749-6632.1997.tb48480.x. [DOI] [PubMed] [Google Scholar]
- 35.Carlson ES, Magid R, Petryk A, Georgieff MK. Iron deficiency alters expression of genes implicated in Alzheimer disease pathogenesis. Brain Res. 2008;1237:75–83. doi: 10.1016/j.brainres.2008.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonda DJ, Lee HG, Blair JA, Zhu X, Perry G, Smith MA. Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics. 2011;3:267–70. doi: 10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Academy of Pediatrics, Committee on Nutrition. Iron. In: Kleinman RE, editor. Pediatric Nutrition Handbook. Elk Grove Village, IL: American Academy of Pediatrics; 2009. pp. 403–22. [Google Scholar]
- 38.Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–63. doi: 10.1093/jn/130.2.254. [DOI] [PubMed] [Google Scholar]
- 39.Rao R, Sperr D, Ennis K, Tran P. Postnatal age influences hypoglycemia-induced Poly(ADP-ribose) polymerase-1 activation in the brain regions of rats. Pediatr Res. 2009;66:642–7. doi: 10.1203/PDR.0b013e3181bbce69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.