Abstract
Neuronavigation has become an ubiquitous tool in the surgical management of brain tumors. This review describes the use and limitations of current neuronavigational systems for brain tumor biopsy and resection. Methods for integrating intraoperative imaging into neuronavigational datasets developed to address the diminishing accuracy of positional information that occurs over the course of brain tumor resection are discussed. In addition, the process of integration of functional MRI and tractography into navigational models is reviewed. Finally, emerging concepts and future challenges relating to the development and implementation of experimental imaging technologies in the navigational environment are explored.
Keywords: brain tumor, frameless stereotaxy, intraoperative MRI, neuronavigation
Clinical localization of CNS lesions became possible as physicians began to understand the spatial organization of functional regions within the nervous system. The precision of anatomical and functional localization was enhanced dramatically as imaging studies such as angiography, air ventriculography and later, cross-sectional imaging modalities including computed tomography (CT) and MRI became available. In addition, the ability to pinpoint tumors and other nonneoplastic surgical targets was standardized and became increasingly precise as the stereotactic coordinate system and headframe emerged through the work of Leksell [1] and Spiegel [2]. The logistical challenges of frame-based stereotactic navigation were dramatically reduced as frameless navigation systems emerged [3]. Currently, data from multiple advanced imaging modalities can be combined within the singular reference frame of frameless stereotactic navigation systems to predict the location of a lesion as well as key neighboring structural and functional regions, which may be at risk during an operation. The use of an updated navigational dataset through the use of intraoperative imaging, diminishes the challenge of maintaining accurate navigational data, even when lesional and nonlesional structures shift over the course of an operation [4]. While there are many important applications of neuronavigation in the fields of functional, vascular and spinal neurosurgery, this review will explore the applications and future directions of frameless stereotactic navigation for neurosurgical oncology.
Conceptual basis for frameless stereotaxy
The components of frameless stereotactic navigation systems include a computer-based image processing module, a reference frame and a pointer that is recognized by an optical or electromagnetic detector. The basic arrangement for frameless stereotactic navigation relies on the spatial registration of anatomic landmarks in the operative environment to identical landmarks in a 3D model based on a reconstruction of cross-sectional imaging studies. The spatial coordinates of anatomic landmarks that are established through the use of a tracking system that pairs an optical or electromagnetic detector with a complimentary probe.
Tracking systems
The most-widely used tracking systems utilize dual infrared cameras that track the position of a probe relative to a fixed reference frame. The main limitation of infrared-based systems is the need for maintaining a direct line of sight between the camera, the reference frame and the probe during navigation [5]. Electromagnetic tracking systems are the major commercially available alternative to optical tracking systems. Electromagnetic-based navigation relies on the tracking of a probe within an electromagnetic field, created by a field generator in a fixed location. Line of sight issues are completely obviated by the use of electromagnetic field-based tracking. Additionally, the electromagnetic navigational probes are lower profile, making use in catheter placement and under the microscope more straightforward. Logistically, the main consideration with electromagnetic tracking is the relatively large size of the field generator and the need for its positioning close to the operative site. In addition, while concerns have been raised about the accuracy of electromagnetic field-based tracking systems given the presence of multiple ferromagnetic instruments in the operative field, the best available evidence suggests that this is rarely a problem in the clinical environment [6].
Navigational accuracy
Detailed clinical studies suggest that frameless stereotactic navigation can achieve positional accuracy comparable with that of frame-based stereotaxy. Electrode-based studies using pre- and post-operative MRI suggested that modern frameless methods for localization yield positional accuracy within 2–3 mm during surgery, which is equivalent to the accuracy of frame-based stereotaxy [7]. The error inherent in frameless stereotactic navigation systems relates to the accuracy of probe tracking as well as the quality of preoperative images and the method of image-to-patient registration [8]. Clinical factors that cause shift of the brain or a lesion, such as cerebrospinal fluid loss, cyst decompression and cerebral edema or sag, may also diminish navigational accuracy [9].
An important practical concern relating to navigational accuracy in the practice of neurosurgical oncology is error introduced by using images obtained days or weeks prior to an intervention [10]. Particularly for rapidly evolving neoplasms, navigation based on preoperative images is prone to error related to the changing geometry of the lesion.
Navigation based on an immediate preoperative scan obtained in the setting of an intraoperative MRI suite optimizes the accuracy of data used for initial surgical planning. Intraoperative MRI also represents a means of addressing the changing position of a lesion, as well as key normal structures throughout the course of an operation [11–13]. Shifts in the position of the brain or tumor related to resection, cyst decompression on removal of cerebrospinal fluid may cause profound errors in navigational data (Figure 1). By obtaining iterative intraoperative MRI, the surgical plan can be altered to account for shift in the position of critical cerebral structures as well as the lesion itself [14–16].
Figure 1. Comparison of preoperative and intraoperative magnetic resonance imaging to demonstrate shift and decompression encountered during surgery.
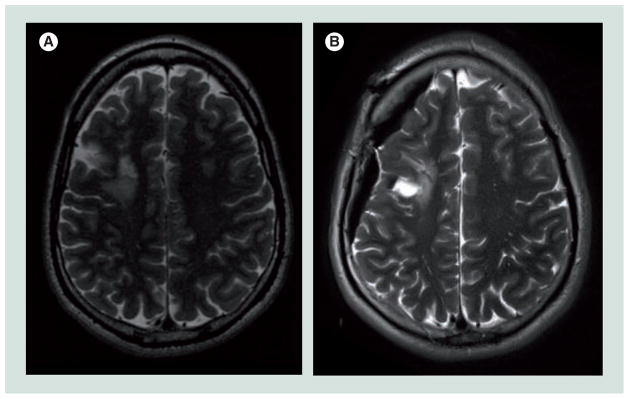
Note the shift in the position of the surface of the right hemisphere and lesion in the (A) preoperative and (B) intraoperative T2-weighted images.
Given the logistic limitations related to intraoperative MRI, ultrasound-based techniques to update navigational data have also been developed. Practically, ultrasound provides an excellent, rapid means of localizing a lesion and predicting the true geometric position of a lesion prior to durotomy during a brain tumor resection. In addition, ultrasound can be used as a substitute for iterative MRI imaging to evaluate for brain shift and other anatomic deformations experienced during brain tumor resection. One prototype device that can be used transcranially has been designed, constructed and shown to be effective in demonstrating positional changes in anatomic landmarks during surgery [17]. While ultrasound is a relatively poor way to assess the extent of tumor resection [18], it provides an excellent means of comparing preoperative and intraoperative anatomic data. The interactive brain imaging system, NeuroNav is one example of a navigational system capable of updating navigational data based on intraoperatively acquired ultrasound data [19]. In addition, the low cost and ease of use of ultrasound also makes it an attractive means of ensuring that navigational data is current [20].
Image registration
Image registration, the process of transforming images into a common coordinate system, is a key component of neuronavigation, especially when multiple modalities and intraoperative imaging data are incorporated into the navigational plan. Image registration can be accomplished by rigid or nonrigid methods. Rigid methods such as rotation and translation provide less accurate registration than nonrigid methods, which are designed to incorporate local shifts in brain and lesional tissue that occur during surgery. When intraoperative imaging data are used to update a navigational plan during surgery, nonrigid registration methods can be used to model the high dimensional spatial deformation caused by operative exposure. The basic process of nonrigid registration of two image datasets requires the creation of a spatial transformation that aligns homologous structures in the two datasets. An expression can be derived to describe the distance or similarity function, which estimates the distance between homologous structures in the transform, and R, the regularization function, which limits the possible transform function to anatomically plausible possibilities. While not commercially available at this time, it is likely that nonrigid registration algorithms will add to the navigational accuracy, especially when incorporating functional and intraoperative imaging datasets [21].
Applications of neuronavigation: brain biopsy
The need for a reliable means of accessing deep brain lesions with high accuracy has been recognized within neurosurgery since the early 1900s [8]. The earliest neuronavigational systems relied on an arc-centered stereotactic frame system in which points of interest in the surgical field could be correlated with points in the imaging data set. Frameless systems eliminate the need for attaching a rather uncomfortable and cumbersome frame to an awake patient’s head for imaging and intervention. Stereotactic frames also limit the possibilities for surgical positioning and exposure. Consequently, while frame-based stereotaxy is still used, particularly, in the setting of deep brain stimulation, frameless systems have been increasingly adopted for brain biopsy [22,23] and brain tumor surgery [24–26]. Importantly, frameless systems provide comparable accuracy with frame-based systems [27,28]. In addition, the use of frameless stereotaxy has been shown to be safe and effective in collecting diagnostic tissues in both eloquent and non-eloquent regions of the brain [29]. Although it is not currently standard practice, some authors have suggested that the accuracy and yield of frameless stereotactic biopsy is so high that intraoperative histopathology may not be necessary [30].
Conventional frameless stereotactic navigational systems require that the patient should be immobilized in a rigid head holder to ensure a fixed relationship between the reference frame and target tissue. Some neurosurgeons prefer to perform brain biopsies in patients under conscious sedation to eliminate the unwanted side effects of administration of general anesthetic. However, fixing an awake patient in a rigid head holder can be challenging. Future stereotactic navigational systems, currently under development, will contain a trackable component that can be affixed directly to the skull [31]. Skull-attached navigational systems allow the patient’s head to be positioned in a nonrigid way on a headrest and, consequently improve the patient’s comfort during the course of the procedure. The performance of skull-attached biopsy systems is acceptable for targets 4 mm or greater in size.
Currently, all systems for stereotactic biopsy rely on navigation based on preoperatively obtained images. As described above, navigation based on preoperatively obtained images can introduce an unacceptable degree of error, thereby decreasing the efficiency of biopsy. In a case series of 33 patients, intraoperative MRI obtained during biopsy was been shown to be effective in enabling real-time tracking of a biopsy needle and yielded diagnostic tissue in 32 patients [32]. While its implementation has been limited by logistical challenges, intraoperative MRI-assisted brain biopsy holds the promise of providing additional reassurance to the surgeon about the accuracy of brain biopsy, particularly for deep or small lesions in regions of the CNS that are difficult to access. A number of systems for brain biopsy in the setting of MRI are under development and are likely to become more widely adopted as intraoperative MRI gains popularity [33].
Applications of neuronavigation: brain tumor resection
Although it has been debated in neurosurgery for decades, complete and, if it is not possible, maximal safe resection of gliomas has long been suspected to have an important prognostic role in patient outcome. Recently, retrospective volumetric data analysis has been used to demonstrate a strong correlation between extent of resection and survival [34,35]. Frameless stereotactic neuronavigation is now a ubiquitous tool in planning a surgical approach for brain tumor resection. Neuronavigational data can be reconstructed in three dimensions to simulate a surgical approach prior to surgery. While the most commonly used platforms for preoperative planning rely on a standard 2D computer screen interface, some have developed 3D virtual reality environments that can be used to better simulate an operative approach [36–40].
Neuronavigation is most useful as an adjunct to other brain-mapping techniques such as awake mapping and electrocorticography in the resection of lesions within eloquent motor and language areas [41]. Neuronavigation is also commonly used in skull base tumors, especially for planning an operative trajectory in regions containing vital neurovascular structures [42] and may be used for cerebrovascular surgery [43].
Interestingly, however, evidence from a unique randomized controlled trial of 45 patients with solitary contrast-enhancing intracerebral tumors suggests that standard frameless neuronavigation does not influence extent of resection [44]. The question arises, if neuronavigation improves the surgeon’s ability to approach, assess and operate on brain tumors, and is widely used in modern neurosurgical practice, why is the effect on surgical outcome difficult to detect? One possible explanation is that the effect of neuronavigation on extent of resection may be attenuated by the known limitations of the technology. Specifically, real-time geometric changes in the anatomy of the brain, as well as the lesion itself are known to affect the accuracy and efficacy of neuronavigation. Some neurosurgeons have attempted to utilize neuronavigational data to insert catheters that can serve as ‘fence-posts’ that establish landmarks near the operative cavity that will shift as the brain and lesion shift over the course of surgery [45].
Alternatively, the use of intraoperative MRI and CT [46] can be used to address the changing geometry of the operative corridor and update the surgical plan. The first intraoperative MRI developed at the Brigham and Women’s Hospital in the 1990s was designed as a ‘double donut’ configuration with the surgeon standing in the gap between the two halves of the magnet. This configuration had the benefit of allowing for intraoperative image acquisition in an iterative fashion [47–50]. This design was the first to have an integrated navigation system that enabled continuous updating of the navigational dataset [51].
As intraoperative MRI has become more widespread, the evidence supporting the use of intraoperative MRI to maximize resection has also grown. A systematic review of existing data on the use of intraoperative MRI for glioma surgery revealed 12 high-quality cohort studies in the literature and concluded that level 2 evidence exists to support the use of intraoperative MRI results for improving extent of resection, quality of life and survival in glioma patients [52]. Moreover, in a unique survival analysis of 156 patients undergoing intraoperative MRI-assisted resection of low-grade gliomas, the risk of death was found to be 4.9-times greater in patients undergoing subtotal resection compared with those in which complete resection was achieved, underscoring the importance of the use to intraoperative imaging to ensure complete resection on patient outcome [53]. Until recently, however, the literature lacked a randomized controlled trial comparing neuronavigation with intraoperative MRI to neuronavigation alone. In 2011, Senft et al. reported a trial with 58 high-grade glioma patients undergoing neuronavigation with or without the use of intraoperative MRI, and demonstrated a dramatic improvement in the percentage of patients in which a radiographically complete resection was achieved (98% for intraoperative MRI group vs 68% for controls) [54].
Unfortunately, intraoperative MRI is also associated with significant drawbacks that have limited its widespread adoption. Among the most serious concerns related to intraoperative MRI are the costs and increased operative duration. Recent series have quantified the increase in operative time related to the use of intraoperative MRI [55,56]. Quantifying the cost–benefit ratio of intraoperative MRI for brain tumor surgery remains an area of active investigation [57].
Applications of neuronavigation: integration of functional imaging data
Advances in MRI imaging have enabled the possibility of incorporating functional data into the neuronavigational datasets [21]. There are currently two major types of functional datasets that are used for navigation: functional MRI (fMRI) and diffusion tensor imaging (DTI).
fMRI is based on blood-oxygen level dependent changes in cortical regions that occur during specific tasks [58]. Regions of blood-oxygen level-dependent changes can be overlayed onto structural MR images to create volumes representing active cortical regions. Active cortical volumes can be created or imported into navigational models (Figure 2B) and used intraoperatively [59]. Alternatively, fMRI navigational data can also be used to localize eloquent cortical and subcortical structures via electrophysiological methods [60].
Figure 2. Integration of diffusion tensor imaging and functional magnetic resonance imaging into 3D models for presurgical planning.
(A) 3D model after tumor segmentation (green). (B) Structural model with diffusion tensor imaging (DTI). (C) Structural model with functional MRI (fMRI). Hand activation (blue), foot activation (purple) and face activation (orange) are shown as volumes. (D) Structural model with both DTI and fMRI.
Colour figure can be found online at www.expert-reviews.com/doi/full/10.1586/erd.12.42
fMRI-based navigation has been proposed as a way to diminish the incidence of operative morbidity associated with both extra-axial [61] and intra-axial [62] tumors. The authors have found incorporating fMRI activation volumes into our neuronavigational models for brain tumor surgeries to be helpful in ensuring a safe surgical trajectory and strategy for resection (Figure 3). While some investigators have reported that fMRI is the most reliable means of localizing functional motor areas [63], others contend that cortical stimulation alone is adequate to guide maximal safe glioma resection [64]. The utility of fMRI for brain tumor resection in the future will rely on the continued correlation and refinement of fMRI activation areas with intraoperative mapping techniques and establishing standardized paradigms for cortical stimulation [65].
Figure 3. Selection of trajectory based on tractography.
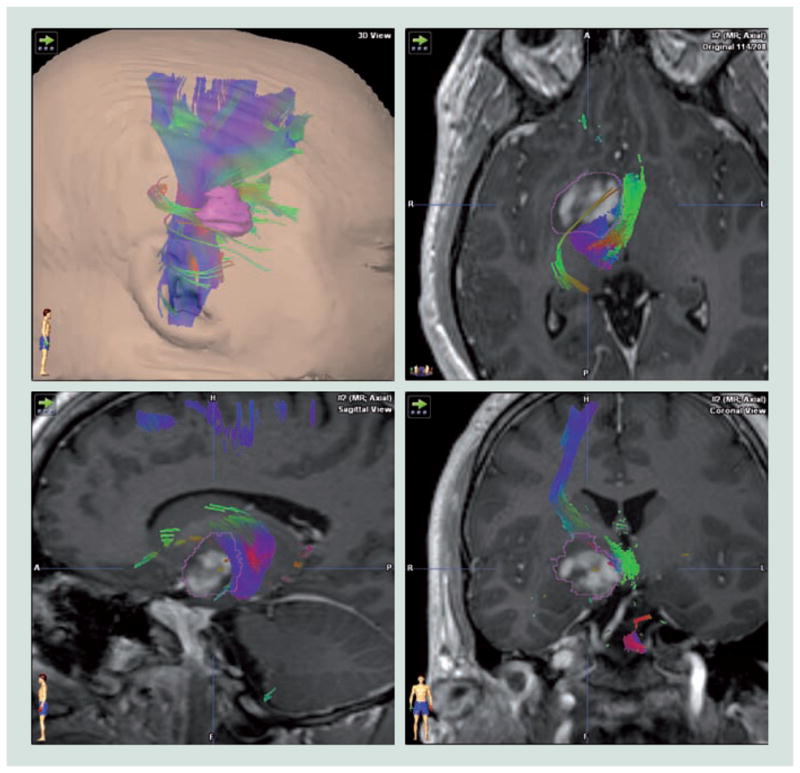
The thalamic region tumor, segmented with a pink outline shifts adjacent fiber tracts posterior and medially. Tractography suggested a lateral approach would be the safest route to access the lesion.
Colour figure can be found online at www.expert-reviews.com/doi/full/10.1586/erd.12.42
While fMRI may be used in a neuronavigational model to highlight key cortical structures, DTI is used to map subcortical anatomy. DTI is based on the preferential diffusion of water in the direction of white matter tracts within the CNS [66]. DTI is a unique tool used by neurosurgeons to better understand the relationship of a lesion to key subcortical fiber tracts that could be vulnerable during surgical exposure and tumor resection [67].
DTI data can be incorporated into navigational datasets to allow surgeons to plan and re-evaluate surgical trajectories in three dimensions to minimize the risk of surgical injury to white matter tracts (Figure 2A). Interestingly, in a randomized controlled trial of 238 patients comparing neuronavigation with or without DTI of the pyramidal tracts, Wu et al. demonstrated that extent of resection was higher and postoperative neurologic deficits were less frequent with the addition of DTI to the navigational dataset [68]. Importantly, DTI can also be acquired intraoperatively to create updated positional information of key white matter tracts. As with other structures in the brain, the position of key fiber tracts changes over the course of an operation [11]. Therefore, for DTI information to retain its value throughout an operation, it must be updated intraoperatively. Work is currently underway to streamline the process of incorporating intraoperative tractography into navigational models using computational methods [67].
Surgeons using DTI must keep in mind that subcortical stimulation remains as the gold-standard for ascertaining the position of functional white matter tracts during surgery. A recent report by Zolal et al. in 36 patients who underwent subcortical white matter stimulation during brain tumor resection suggests that DTI provides a rough estimate of the true location of the corticospinal tract [69]. Generally, a response can be elicited within 8 mm of the location of the DTI-predicted corticospinal tract but distances of 0–15 mm for stimulation to tract distance have been reported [70,71]. Many factors such as tissue conductance, anatomical stimulation point (subcortical versus, internal capsule and brainstem) and stimulation parameters also affect the accuracy of subcortical white matter mapping. Similarly, MRI parameters and artifacts may also affect the accuracy of DTI. Consequently a combination of subcortical stimulation and DTI-informed navigation is likely to provide the most robust data for decision-making during the resection of lesions near key white matter tracts.
Applications of neuronavigation: pituitary surgery
While the transphenoidal route for resection of pituitary adenomas eliminates the need for craniotomy to expose the pituitary gland, it creates narrow access to a deep region of the skull rich with critical neurovascular structures. To ensure an appropriate trajectory, image-guidance has been used in transphenoidal surgeries for decades. The evolution of image-guidance in pituitary surgery has included the use of intraoperative fluoroscopy, CT, ultrasound and MRI. Both electromagnetic [72] and optical [73] navigational systems have been validated and used successfully in the setting of pituitary adenoma resection. The greatest advantages of the use of navigational systems in pituitary surgery include improvements in safety, a decrease in the duration of surgery and radiation exposure.
In a recent review of 208 pituitary adenoma cases, Eboli et al. reported integration of intraoperative CT data with preoperative MR data into an electromagnetic-based navigational system and demonstrated 100% concordance between bony landmarks in the imaging dataset with the probe location intraoperatively. In addition, the use of intraoperative CT-based navigation decreased operative duration by 10 min [74]. The use of intra-operative MRI in pituitary surgery affords surgeons the added benefit of evaluating the extent of resections during surgery. Similar to intraoperative CT data, intraoperative MRI data can be incorporated into the navigational dataset. In a case series of 32 patients undergoing pituitary surgery with the assistance of low-field intraoperative MRI, subtotal resection was encountered intraoperatively in 15 patients, eight of whom underwent additional resection [75]. A larger study of 40 patients undergoing pituitary surgery with the guidance of intraoperative MRI found a lower rate of subtotal resection (17.5%) and found that decompression of the optic chiasm, an important objective of pituitary surgery was achieved in all of the patients in their series [76]. When intraoperative CT or MR is not available, highly accurate 3D navigational datasets may also be acquired by the use of a C-arm alone [77].
The use of endoscopy to improve visualization in the trans-sphenoidal approach has become widespread in neurosurgery and has recently been reviewed elsewhere [78]. The integration of endoscopes with the navigational system offers the surgeon the opportunity to view a surgical view alongside navigational data and has been shown to be feasible in several large retrospective case series studies of operations for sellar lesions [79,80]. In the future, active tracking of the endoscope, developed for other neurosurgical applications [81], may also be helpful in improving navigation during pituitary surgeries.
Neuronavigation based on emerging imaging modalities
The goal of brain tumor resection is to safely reduce the number of tumor cells. However, surgeons currently lack a means for visualizing tumors at the cellular level. Several recent reports suggest a possible solution to this problem: intraoperative confocal microscopy [82,83]. After the administration of a fluorescent contrast agent, such as fluorescein, a handheld confocal probe can be inserted into the operative field to generate histologic images. With the confocal probe or point of interest registered to the navigational dataset, the histologic appearance can be correlated with the MR appearance of the lesion [84]. The limitations of confocal microscopy-based neuronavigation lie in the limited distribution and performance of contrast agents as well as the quality of the confocal images achieved.
Mass spectrometry has also been recently proposed as a method uniting neuronavigation with molecular imaging. Recent advances in mass spectrometry technology have created the possibility of creating a clinical probe, which could be registered within the neuronavigational environment to generate a chemical map of points within an operative cavity [85]. Specific points of interest in a navigational dataset could be sampled to determine whether their chemical signal is more consistent with normal brain or lesional tissue. As the mass spectral characteristics of brain tumors are increasingly well understood, it may be possible to make a decision about whether a given region of tissue contains resectable tumor [86]. The clinical success of mass spectrometry in brain tumor resection will require further validation of the technology in clinical studies.
Another nonimage-based dataset that has been incorporated into neuronavigational models is transcranial magnetic stimulation (TMS). TMS systems rely on correlating the activation of corticospinal tracts via magnetic stimulation of specific foci within the motor cortex and distal muscular activation. Points of activation within the motor cortex can be incorporated into navigational datasets at the time of surgery. TMS systems produce navigable datasets that are closely correlated with activation areas determined by direct cortical stimulation [87]. Additional clinical evidence suggests that TMS may also be used for mapping and navigation within language areas [88].
Integrating neuronavigational information into the operative workflow
The use of neuronavigation for planning an appropriately positioned and sized craniotomy cannot be underestimated. Some authors argue that this is the central role of a neuronavigational system in neurosurgery [89,90]. However, placement of a craniotomy based heavily on neuronavigational systems may be flawed. Virtual reality systems allowing 3D planning environments can diminish the error related to projecting a lesion onto the surface of the skin for estimating the size of a craniotomy [36]. Moreover, 3D multimodality data, such as fMRI and DTI, can be incorporated into 3D models to plan ideal approaches. By allowing a unique perspective into navigational data, virtual reality planning systems enable the possibility of neurosurgical approaches that are minimally invasive [91].
One of the limitations of most navigational systems is that positional information used during surgery is displayed outside of the operative field. Therefore, to access navigational information, surgeons must take their attention away from the operative field and focus elsewhere on a computer monitor. Increasingly, commonly utilized navigational systems produced by Brainlab and Medtronic are enabling navigational information to be injected into the display within an operating microscope. In our experience, commercially available systems for navigational data injection are helpful but have not been widely embraced by neurosurgeons (Figure 4). Advancements in data rendering within the microscopic view will be required for data injection to become a true solution to the surgeon’s challenge of trying to integrate information displayed on a computer screen into the operative field.
Figure 4. Injection of navigational information into microscopic view.
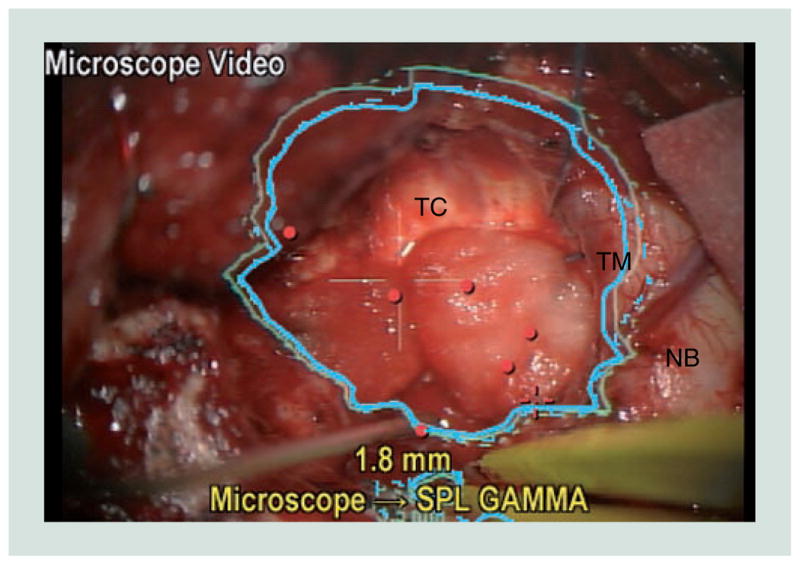
In this view generated by the neuronavigational system, an image of the microscope video feed in the bottom left-hand corner demonstrates the surgeon’s view of the outline (blue) of the preoperatively segmented lesion that is being resected.
NB: Normal brain adjacent to the operative cavity; TC: Tumor cavity; TM: Tumor margin.
Colour figure can be found online at www.expert-reviews.com/doi/full/10.1586/erd.12.42
Some have suggested integrating auditory feedback into the navigational environment as a means of eliminating the need to consult a computer screen to determine if an operative approach is threatening to involve key structures. In one such system, the surgeon would hear a warning sign if registered instruments strayed into predetermined eloquent regions [92].
In the future, it may be possible for surgeons to use ‘smart’ tools that can integrate navigational information as they are deployed. While a number of robots have been developed for surgical applications, the MR-compatible NeuroArm robotic arm system has specific characteristics that make it well suited for application within a neurosurgical environment. First, the NeuroArm system can integrate pre- and intra-operative imaging data to determine a trajectory to perform stereotactic and microneurosurgical maneuvers. In addition, NeuroArm was designed to offer easy tool exchange and the possibility of using standard neurosurgical instruments. NeuroArm is designed to recreate the multisensory cues that surgeons experience, including auditory, visual and tactile feedback. Tactile feedback is achieved via haptic technology incorporating titanium multi-axis torque/force sensors, which increases efficiency and decreases errors in the operative environment. The NeuroArm can also be programmed to prevent a surgeon from entering a ‘no-go zone’ or predefined region, such as the internal capsule, that may cause neurologic deficit if manipulated [93]. Initial studies have developed the feasibility of using NeuroArm to assist in brain tumor resection [94]. Further clinical studies are required to determine the feasibility of integrating robotics into the workflow of neurosurgery.
Critical interpretation of navigational data
Frameless stereotaxy has revolutionized the workflow for brain tumor surgery. Nonetheless, neuronavigational systems and the data they generate are fallible. Navigational errors can be introduced in each step of the use of a neuronavigational platform. Specifically, the type of imaging modality (CT/MR/ultrasound) [95], slice thickness of cross-sectional imaging, tracking modality, image to patient registration and brain shift are among the key sources of navigational inaccuracy [9,96]. A quantitative description of various sources of error has recently been reviewed [8]. Given the multiple sources of error, and the importance of maintaining an accurate navigational dataset, it is essential that the surgeon relies on specific landmarks, both superficial and deep, that can be used to verify navigational accuracy. Large cortical veins are useful anatomic landmarks that shift with the brain during surgery. Dural anatomy such as the tentorium and falx are similarly useful and are largely static over the course of an operation. Incorporating both navigational data and the intraoperative assessment of key landmarks is likely to result in the highest quality positional information for surgical decision-making. There remains no substitute for common sense for guiding surgical intervention regardless of tools, such as neuronavigational systems that may be of assistance.
Expert commentary & five-year view
In the coming years, we anticipate that the evidence supporting the importance of achieving maximal resection of brain tumors will continue to accumulate. It is also likely that the data will continue to support the hypothesis that technologic innovations are vital to ensuring that optimal surgical outcomes are achieved. The most widely used neuronavigational systems today are limited by their reliance on preoperative images. Future systems will integrate updated imaging data, most likely acquired via intra-operative MRI, to improve navigational accuracy. Consequently, neuronavigational systems that interface well with intraoperative MRI, both from a data standpoint, and from the standpoint of operative workflow, are likely to become the preferred tools for brain tumor biopsy and resection.
In the future, as the capacity for multimodality neuronavigation becomes incorporated into commercially available clinical systems, surgeons will have a growing armamentarium of tools to facilitate the biopsy and resection of brain tumors. We feel that multimodality neuronavigation incorporating ultrasound, functional MR imaging and tractography will become increasingly used as adjuncts to structural MRI in planning and executing safe, efficient interventions for brain tumors located in eloquent regions. In addition, integration of optical and molecular imaging technologies into the navigational environment holds great promise for improving the safety and efficiency of brain tumor resections.
Given the increasing necessity for cost-effective delivery of medical care, the importance of well-designed studies to quantify the value of specific neuronavigational technologies will be vital. By improving surgical accuracy, we feel that the use of neuronavigational technology can, in some cases, reduce cost by obviating the need for reoperation for brain tumor biopsy or brain tumor re-resection. In addition, by enabling gross total resection among patients with resectable tumors, neuronavigational systems may diminish costs by decreasing the likelihood of recurrence. The ultimate validation of evolving technologies in neuronavigation will be designed to measure the clinical and financial impact of the surgeon’s ability to safely deliver maximal resection of brain tumors.
Key issues.
Navigation based on preoperative imaging alone, is limited by decreasing accuracy as an operation progresses. Therefore, maintaining an updated navigational dataset through continuous or iterative imaging is required for maximal accuracy during brain tumor operations.
Combining ultrasound, structural MRI, functional MRI and diffusion tensor imaging provides important multimodal information that can improve the safety and efficacy of brain tumor surgery.
Integration of currently available and emerging multimodality imaging techniques in a user-friendly interface is likely to improve the accuracy and usefulness of the navigational dataset.
Emerging strategies for navigation such as navigated intraoperative microscopy and molecular imaging should be validated through clinical studies examining their efficacy and cost efficiency.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants P41EB015898 (F Jolesz), P41RR019703 (F Jolesz) and R25CA089017 (DA Orringer). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact reprints@expert-reviews.com
References
- 1.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316–319. [PubMed] [Google Scholar]
- 2.Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic apparatus for operations on the human brain. Science. 1947;106(2754):349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 3.Roberts DW, Strohbehn JW, Hatch JF, Murray W, Kettenberger H. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65(4):545–549. doi: 10.3171/jns.1986.65.4.0545. [DOI] [PubMed] [Google Scholar]
- 4.Jolesz FA, Kikinis R, Talos IF. Neuronavigation in interventional MR imaging. Frameless stereotaxy. Neuroimaging Clin N Am. 2001;11(4):685–693. ix. [PubMed] [Google Scholar]
- 5.Cleary K, Peters TM. Image-guided interventions: technology review and clinical applications. Annu Rev Biomed Eng. 2010;12:119–142. doi: 10.1146/annurev-bioeng-070909-105249. [DOI] [PubMed] [Google Scholar]
- 6.Mascott CR. Comparison of magnetic tracking and optical tracking by simultaneous use of two independent frameless stereotactic systems. Neurosurgery. 2005;57(4 Suppl):295–301. doi: 10.1227/01.neu.0000176411.55324.1e. discussion 295. [DOI] [PubMed] [Google Scholar]
- 7.Mascott CR. In vivo accuracy of image guidance performed using optical tracking and optimized registration. J Neurosurg. 2006;105(4):561–567. doi: 10.3171/jns.2006.105.4.561. [DOI] [PubMed] [Google Scholar]
- 8.Widmann G, Schullian P, Ortler M, Bale R. Frameless stereotactic targeting devices: technical features, targeting errors and clinical results. Int J Med Robot. 2012;8(1):1–16. doi: 10.1002/rcs.441. [DOI] [PubMed] [Google Scholar]
- 9.Spetzger U, Hubbe U, Struffert T, et al. Error analysis in cranial neuronavigation. Minim Invasive Neurosurg. 2002;45(1):6–10. doi: 10.1055/s-2002-23583. [DOI] [PubMed] [Google Scholar]
- 10.Gasser T, Szelenyi A, Senft C, et al. Intraoperative MRI and functional mapping. Acta Neurochir Suppl. 2011;109:61–65. doi: 10.1007/978-3-211-99651-5_10. [DOI] [PubMed] [Google Scholar]
- 11.Romano A, D’Andrea G, Calabria LF, et al. Pre- and intraoperative tractographic evaluation of corticospinal tract shift. Neurosurgery. 2011;69(3):696–704. doi: 10.1227/NEU.0b013e31821a8555. discussion 704. [DOI] [PubMed] [Google Scholar]
- 12.Nabavi A, Black PM, Gering DT, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48(4):787–797. doi: 10.1097/00006123-200104000-00019. discussion 797. [DOI] [PubMed] [Google Scholar]
- 13.Warfield SK, Haker SJ, Talos IF, et al. Capturing intraoperative deformations: research experience at Brigham and Women’s Hospital. Med Image Anal. 2005;9(2):145–162. doi: 10.1016/j.media.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Ferrant M, Nabavi A, Macq B, Jolesz FA, Kikinis R, Warfield SK. Registration of 3-D intraoperative MR images of the brain using a finite-element biomechanical model. IEEE Trans Med Imaging. 2001;20(12):1384–1397. doi: 10.1109/42.974933. [DOI] [PubMed] [Google Scholar]
- 15.Ferrant M, Nabavi A, Macq B, et al. Serial registration of intraoperative MR images of the brain. Med Image Anal. 2002;6(4):337–359. doi: 10.1016/s1361-8415(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 16.Hartkens T, Hill DL, Castellano-Smith AD, et al. Measurement and analysis of brain deformation during neurosurgery. IEEE Trans Med Imaging. 2003;22(1):82–92. doi: 10.1109/TMI.2002.806596. [DOI] [PubMed] [Google Scholar]
- 17.White PJ, Whalen S, Tang SC, Clement GT, Jolesz F, Golby AJ. An intraoperative brain shift monitor using shear mode transcranial ultrasound: preliminary results. J Ultrasound Med. 2009;28(2):191–203. doi: 10.7863/jum.2009.28.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rygh OM, Selbekk T, Torp SH, Lydersen S, Hernes TA, Unsgaard G. Comparison of navigated 3D ultrasound findings with histopathology in subsequent phases of glioblastoma resection. Acta Neurochir (Wien) 2008;150(10):1033–1041. doi: 10.1007/s00701-008-0017-3. discussion 1042. [DOI] [PubMed] [Google Scholar]
- 19.Mercier L, Del Maestro RF, Petrecca K, et al. New prototype neuronavigation system based on preoperative imaging and intraoperative freehand ultrasound: system description and validation. Int J Comput Assist Radiol Surg. 2011;6(4):507–522. doi: 10.1007/s11548-010-0535-3. [DOI] [PubMed] [Google Scholar]
- 20.Moiyadi A, Shetty P. Objective assessment of utility of intraoperative ultrasound in resection of central nervous system tumors: A cost-effective tool for intraoperative navigation in neurosurgery. J Neurosci Rural Pract. 2011;2(1):4–11. doi: 10.4103/0976-3147.80077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risholm P, Golby AJ, Wells W., 3rd Multimodal image registration for preoperative planning and image-guided neurosurgical procedures. Neurosurg Clin N Am. 2011;22(2):197–206. viii. doi: 10.1016/j.nec.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriarty TM, Quinones-Hinojosa A, Larson PS, et al. Frameless stereotactic neurosurgery using intraoperative magnetic resonance imaging: stereotactic brain biopsy. Neurosurgery. 2000;47(5):1138–1145. doi: 10.1097/00006123-200011000-00023. discussion 1145. [DOI] [PubMed] [Google Scholar]
- 23.Martin AJ, Hall WA, Roark C, Starr PA, Larson PS, Truwit CL. Minimally invasive precision brain access using prospective stereotaxy and a trajectory guide. J Magn Reson Imaging. 2008;27(4):737–743. doi: 10.1002/jmri.21318. [DOI] [PubMed] [Google Scholar]
- 24.McInerney J, Roberts DW. Frameless stereotaxy of the brain. Mt Sinai J Med. 2000;67(4):300–310. [PubMed] [Google Scholar]
- 25.Kettenbach J, Wong T, Kacher D, et al. Computer-based imaging and interventional MRI: applications for neurosurgery. Comput Med Imaging Graph. 1999;23(5):245–258. doi: 10.1016/s0895-6111(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 26.Chinzei Warfield, Hata Tempany, Jolesz Kikinis. Planning, simulation and assistance with intraoperative MRI. Minim Invasive Ther Allied Technol. 2003;12(1):59–64. doi: 10.1080/13645700310001531. [DOI] [PubMed] [Google Scholar]
- 27.Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJ, Vincent AJ. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien) 2008;150(1):23–29. doi: 10.1007/s00701-007-1473-x. [DOI] [PubMed] [Google Scholar]
- 28.Lobão CA, Nogueira J, Souto AA, Oliveira JA. Cerebral biopsy: comparison between frame-based stereotaxy and neuronavigation in an oncology center. Arq Neuropsiquiatr. 2009;67(3B):876–881. doi: 10.1590/s0004-282x2009000500018. [DOI] [PubMed] [Google Scholar]
- 29.Air EL, Leach JL, Warnick RE, McPherson CM. Comparing the risks of frameless stereotactic biopsy in eloquent and noneloquent regions of the brain: a retrospective review of 284 cases. J Neurosurg. 2009;111(4):820–824. doi: 10.3171/2009.3.JNS081695. [DOI] [PubMed] [Google Scholar]
- 30.Shooman D, Belli A, Grundy PL. Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J Neurosurg. 2010;113(2):170–178. doi: 10.3171/2009.12.JNS09573. [DOI] [PubMed] [Google Scholar]
- 31.Amin DV, Lozanne K, Parry PV, Engh JA, Seelman K, Mintz A. Image-guided frameless stereotactic needle biopsy in awake patients without the use of rigid head fixation. J Neurosurg. 2011;114(5):1414–1420. doi: 10.3171/2010.7.JNS091493. [DOI] [PubMed] [Google Scholar]
- 32.Quinn J, Spiro D, Schulder M. Stereotactic brain biopsy with a low-field intraoperative magnetic resonance imager. Neurosurgery. 2011;68(1 Suppl Operative):217–224. doi: 10.1227/NEU.0b013e31820826c2. discussion 224. [DOI] [PubMed] [Google Scholar]
- 33.Schulder M, Spiro D. Intraoperative MRI for stereotactic biopsy. Acta Neurochir Suppl. 2011;109:81–87. doi: 10.1007/978-3-211-99651-5_13. [DOI] [PubMed] [Google Scholar]
- 34.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 35.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 36.Stadie AT, Kockro RA, Serra L, et al. Neurosurgical craniotomy localization using a virtual reality planning system versus intraoperative image-guided navigation. Int J Comput Assist Radiol Surg. 2011;6(5):565–572. doi: 10.1007/s11548-010-0529-1. [DOI] [PubMed] [Google Scholar]
- 37.Gering DT, Nabavi A, Kikinis R, et al. An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imaging. 2001;13(6):967–975. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 38.Nabavi A, Gering DT, Kacher DF, et al. Surgical navigation in the open MRI. Acta Neurochir Suppl. 2003;85:121–125. doi: 10.1007/978-3-7091-6043-5_17. [DOI] [PubMed] [Google Scholar]
- 39.Gleason PL, Kikinis R, Altobelli D, et al. Video registration virtual reality for nonlinkage stereotactic surgery. Stereotact Funct Neurosurg. 1994;63(1–4):139–143. doi: 10.1159/000100305. [DOI] [PubMed] [Google Scholar]
- 40.Kikinis R, Gleason PL, Moriarty TM, et al. Computer-assisted interactive three-dimensional planning for neurosurgical procedures. Neurosurgery. 1996;38(4):640–649. discussion 649. [PubMed] [Google Scholar]
- 41.Walter J, Kuhn SA, Waschke A, Kalff R, Ewald C. Operative treatment of subcortical metastatic tumours in the central region. J Neurooncol. 2011;103(3):567–573. doi: 10.1007/s11060-010-0420-5. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura M, Krauss JK. Image-guided resection of small lesions in the cavernous sinus and Meckel’s cave. Eur J Surg Oncol. 2010;36(2):208–213. doi: 10.1016/j.ejso.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima S, Atsumi H, Bhalerao AH, et al. Computer-assisted surgical planning for cerebrovascular neurosurgery. Neurosurgery. 1997;41(2):403–409. doi: 10.1097/00006123-199708000-00013. discussion 409. [DOI] [PubMed] [Google Scholar]
- 44.Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg. 2006;104(3):360–368. doi: 10.3171/jns.2006.104.3.360. [DOI] [PubMed] [Google Scholar]
- 45.Kajiwara K, Yoshikawa K, Ideguchi M, et al. Navigation-guided fence-post tube technique for resection of a brain tumor: technical note. Minim Invasive Neurosurg. 2010;53(2):86–90. doi: 10.1055/s-0030-1249053. [DOI] [PubMed] [Google Scholar]
- 46.Uhl E, Zausinger S, Morhard D, et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009;64(5 Suppl 2):231–239. doi: 10.1227/01.NEU.0000340785.51492.B5. discussion 239. [DOI] [PubMed] [Google Scholar]
- 47.Black PM, Moriarty T, Alexander E, 3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41(4):831–842. doi: 10.1097/00006123-199710000-00013. discussion 842. [DOI] [PubMed] [Google Scholar]
- 48.Jolesz FA, Nabavi A, Kikinis R. Integration of interventional MRI with computer-assisted surgery. J Magn Reson Imaging. 2001;13(1):69–77. doi: 10.1002/1522-2586(200101)13:1<69::aid-jmri1011>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 49.Black PM, Alexander E, 3rd, Martin C, et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45(3):423–431. doi: 10.1097/00006123-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz RB, Hsu L, Wong TZ, et al. Intraoperative MR imaging guidance for intracranial neurosurgery: experience with the first 200 cases. Radiology. 1999;211(2):477–488. doi: 10.1148/radiology.211.2.r99ma26477. [DOI] [PubMed] [Google Scholar]
- 51.Schenck JF, Jolesz FA, Roemer PB, et al. Superconducting open-configuration MR imaging system for image-guided therapy. Radiology. 1995;195(3):805–814. doi: 10.1148/radiology.195.3.7754014. [DOI] [PubMed] [Google Scholar]
- 52.Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 2011;12(11):1062–1070. doi: 10.1016/S1470-2045(11)70130-9. [DOI] [PubMed] [Google Scholar]
- 53.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 54.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 55.Shah MN, Leonard JR, Inder G, et al. Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr. 2012;9(3):259–264. doi: 10.3171/2011.12.PEDS11227. [DOI] [PubMed] [Google Scholar]
- 56.Hirschberg H, Samset E, Hol PK, Tillung T, Lote K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg. 2005;48(2):77–84. doi: 10.1055/s-2004-830225. [DOI] [PubMed] [Google Scholar]
- 57.Makary M, Chiocca EA, Erminy N, et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging. 2011;34(5):1022–1030. doi: 10.1002/jmri.22739. [DOI] [PubMed] [Google Scholar]
- 58.Belliveau JW, Kennedy DN, Jr, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 59.Nimsky C, Kuhnt D, Ganslandt O, Buchfelder M. Multimodal navigation integrated with imaging. Acta Neurochir Suppl. 2011;109:207–214. doi: 10.1007/978-3-211-99651-5_32. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi F, Takahashi H, Teramoto A. Navigation-assisted subcortical mapping: intraoperative motor tract detection by bipolar needle electrode in combination with neuronavigation system. J Neurooncol. 2009;93(1):121–125. doi: 10.1007/s11060-009-9847-y. [DOI] [PubMed] [Google Scholar]
- 61.Li F, Lin J, Zhu G, et al. Neuroimaging and functional navigation as potential tools to reduce the incidence of surgical complications of lateral ventricular meningiomas. Clin Neurol Neurosurg. 2011;113(7):564–569. doi: 10.1016/j.clineuro.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 62.Xie J, Chen XZ, Jiang T, et al. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with gliomas involving the motor cortical areas. Chin Med J. 2008;121(7):631–635. [PubMed] [Google Scholar]
- 63.Shinoura N, Yamada R, Tabei Y, Saito K, Suzuki Y, Yagi K. Advantages and disadvantages of awake surgery for brain tumours in the primary motor cortex: institutional experience and review of literature. Br J Neurosurg. 2011;25(2):218–224. doi: 10.3109/02688697.2010.505671. [DOI] [PubMed] [Google Scholar]
- 64.Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within ‘noneloquent’ areas in the left dominant hemisphere: toward a ‘supratotal’ resection. Clinical article J Neurosurg. 2011;115(2):232–239. doi: 10.3171/2011.3.JNS101333. [DOI] [PubMed] [Google Scholar]
- 65.Vlieger EJ, Majoie CB, Leenstra S, Den Heeten GJ. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. Eur Radiol. 2004;14(7):1143–1153. doi: 10.1007/s00330-004-2328-y. [DOI] [PubMed] [Google Scholar]
- 66.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 67.Elhawary H, Liu H, Patel P, et al. Intraoperative real-time querying of white matter tracts during frameless stereotactic neuronavigation. Neurosurgery. 2011;68(2):506–516. doi: 10.1227/NEU.0b013e3182036282. discussion 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935–948. doi: 10.1227/01.neu.0000303189.80049.ab. discussion 948. [DOI] [PubMed] [Google Scholar]
- 69.Zolal A, Hejcl A, Vachata P, et al. The use of diffusion tensor images of the corticospinal tract in intrinsic brain tumor surgery: a comparison with direct subcortical stimulation. Neurosurgery. 2012;71(2):331–340. doi: 10.1227/NEU.0b013e31825b1c18. [DOI] [PubMed] [Google Scholar]
- 70.Maesawa S, Fujii M, Nakahara N, Watanabe T, Wakabayashi T, Yoshida J. Intraoperative tractography and motor evoked potential (MEP) monitoring in surgery for gliomas around the corticospinal tract. World Neurosurg. 2010;74(1):153–161. doi: 10.1016/j.wneu.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 71.Prabhu SS, Gasco J, Tummala S, Weinberg JS, Rao G. Intraoperative magnetic resonance imaging-guided tractography with integrated monopolar subcortical functional mapping for resection of brain tumors. Clinical article J Neurosurg. 2011;114(3):719–726. doi: 10.3171/2010.9.JNS10481. [DOI] [PubMed] [Google Scholar]
- 72.Hayhurst C, Byrne P, Eldridge PR, Mallucci CL. Application of electromagnetic technology to neuronavigation: a revolution in image-guided neurosurgery. J Neurosurg. 2009;111(6):1179–1184. doi: 10.3171/2008.12.JNS08628. [DOI] [PubMed] [Google Scholar]
- 73.Elias WJ, Chadduck JB, Alden TD, Laws ER., Jr Frameless stereotaxy for transsphenoidal surgery. Neurosurgery. 1999;45(2):271–275. doi: 10.1097/00006123-199908000-00015. discussion 275. [DOI] [PubMed] [Google Scholar]
- 74.Eboli P, Shafa B, Mayberg M. Intraoperative computed tomography registration and electromagnetic neuronavigation for transsphenoidal pituitary surgery: accuracy and time effectiveness. J Neurosurg. 2011;114(2):329–335. doi: 10.3171/2010.5.JNS091821. [DOI] [PubMed] [Google Scholar]
- 75.Berkmann S, Fandino J, Zosso S, Killer HE, Remonda L, Landolt H. Intraoperative magnetic resonance imaging and early prognosis for vision after transsphenoidal surgery for sellar lesions. J Neurosurg. 2011;115(3):518–527. doi: 10.3171/2011.4.JNS101568. [DOI] [PubMed] [Google Scholar]
- 76.Gerlach R, du Mesnil de Rochemont R, Gasser T, et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63(2):272–284. doi: 10.1227/01.NEU.0000312362.63693.78. discussion 84–85. [DOI] [PubMed] [Google Scholar]
- 77.Fox WC, Wawrzyniak S, Chandler WF. Intraoperative acquisition of three-dimensional imaging for frameless stereotactic guidance during transsphenoidal pituitary surgery using the Arcadis Orbic System. J Neurosurg. 2008;108(4):746–750. doi: 10.3171/JNS/2008/108/4/0746. [DOI] [PubMed] [Google Scholar]
- 78.Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic skull base surgery: a comprehensive comparison with open transcranial approaches. Br J Neurosurg. 2012;26(5):637–648. doi: 10.3109/02688697.2012.654837. [DOI] [PubMed] [Google Scholar]
- 79.Yano S, Kawano T, Kudo M, et al. Endoscopic endonasal transsphenoidal approach through the bilateral nostrils for pituitary adenomas. Neurol Med Chir (Tokyo) 2009;49(1):1–7. doi: 10.2176/nmc.49.1. [DOI] [PubMed] [Google Scholar]
- 80.Zhao B, Wei YK, Li GL, et al. Extended transsphenoidal approach for pituitary adenomas invading the anterior cranial base, cavernous sinus, and clivus: a single-center experience with 126 consecutive cases. J Neurosurg. 2010;112(1):108–117. doi: 10.3171/2009.3.JNS0929. [DOI] [PubMed] [Google Scholar]
- 81.Schulz M, Bohner G, Knaus H, Haberl H, Thomale UW. Navigated endoscopic surgery for multiloculated hydrocephalus in children. J Neurosurg Pediatr. 2010;5(5):434–442. doi: 10.3171/2010.1.PEDS09359. [DOI] [PubMed] [Google Scholar]
- 82.Martirosyan NL, Cavalcanti DD, Eschbacher JM, et al. Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. J Neurosurg. 2011;115(6):1131–1138. doi: 10.3171/2011.8.JNS11559. [DOI] [PubMed] [Google Scholar]
- 83.Eschbacher J, Martirosyan NL, Nakaji P, et al. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J Neurosurg. 2012;116(4):854–860. doi: 10.3171/2011.12.JNS11696. [DOI] [PubMed] [Google Scholar]
- 84.Sanai N, Eschbacher J, Hattendorf G, et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery. 2011;68(2 Suppl Operative):282–290. doi: 10.1227/NEU.0b013e318212464e. discussion 290. [DOI] [PubMed] [Google Scholar]
- 85.Agar NY, Golby AJ, Ligon KL, et al. Development of stereotactic mass spectrometry for brain tumor surgery. Neurosurgery. 2011;68(2):280–289. doi: 10.1227/NEU.0b013e3181ff9cbb. discussion 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eberlin LS, Norton I, Dill AL, et al. Classifying human brain tumors by lipid imaging with mass spectrometry. Cancer Res. 2012;72(3):645–654. doi: 10.1158/0008-5472.CAN-11-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forster MT, Hattingen E, Senft C, Gasser T, Seifert V, Szelényi A. Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: advanced adjuncts in preoperative planning for central region tumors. Neurosurgery. 2011;68(5):1317–1324. doi: 10.1227/NEU.0b013e31820b528c. discussion 1324. [DOI] [PubMed] [Google Scholar]
- 88.Shamov T, Spiriev T, Tzvetanov P, Petkov A. The combination of neuronavigation with transcranial magnetic stimulation for treatment of opercular gliomas of the dominant brain hemisphere. Clin Neurol Neurosurg. 2010;112(8):672–677. doi: 10.1016/j.clineuro.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Wagner W, Gaab MR, Schroeder HW, Tschiltschke W. Cranial neuronavigation in neurosurgery: assessment of usefulness in relation to type and site of pathology in 284 patients. Minim Invasive Neurosurg. 2000;43(3):124–131. doi: 10.1055/s-2000-8332. [DOI] [PubMed] [Google Scholar]
- 90.Spivak CJ, Pirouzmand F. Comparison of the reliability of brain lesion localization when using traditional and stereotactic image-guided techniques: a prospective study. J Neurosurg. 2005;103(3):424–427. doi: 10.3171/jns.2005.103.3.0424. [DOI] [PubMed] [Google Scholar]
- 91.Stadie AT, Kockro RA, Reisch R, et al. Virtual reality system for planning minimally invasive neurosurgery. Technical note J Neurosurg. 2008;108(2):382–394. doi: 10.3171/JNS/2008/108/2/0382. [DOI] [PubMed] [Google Scholar]
- 92.Woerdeman PA, Willems PW, Noordmans HJ, van der Sprenkel JW. Auditory feedback during frameless image-guided surgery in a phantom model and initial clinical experience. J Neurosurg. 2009;110(2):257–262. doi: 10.3171/2008.3.17431. [DOI] [PubMed] [Google Scholar]
- 93.Sutherland GR, Latour I, Greer AD. Integrating an image-guided robot with intraoperative MRI: a review of the design and construction of NeuroArm. IEEE Eng Med Biol Mag. 2008;27(3):59–65. doi: 10.1109/EMB.2007.910272. [DOI] [PubMed] [Google Scholar]
- 94.Pandya S, Motkoski JW, Serrano-Almeida C, Greer AD, Latour I, Sutherland GR. Advancing neurosurgery with image-guided robotics. J Neurosurg. 2009;111(6):1141–1149. doi: 10.3171/2009.2.JNS081334. [DOI] [PubMed] [Google Scholar]
- 95.Mascott CR, Sol JC, Bousquet P, Lagarrigue J, Lazorthes Y, Lauwers-Cances V. Quantification of true in vivo (application) accuracy in cranial image-guided surgery: influence of mode of patient registration. Neurosurgery. 2006;59(1 Suppl 1):ONS146–56. doi: 10.1227/01.NEU.0000220089.39533.4E. discussion ONS146. [DOI] [PubMed] [Google Scholar]
- 96.Grunert P, Darabi K, Espinosa J, Filippi R. Computer-aided navigation in neurosurgery. Neurosurg Rev. 2003;26(2):73–99. doi: 10.1007/s10143-003-0262-0. discussion 100. [DOI] [PubMed] [Google Scholar]



