Abstract
Over the last thirty years, epidemiologic and molecular studies indicate a strong and synergist relationship between the dual epidemics of herpes simplex type 2 (HSV-2) and HIV-1 infection. While prospective studies show that HSV-2 infection increases the risk for HIV-1 acquisition by two-three fold, HSV-2 suppression with standard prophylactic doses of HSV-2 therapy did not prevent HIV-1 acquisition. Reconciling these discrepancies requires understanding recent HSV-2 pathogenesis research, which indicates HSV-2 infection is not a latent infection with infrequent recurrence but a near constant state of reactivation and viral shedding which is not completely suppressed by standard antivirals. Because current antivirals do not prevent or fully suppress HSV-2 replication, priorities are HSV-2 vaccine development and antivirals that reach high concentrations in the genital mucosa and suppress the persistent genital inflammation associated with genital herpes reactivation in order to reduce the increased susceptibility to HIV-1 infection associated with HSV-2. HIV-1 and HSV-2 synergy is also seen among co-infected individuals who exhibit higher HIV-1 viral load compared to HSV-2 uninfected individuals. Standard HSV-2 therapy modestly lowers HIV-1 viral load and is associated with slower HIV-1 disease progression. A promising area of research is higher doses of HSV-2 suppressive therapy achieving a greater reduction in plasma HIV-1 RNA, which could translate to greater reductions in HIV-1 disease progression and infectiousness. However, many questions remain to be answered including potential effectiveness and cost-effectiveness of higher dose HSV-2 suppressive therapy. Mathematical models of HSV-2 and HIV-1 at a population level would be useful tools to estimate the potential impact and cost-effectiveness of higher dose HSV-2 suppressive therapy.
Keywords: HIV, HSV-2, HIV-1 viral load, HIV-1 RNA, epidemiologic synergy, valacyclovir
Introduction
Over the last 30 years, the global, intersecting epidemics of herpes simplex virus type 2 (HSV-2) and HIV-1 have demonstrated a complex relationship between pathogens. The synergy between these two sexually transmitted infections goes beyond similar risk factors for acquisition; they interact both in their epidemiologic niche and at a pathogenesis level driving viral replication and facilitating transmission (1). Moreover, exploiting this synergy for HIV-1 prevention interventions has not proved straight-forward due to both viral and host factors. Indeed, because trials of standard doses of HSV-2 suppressive therapy (i.e., acyclovir 400 mg twice daily) did not reduce acquisition or transmission of HIV-1, research in this area slowed. However, recent epidemiologic and laboratory studies have shed new light on the role of HSV-2 in transmission of HIV-1. Recent intervention trials have indicated that standard dose HSV-2 suppressive therapy for HIV-1 infected persons at intermediate CD4 T cell counts delays HIV-1 disease progression pre-ART initiation and higher doses of HSV-2 suppressive therapy achieve substantially greater reductions in plasma HIV-1 levels, potentially leading to greater clinical and prevention benefits which require further evaluation. Below we review the evidence for HSV-2 and HIV-1 synergy, describe new insights, and propose future research directions.
Epidemiology
HSV-2, the etiologic agent of genital herpes and the most common cause of genital ulcer disease (GUD), is a highly prevalent sexually transmitted infection (STI) globally. Sixteen percent of the world’s population age 15 to 49 years, 536 million people, were estimated to be living with HSV-2 in 2003 (2). The prevalence of HSV-2 varies by geographic region, age, gender, study population (3) and HIV-1 serostatus. Sub-Saharan Africa has a higher reported HSV-2 seroprevalence compared to the Americas, Europe, Australia, Asia and the Middle East and North Africa (MENA). HSV-2 prevalence estimates among adults in the general population (HIV-1 uninfected) in sub-Saharan Africa range from 30% to 80% in women, and 10% to 50% in men (4). Women in Central and South America have HSV-2 prevalence ranges from about 20% to 40%, with lower prevalence seen in developing Asian countries (10–30%) (4). In the United States, HSV-2 seroprevalence was estimated to be 16% between 2005 and 2008 in a national survey of adults in the general population age 14 to 49 years--12% in men and 21% in women (5). Estimates from the MENA regions are some of the lowest seen for the general population (13% in women attending antenatal clinics and 9% for men in a HIV-1 sentinel surveillance study in Morocco (6)) with higher prevalence seen among risk groups (7). In all settings HSV-2 seropositivity is higher in women than in men, as seen in the data summarized above.
HSV-2 seroprevalence increases steadily after sexual debut and with age. In the United States, the seroprevalence of HSV-2 rose steadily from 1.4% among 14 to 19 year olds to 26.1% among 40 to 49 year olds (5); a trend that has not changed since the inception of the national health surveys with HSV-2 testing in 1976. In sub-Saharan Africa, 50% of HIV-1 uninfected persons are HSV-2 seropositive by age 25 years (4). Sexual network structures contribute to higher HSV-2 prevalence seen among STI clinic clients and commercial sex workers (4). HIV-1 infected individuals have higher rates of HSV-2 antibodies compared to HIV-1 uninfected persons; 85% among HSV-2 and HIV-1 co-infected individuals in sub-Saharan Africa (4), 65% among men who have sex with men (MSM) in San Francisco (8) and 80% of HIV-1 infected men in combined data from a US national survey (9).
A recent household serosurvey in Kenya, notable as the first nationally representative estimate of HSV-2 prevalence and risk factors for that country, clearly demonstrated these epidemiologic patterns. Of the 17,940 participants who consented, 15,707 individuals (87.5%) were tested for HSV-2 and HIV-1 (10). They found a HSV-2 seroprevalence of 35% with 42% HSV-2 seropositivity among women and 26% among men. For women and men age 15 to 24 years, HSV-2 prevalence increased from 7% to 30% and 3% to 14% respectively. The seroprevalence of HSV-2 among HIV-1 co-infected individuals was 81%. Among HSV-2 seropositive persons, HIV-1 prevalence was 16% and 2% among HSV-2 seronegative individuals. The synergy between HSV-2 and HIV-1 seen in these epidemiologic patterns is supported by the pathogenesis of both infections.
Pathogenesis
HSV-2, a neurotropic virus, gains entry either at the mucosal skin or through skin abrasions and replicates in the epidermal or dermal cells (3). Primary infection can be asymptomatic, but can also cause ulcer disease accompanied by systemic symptoms and rarely manifests as disseminated disease. Following initial infection, the virus travels down the nerve axon and establishes latency within the dorsal root ganglia. Viral replication initiates reactivation which occurs as either new blister formation or, more frequently, asymptomatic shedding (11). Recent studies have illustrated that the major burden of HSV-2 infection lies in frequent recurrences – ≥80% of which are asymptomatic or unrecognized. Studies that use daily self-collected anogenital swabs to detect HSV-2 reactivation demonstrated that the median shedding rate was 25% of days (range 2% to 75% of days) (12). A study that used six hourly swabbing found that 49% of episodes lasted less than 12 hours and 29% lasted less than 6 hours (13). Even these short bursts of HSV-2 replication have viral copy numbers sufficient for HSV-2 transmission. An intra-host model of HSV-2 pathogenesis estimated that the dorsal root ganglion releases small amounts of virus in a near constant pattern to replicate the shedding patterns seen in clinical studies (14). A key characteristic of HSV-2 infection is now understood to be near-constant viral shedding--not at all the quiescent latent phase previously ascribed to HSV-2 between episodes of symptomatic genital ulcer disease, which were described to be infrequent (11).
Impact of HSV-2 therapy on HSV-2 natural history
Acyclovir and its pro-drug, valacyclovir, treat herpes outbreaks and suppress HSV-2 shedding. Valacyclovir achieves higher plasma concentrations, has 3 – 5 times higher bioavailability and a longer half-life than acyclovir, which makes less frequent valacyclovir dosing possible compared to acyclovir (15). Valacyclovir is converted to acyclovir in vivo. Acyclovir is an HSV-specific drug, which requires HSV thymidine kinase for phosphorylation. This intracellular phosphorylation step activates acyclovir, which is incorporated by HSV DNA polymerase, triggering chain termination and ending HSV replication. Acyclovir and valacyclovir decrease the severity of genital lesions in immunocompetent and immunosuppressed individuals, if given early in the disease course. Suppressive therapy at low doses (acyclovir 400mg twice daily and valacyclovir 500mg daily) in HIV-uninfected persons decreases genital ulcer rates by ~75% and shedding rates by 80% (16). No further decrease in the shedding rate was seen when high dose therapy (valacyclovir 1g thrice daily) was compared to standard dose HSV-2 suppressive therapy (acyclovir 400mg twice daily and valacyclovir 500mg daily) (17). Regardless of HSV-2 suppressive therapy dose, breakthrough episodes had a median length of 7–10 hours and 80% were subclinical (17).
Natural history of HSV-2 in HIV-1 co-infected individuals
Deficits in host innate or cellular immunity result in more frequent clinical disease, which can be persistent or disseminated. Indeed, persistent HSV-2 is a common presentation of advanced HIV-1 infection; low CD4 counts and high viral load are associated with increased frequency of HSV-2 shedding (18, 19). Use of antiretroviral therapy with subsequent increases in CD4 cells does not completely eliminate the effect of HIV-1 infection on HSV-2 shedding and GUD (19, 20). The synergy between HIV-1 and HSV-2 goes beyond increased severity of clinical HSV-2 disease among HIV-1 infected individuals; HSV-2 infection is associated with both increased risk of HIV-1 transmission and acquisition; mechanisms of which are addressed below.
HSV-2 and HIV-1 synergy
Multiple observational studies from 4 continents suggest a 2–3 fold increased risk of HIV-1 acquisition associated with prevalent HSV-2 infection and an up to 7-fold increased risk with incident HSV-2 infection (21–23). A meta-analysis of prospective studies, adjusted for age and sexual behavior, found an increased risk of HIV-1 acquisition among HSV-2 seropositive individuals for women (relative risk (RR) =3.4; 95% CI: 2.4, 4.8), men (RR =2.8; 95% CI: 2.1, 3.7), and MSM (RR =1.6; 95% CI: 1.2, 2.0) (23). Re-enforcing this synergy, HIV-1 infection increases acquisition of HSV-2 approximately 3 to 5-fold for the minority of HIV-1 infected persons who are HSV-2 seronegative when they acquire HIV-1 (24, 25). At the population level, a mathematical model of HSV-2/HIV-1 co-infection in a high HSV-2 prevalence setting (Kisumu, Kenya) estimated that more than 25% of HIV-1 infections were attributable to HSV-2 and that HSV-2 facilitated the spread of HIV-1 into low-risk populations (26). Further, modeling studies that examined the relationship between HSV-2 and HIV-1 suggested that an HSV-2 prevalence of >20% would be necessary to amplify an HIV-1 epidemic (7).
Mechanisms through which HSV-2 increases HIV-1 incidence
Three biological mechanisms likely contribute to increased HIV-1 incidence among HSV-2 seropositive individuals: (1) physical disruption of the epithelial surface by HSV-2, (2) recruitment and persistence of inflammatory cells in the genital tract during HSV-2 reactivation at mucosal surfaces, and (3) increase in plasma HIV-1 RNA with HSV-2 co-infection (Table 1). Macro and micro HSV-2 ulceration create a portal of entry for HIV-1 (27). Further, HSV-2 reactivation increases the recruitment and persistence of inflammatory cells targeted by HIV-1 (28, 29). Recent studies have demonstrated an increase and persistence of HIV-1 target cells (CCR-5-expressing CD4 T cells and CD-SIGN-expressing dendritic cells) that are present after ulcer healing in normal skin, are minimally affected by antiviral therapy, and increase susceptibility for HIV-1 (29).
Table 1.
Mechanisms through which HSV-2 increases HIV-1 incidence
| HSV-2 mechanism | HIV-1 transmission | HIV-1 acquisition |
|---|---|---|
| Genital shedding, micro- ulcerations, and GUD | x | x |
| Increase in HIV-1 target cells in the genital mucosa | x | x |
| Increase HIV-1 replication and genital and plasma viral load | x |
In addition to increasing the risk of HIV-1 acquisition, herpetic lesions are accompanied by HIV-1 shedding from the genital mucosal surface of co-infected individuals (30), thereby increasing the risk of HIV-1 transmission. Higher HIV-1 titers are found in genital secretions during episodes of HSV-2 reactivation (30, 31). HSV-2 up-regulates HIV-1 replication at the cellular level through different pathways including transactivation of the HIV-1 long terminal repeat by HSV-1-infected cell protein (ICP)0, ICP4, ICP27 and US11 gene products and cytokine release and antigen presentation from HSV-2 infected cells (32–34). This up-regulation increases HIV-1 viral load, the primary determinant of HIV-1 transmission (35, 36). Plasma HIV-1 RNA levels in HIV-1/HSV-2 co-infected individuals pre-ART are 0.20–0.55 log10 copies/ml higher than those without HSV-2 co-infection (37–40), which may translate to more rapid progression to AIDS and/or increased HIV-1 infectiousness for sexual partners (36, 41, 42). Recently we reviewed studies that adjusted for CD4 count or time since HIV-1 infection and found that HSV-2 infection was associated with increased plasma HIV-1 RNA by a mean of 0.18 log10 copies/mL (95% CI: 0.01, 0.34), and HSV suppressive therapy decreased plasma HIV-1 levels by -0.28 log10 copies/mL (95% CI: -0.36, -0.19) (43).
Episodic therapy for GUD, which was evaluated in three randomized control trials (RCTs) in Ghana, Central African Republic, South Africa (acyclovir 400mg thrice daily) and Malawi (acyclovir 800mg twice daily), can shorten the disease course of GUD but is often started late (median duration of symptoms at presentation was 6 days) (44–46). Analysis of pooled data from all three trials among 1,478 participants with GUD found 63% of ulcers were herpetic, based on laboratory testing (47). Among 726 HIV-1 infected participants randomized to episodic acyclovir, 92% had HIV-1 RNA detected from genital ulcers at study entry, and those on acyclovir had lower levels of lesional HIV-1 RNA on day seven of follow-up compared to participants receiving placebo (adjusted risk ratio (aRR)= 0.73, 95% CI: 0.55,0.98) (47). Thus, the reduction in HIV-1 shedding from genital lesions after short course episodic acyclovir showed a marginal benefit. Further, our meta-analysis found that episodic therapy for GUD was not associated with a change in plasma HIV-1 RNA (43). In summary, short course episodic treatment for HSV-2 GUD and suppressive therapy of acyclovir 400mg twice daily have been found to be insufficient to reduce the persistent genital inflammation associated with HSV-2 reactivation.
Higher HIV-1 viral load in the setting of HSV-2 co-infection translated to increase risk of transmission: a study among HIV-1 serodiscordant couples in Uganda estimated that the per-coital act transmission probability of HIV-1 was higher for participants reporting GUD history than those without (48). Also, studies indicate that HSV-2 increases mother to child transmission (MTCT) of HIV-1 (49, 50). Among 307 HIV-1 infected pregnant Thai women, HSV-2 shedding was associated with higher HIV-1 RNA (4.2 vs. 4.1 log10 copies/mL; p=0.05) and increased risk of intrapartum transmission (adjusted odds ratio (aOR) = 2.9; 95% CI: 1.0, 8.5) (51). This increase in both genital HSV-2 shedding and HIV-1 viral load during late pregnancy would support the increased risk of MTCT of HIV-1 associated with HSV-2.
Impact of HSV-2 suppressive therapy on HIV-1 transmission
Supported by evidence of the role of HSV-2 infection in HIV-1 acquisition and transmission, three RCTs evaluated the impact of acyclovir suppressive therapy 400 mg twice daily, two RCTs on HIV-1 acquisition (the multicenter HPTN 039 trial and the London School of Hygiene and Tropical Medicine trial in Mwanza, Tanzania) and one RCT (the Partners in Prevention HSV-2/HIV-1 Transmission Study) on HIV-1 transmission; no significant effect was found on HIV-1 incidence (52–54) despite modest reductions in genital ulcer incidence, and an average 0.25 log10 reduction in plasma HIV-1 RNA in the Partners in Prevention HSV-2/HIV-1 Transmission Study. The reasons for these null findings have been better understood since the trials reported their results. First, as described above, HSV-2 pathogenesis studies have demonstrated a near constant state of HSV-2 reactivation with shedding not completely suppressed by even high doses of HSV-2 suppressive therapy (11, 17). Second, persistence of HIV-1 susceptible T cells (i.e., CCR5+ cells) in the genital epithelium even after lesion healing and prolonged acyclovir therapy increases available target cells for HIV-1 attachment and cell entry (29). Third, while we found that episodic acyclovir initiated promptly after GUD recognition had similar virologic and clinical response as in previous episodic GUD studies (55), we also observed lower acyclovir levels in a pharmacokinetic study in black African women compared to historical non-Africans, which requires further study (56). The fourth possible reason for the findings was suboptimal adherence; adherence has been shown to be challenging for HIV-1 prevention trials requiring daily or coitally dependent dosing and the HSV-2 suppression trials required twice daily dosing.
Given the challenges of HSV-2 suppression for HIV-1 prevention, key areas of further research are HSV-2 vaccine development and microbicides that inhibit both HIV-1 and HSV-2. An HSV-2 vaccine would be of benefit by reducing the excess HIV-1 incident cases associated with HSV-2 infection, estimated to be up to one third of HIV-1 cases in sub-Saharan Africa (26). Vaccine candidates to date have not demonstrated efficacy; initial recombinant vaccine candidates were thought to protect HSV-1 and HSV-2 negative women (57) but that finding was not borne out by a RCT (58). New strategies, including live attenuated HSV-2 virus (59), are in early development. A better understanding of the T cell response needed to clear initial HSV-2 infection would bolster HSV-2 vaccine development (60). In addition to HSV-2 vaccines, 1% vaginal tenofovir-based gel reduced the incidence of HIV-1 by 39% and HSV-2 by 51% among women in a study by the Centre for the AIDS Programme of Research in South Africa (CAPRISA) (61). Further, tenofovir gel was shown to achieve high intra-vaginal levels when applied topically (compared with oral administration) with direct anti-herpetic effects (inhibiting HSV DNA polymerase) (62). Recently, daily vaginal 1% tenofovir gel was reported to have no efficacy against HIV-1 acquisition in the Microbicide Trial Network VOICE trial; efficacy against HSV-2 in the daily 1% tenofovir gel has not yet been analyzed (63).
Impact of HSV-2 suppressive therapy on HIV-1 disease progression
Mediated through decreases in HIV-1 viral load, the key determinant of HIV-1 disease progression (64), HSV-2 suppressive therapy slows the clinical course of HIV-1. Longstanding evidence has demonstrated a survival advantage with HSV-2 suppressive therapy across high, moderate and low resource populations: First seen in early studies of HIV-1 treatment almost 20 years ago, high dose acyclovir added to zidovudine monotherapy was found to reduce the risk of HIV-related mortality (65). Further, in a meta-analysis of randomized trials from North America and Europe, high dose acyclovir prophylaxis (>3,200mg per day) demonstrated a reduction in the risk of mortality (66). A systematic review found that a 1 log10 increase in viral load was associated with a 2-fold increase in HIV-1 disease progression (67). The Partners in Prevention HSV-2/HIV-1 Transmission Study of acyclovir suppressive therapy (400mg bid) among HIV-1 serodiscordant couples in East and southern Africa found a 16% (95% CI: 2%, 29%) reduction in disease progression (using a composite endpoint of CD4 count <200, ART initiation and non-trauma related death) and 0.25 log10 copies/mL decrease in viral load (68). Further, among the HIV-1 infected participants with a CD4 count >350 cells/μL at study entry, a 19% (95% CI: 7%, 29%) reduction in time to CD4<350 cells/μL was seen (68).
Similarly, a recent randomized placebo controlled study among 440 HIV-1 and HSV-2 co-infected individuals with CD4 counts 300–400 cells/μL in Uganda found 400mg of acyclovir twice daily decreased HIV-1 disease progression by 27% (aHR 0.73, 95% CI: 0.56, 0.97). When stratified by baseline viral load quintile, a stronger treatment effect of 36% (HR 0.64, 95% CI: 0.43, 0.96) was seen among the highest two baseline plasma viral load quintiles (i.e., HIV-1 RNA > 50 000 copies/mL), while the lowest two lowest viral load quintiles showed no efficacy (HR 0.90, 95% CI: 0.54, 1.50) (69). Across all study participants, plasma HIV-1 viral load was 0.46 log10 copies/mL lower in the acyclovir group compared to the placebo group.
A recent study evaluated the role of valacyclovir to reduce plasma and breast milk HIV-1 shedding among HIV-1, HSV-2 co-infected women post-partum; Roxby and colleagues conducted a randomized, double-blind, placebo controlled trial of HSV-2 suppression with 500 mg valacyclovir twice daily among 148 pregnant Kenyan women from 34 weeks gestation to 12 months postpartum. At 12 months, plasma HIV-1 RNA in the valacyclovir arm was 0.4 log10 copies/ml lower in the valacyclovir arm compared to placebo at 12 months postpartum. CD4 count increased from prenatal baseline levels in both groups due to post-pregnancy effects, but the CD4 count increased by 73 cells/μl more in the valacyclovir arm than in the placebo arm (p=0.03) (70). Valacyclovir reduced risk of breast milk HIV-1 RNA detection by 30% (RR 0.70, 95% CI: 0.49, 0.99) at 6 weeks postpartum, compared to placebo (71). Mean plasma HIV-1 RNA levels were 0.51 log10 copies/mL lower (95% CI: 0.73 to 0.30, p<.001) in the valacyclovir arm compared to the placebo arm between 6 weeks and twelve months postpartum. The study was too small to assess the impact of valacyclovir on MTCT of HIV-1. Thus, post-partum valacyclovir suppressive therapy could be evaluated for reduction of maternal to child HIV-1 transmission in countries that have high breastfeeding rates for HIV-1 infected women who do not continue ART post-partum.
To evaluate whether HSV-2 suppressive dose is associated with different magnitude of plasma HIV-1 reduction, two studies recently investigated high dose valacyclovir. Perti and colleagues reported valacyclovir 1g twice daily decreased HIV-1 RNA by 0.40 log10 copies/mL (95% CI: −0.64 to −0.21 log10 copies/mL) among HIV-1, HSV-2 dually infected persons in Seattle who were not on ART (72). Mugwanya and colleagues reported the results of a randomized cross over trial of higher dose HSV-2 suppressive therapy, valacyclovir 1.5g compared to acyclovir 400mg (both twice daily) among 32 HIV-1-HSV-2 co-infected participants in Kenya (73). They found mean plasma HIV-1 viral load was significantly lower on valacyclovir compared to acyclovir by 0.62 log10 copies/mL (95% CI: −0.68, −0.55 log10 copies/mL) and compared to baseline by 1.23 log10 copies/mL (95% CI: −1.38, −1.07 log10 copies/mL). Also, CD4 cell counts increased by a median of 86 cells/μL (Mann-Whitney U test: P = 0.3 vs. baseline) and 69 cells/μL (Mann-Whitney U test: P = 0.7 vs. baseline) over the 12 weeks of therapy for valacyclovir and acyclovir respectively. These studies suggest that higher-dose suppression may have substantially greater clinical HIV-1 benefits and reduce risk of HIV-1 transmission, based on a modeled 50% reduction in HIV-1 transmission risk with an average of 0.74 log10 reduction in plasma HIV-1 RNA (74). Based on the 1 log10 reduction in plasma viral load observed in the high dose valacyclovir arm in the Mugwanya study, this is estimated to reduce HIV-1 incidence by 55% in a high HSV-2 prevalence region (75).
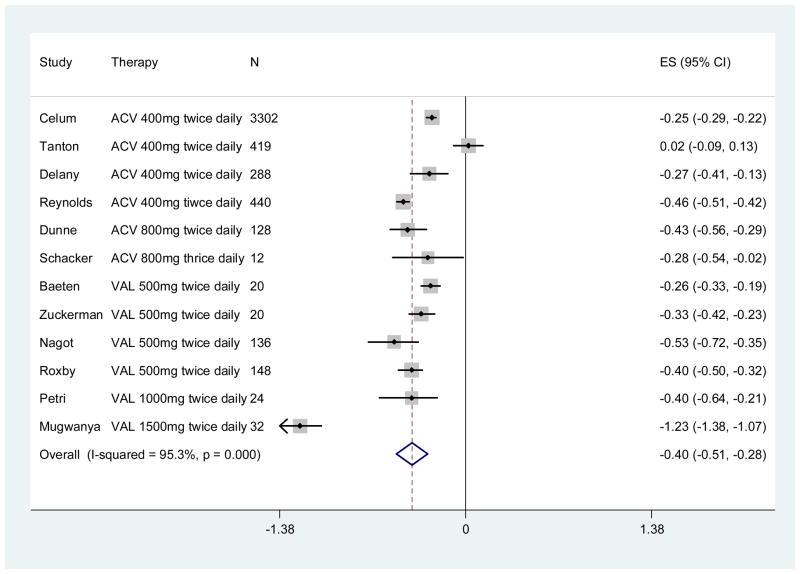
The impact of acyclovir on disease progression is mediated through decreases in HIV-1 viral load, the key determinant of HIV-1 disease progression (64). Standard doses of acyclovir have been demonstrated to modestly decrease HIV-1 RNA by 0.2 – 0.25 log10 copies/mL (43) and slow HIV-1 disease progression 16% to 27% (68, 76). Since our initial meta-analysis, four more studies (described above) demonstrate the impact of HSV-2 suppressive therapy on HIV-1 viral load. In total, there are now 12 published studies which enrolled a total of 4,969 participants that have assessed the impact of acyclovir or valacyclovir HSV-2 suppressive therapy on plasma HIV-1 RNA (41, 53, 70, 72, 73, 76–82); the updated summary estimate for decrease in plasma HIV-1 RNA associated with HSV-2 suppressive therapy is 0.40 log10 copies/mL, (95% CI: −0.51 to −0.28) (Figure 1).
Figure 1. Forest plot of association between HSV-2 suppressive therapy and plasma HIV-1 RNA (log10 copies/mL).
CI, confidence interval; ES, effect size; References (41, 53, 70, 72, 73, 76–82)
A clear dose-dependent effect of suppressive regimens on plasma HIV-1 viral load is evident with higher-dose HSV-2 suppression (Figure 2). At the lowest dose (acyclovir 400 mg twice daily), two randomized trials have recently demonstrated statistically significant delays in HIV-1 disease progression (68, 76), although this dose has not been adopted into clinical practice given the perception that a greater magnitude of effect is needed to warrant the cost (83). A recent study shows that dose of HSV-2 suppression is important; high-dose valacyclovir suppressive therapy (1.5g twice daily) decreased HIV-1 RNA by 1.23 log10 copies/mL (95% CI: −1.38 to −0.96 log10 copies/mL) compared to the baseline plasma HIV-1 RNA in HIV-1/HSV-2 dually infected persons with CD4>250 who were not eligible for ART based on national guidelines (73). Higher-dose suppression should be evaluated for HIV-1 disease progression and infectiousness outcomes now that valacyclovir is generic and drug costs are decreasing significantly.
Fig. 2. Forest plot of association between HSV-2 suppressive therapy at different doses and change in plasma HIV-1 RNA (log10 copies/mL). CI, confidence interval; ES, effect size.
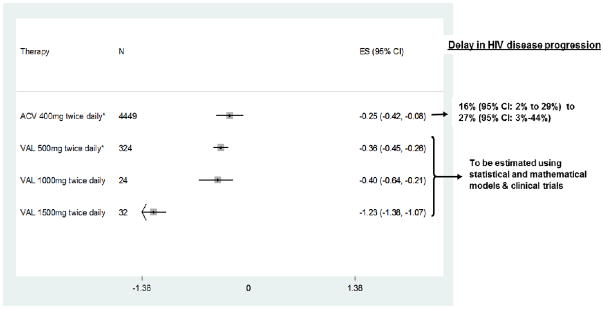
*Summary estimates of published studies; ACV – acyclovir; VAL – valacyclovir;
400mg ACV twice daily (53, 76, 78, 81); 500mg VAL twice daily (70, 77, 80, 82); VAL 1000mg twice daily (72); VAL 1500mg twice daily (73)
Mechanism of action of acyclovir and resistance
The mechanism of action of acyclovir and related drugs on HIV-1 viral load, thought initially to be indirect, is still to be elucidated. It was hypothesized that acyclovir acted indirectly on HIV-1 viral load by decreasing the overall levels of T cell activation by limiting HSV-2 reactivation. However, in vitro studies have suggested that acyclovir may act directly on HIV-1 reverse transcriptase, and that the effects of acyclovir on reducing HIV-1 viral load could be in part through direct inhibitory effects on HIV-1 replication as well as through indirect effects through HSV-2 suppression (84, 85). Clinical studies of acyclovir or valacyclovir suppression on plasma HIV-1 reduction in HSV-seronegative, HIV-1 infected persons would be informative in terms of confirming these in vitro observations, but are challenging to conduct, given the high rate of HIV-1 and HSV co-infection. Importantly, while the in-vitro studies identified a HIV-1 resistance mutation, V75I, which was selected after high doses of acyclovir in vitro, the V75I mutation was not seen in clinical studies over two years of HSV-2 suppressive therapy with acyclovir (86, 87).
Cost-effectiveness of valacyclovir suppressive therapy
Using the Partners in Prevention HSV-2/HIV-1 Transmission Study results of delayed disease progression, a cost-effectiveness analysis (from the perspective of an intervention to delay HIV-1 disease progression in South Africa) found that suppressive acyclovir therapy could be an affordable strategy for reducing HIV-1 disease progression. At the lowest international pricing for acyclovir (US $ 0.026 per day for 400mg acyclovir twice daily), the cost per life year gained (LYG) was US $737 (95% CI: US $ 373/LYG, US $ 2,489/LYG) under ART eligibility criteria of CD4<350 cell/μL. This estimate is cost saving compared to ART, for which the cost-effectiveness is estimated to be US $12,000/LYG. However, under South African pricing for acyclovir (US $ 0.14 per day for 400mg acyclovir twice daily) suppressive acyclovir costs US $ 1130/LYG (95% CI: US $ 616/LYG, US $ 3,785/LYG) (88). Given the greater impact on HIV-1 disease progression recently reported from the Rakai trial with the same dose of acyclovir (400 mg twice daily), the cost-effectiveness would be modestly greater. However, the marked variability of acyclovir and valacyclovir costs substantially impacts the cost-effectiveness of HSV-2 suppressive therapy for HIV-1, HSV-2 dually infected persons for clinical and potentially public benefits.
Corbell and colleagues reviewed the access to acyclovir in 8 African countries. They found that while the country-specific (government) purchasing price of acyclovir was 1 to 2 times the median international reference price, the retail cost in the private sector was 6 to 10 times the median international reference price (89). Drug pricing is highly variable depending on the underlying cost of production, volume required (demand) and supply. Transparency in drug pricing would help cost-effectiveness analyses better balance the effectiveness of interventions against their true costs; particularly for a drug like valacyclovir which is no longer under patent. The Clinton Health Access Initiative (CHAI) is helping to bridge the gap between pharmaceutical companies and drugs for public health use, including valacyclovir
Future directions
Valacyclovir is an excellent candidate for HIV-1 treatment pre-ART to delay disease progression, given its safety, tolerability and lack of evidence of selection of HIV-1 resistance in vivo (86, 87). In addition, daily medication (e.g., co-trimoxazole prophylaxis) has been shown to improve retention in care (90) and may facilitate transition to ART and good ART adherence. Adding choice to our HIV-1 treatment armamentarium is important; HIV-1 infected persons who are not ready to start antiretroviral therapy could use valacyclovir therapy as a means to engage in care, reduce plasma HIV-1 levels and delay disease progression, and learn strategies for adherence until they are ready to start ART. For patients in low resource settings who are diagnosed before they are ART eligible, valacyclovir would provide a valuable intervention pre-ART. Currently there are >12 million HIV+ individuals in low resource countries who are not yet ART eligible by current WHO guidelines (91); valacyclovir could delay their disease progression and engage them in pre-ART care, providing clinical benefits and facilitating their linkage to ART when they become eligible for ART.
HSV-2 is an excellent candidate as a highly prevalent co-infection among HIV-1 infected persons for which we have antivirals that can reduce their frequency of subclinical and clinical HSV-2 reactivation and delay HIV-1 disease progression. The potential impact of co-infections on total community viral load over time is a function not only of the magnitude of the increase in HIV-1 viral load observed in co-infected individuals, but also of the prevalence and the average duration of the co-infection among HIV-positive individuals. The magnitude of the increase in HIV-1 viral load seen with HSV-2 is modest (0.18 log(10) copies/ml) compared to other HIV co-infections such as acute malaria and active TB, which have an observed 2–3 times higher increase in HIV viral load (43). However, because HSV-2 is highly prevalent (80% – 90% among HIV-1 infected individuals) and infection is life-long, the potential impact of HSV-2 co-infection on total community viral load over time is likely to be greater than that of other HIV co-infections.
An ongoing multicenter, randomized double blind trial in the Americas is evaluating the impact of valacyclovir 500mg twice daily versus placebo on HIV-1 disease progression, defined as CD4 <350 cell/μL or ART initiation for any reason (92). While this study will provide further data, questions remain regarding what valacyclovir dose should be used, cost-effectiveness and epidemiologic context in which valacyclovir therapy will be a useful approach to pre-ART HIV-1 treatment. In addition, the net effect of HSV-2 suppression on achieving partial viral suppression and the population-level impact on HIV-1 transmission needs to be explored. The potential role of valacyclovir suppression as a novel HIV-1 treatment and prevention strategy warrants study.
Statistical and mathematical models are powerful tools that could be used to estimate effectiveness and cost-effectiveness of valacyclovir as pre-ART HIV-1 treatment and identify highest priority populations for evaluation of this strategy. Careful modeling analysis could identify the price point at which valacyclovir therapy would be cost-effective and would contribute to clinical trial design. Integrating cost-effectiveness analyses into effectiveness modeling also can speed the translation from clinical trial results to implementation with costing data available for decision makers (93–95).
In summary, over the last three decades a substantial body of epidemiologic and molecular evidence has demonstrated the epidemiologic synergy between HSV-2 and HIV-1. Recent studies have expanded our understanding of HSV-2 pathogenesis and provided a paradigm shift about HSV-2 pathogenesis--from a latent infection with infrequent recurrence to a near constant state of reactivation and viral shedding, which is not completely suppressed by current antivirals. In the absence of interventions that suppress HSV-2 replication, HSV-2 vaccine development is a priority to reduce the increased risk of HIV-1 infection associated with HSV-2. A second priority is to obtain confirmatory data on tenofovir gel for prevention of HSV-2. Lastly, a promising area of research that warrants further research is higher doses of valacyclovir for HSV-2 suppressive therapy on HIV-1 disease progression and infectiousness, given the higher reduction in plasma HIV-1 viral load compared to lower doses of HSV-2 suppression. However, many questions remain to be answered including potential effectiveness and cost-effectiveness of valacyclovir interventions.
References
- 1.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually transmitted diseases [Review] 1992 Mar-Apr;19(2):61–77. [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bulletin of the World Health Organization. 2008 Oct;86(10):805–12A. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010. [Google Scholar]
- 4.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes: the journal of the IHMF. 2004 Apr;11( Suppl 1):24A–35A. [Review] [PubMed] [Google Scholar]
- 5.Xu F, Sternberg M, Gottlieb S, Berman S, Markowitz L, Forhan S, et al. Seroprevalence of Herpes Simplex Virus Type 2 Among Persons Aged 14--49 Years --- United States, 2005--2008. Morbidity and Mortality Weekly Report. 2010 Apr 23;59(15):456–9. [PubMed] [Google Scholar]
- 6.Cowan FM, French RS, Mayaud P, Gopal R, Robinson NJ, de Oliveira SA, et al. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sexually transmitted infections. 2003 Aug;79(4):286–90. doi: 10.1136/sti.79.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Schiffer JT, Ashley R, Mumtaz G, Alsallaq RA, Akala FA, et al. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics. 2010 Dec;2(4):173–82. doi: 10.1016/j.epidem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohl DD, Katz KA, Bernstein K, Wong E, Raymond HF, Klausner JD, et al. Prevalence and correlates of herpes simplex virus type-2 infection among men who have sex with men, san francisco, 2008. Sexually Transmitted Diseases. 2011 Jul;38(7):617–21. doi: 10.1097/OLQ.0b013e31820a8c10. [DOI] [PubMed] [Google Scholar]
- 9.McQuillan GM, Kruszon-Moran D, Granade T, Feldman JW. Seroprevalence of Human Immunodeficiency Virus in the US Household Population Aged 18–49 Years: The National Health and Nutrition Examination Surveys, 1999–2006. J Acquir Immune Defic Syndr. 2009 Aug 25; doi: 10.1097/QAI.0b013e3181b3a8e3. [DOI] [PubMed] [Google Scholar]
- 10.Mugo N, Dadabhai SS, Bunnell R, Williamson J, Bennett E, Baya I, et al. Prevalence of Herpes Simplex Virus Type 2 Infection, Human Immunodeficiency Virus/Herpes Simplex Virus Type 2 Coinfection, and Associated Risk Factors in a National, Population-Based Survey in Kenya. Sex Transm Dis. 2011 Nov;38(11):1059–66. doi: 10.1097/OLQ.0b013e31822e60b6. [DOI] [PubMed] [Google Scholar]
- 11.Schiffer JT, Corey L. Herpes Simplex Virus-2 Infection: New understandings in the dynamics of viral-host interactions and their implications for developing novel antivirals, vaccines and immunotherapies. Nat Med. 2011 Under review. [Google Scholar]
- 12.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–95. doi: 10.1146/annurev.med.59.061606.095540. [Review] [DOI] [PubMed] [Google Scholar]
- 13.Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. The Journal of infectious diseases. 2008 Oct 15;198(8):1141–9. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Magaret A, et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med. 2009 Nov 18;1(7):7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granero GE, Amidon GL. Stability of valacyclovir: implications for its oral bioavailability. Int J Pharm. 2006 Jul 6;317(1):14–8. doi: 10.1016/j.ijpharm.2006.01.050. [In Vitro] [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Wald A, Krantz E, Selke S, Warren T, Vargas-Cortes M, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004 Oct 15;190(8):1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 17.Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang M, et al. Ineffectiveness of daily standard and high-dose antiviral therapy in preventing short episodes of genital HSV-2 reactivation: three randomized, open-label cross-over trials. Lancet. 2011 doi: 10.1016/S0140-6736(11)61750-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClelland RS, Wang CC, Overbaugh J, Richardson BA, Corey L, Ashley RL, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002 Dec 6;16(18):2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 19.Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. The Journal of infectious diseases. 2004 Aug 15;190(4):693–6. doi: 10.1086/422755. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 20.Ameli N, Bacchetti P, Morrow RA, Hessol NA, Wilkin T, Young M, et al. Herpes simplex virus infection in women in the WIHS: epidemiology and effect of antiretroviral therapy on clinical manifestations. AIDS. 2006 Apr 24;20(7):1051–8. doi: 10.1097/01.aids.0000222078.75867.77. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006 Jan 2;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 22.Sobngwi-Tambekou J, Taljaard D, Lissouba P, Zarca K, Puren A, Lagarde E, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009 Apr 1;199(7):958–64. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynn JR, Carael M, Auvert B, Kahindo M, Chege J, Musonda R, et al. Why do young women have a much higher prevalence of HIV than young men? A study in Kisumu, Kenya and Ndola, Zambia. AIDS. 2001 Aug;15( Suppl 4):S51–60. doi: 10.1097/00002030-200108004-00006. [DOI] [PubMed] [Google Scholar]
- 24.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004 Apr 15;35(5):435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Mahiane SG, Legeai C, Taljaard D, Latouche A, Puren A, Peillon A, et al. Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. Aids. 2009 Jan 28;23(3):377–83. doi: 10.1097/qad.0b013e32831c5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008;3(5):e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celum C, Levine R, Weaver M, Wald A. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ. 2004 Jun;82(6):447–53. [PMC free article] [PubMed] [Google Scholar]
- 28.Celum CL. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes. 2004 Apr;11( Suppl 1):36A–45A. [PubMed] [Google Scholar]
- 29.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009 Aug;15(8):886–92. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998 Jul 1;280(1):61–6. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 31.Mbopi Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Brown DW, et al. Genital herpes simplex virus type 2 shedding is increased in HIV-infected women in Africa. AIDS. 1999 Mar 11;13(4):536–7. doi: 10.1097/00002030-199903110-00021. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht MA, DeLuca NA, Byrn RA, Schaffer PA, Hammer SM. The herpes simplex virus immediate-early protein, ICP4, is required to potentiate replication of human immunodeficiency virus in CD4+ lymphocytes. J Virol. 1989 May;63(5):1861–8. doi: 10.1128/jvi.63.5.1861-1868.1989. [In Vitro] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golden MP, Kim S, Hammer SM, Ladd EA, Schaffer PA, DeLuca N, et al. Activation of human immunodeficiency virus by herpes simplex virus. The Journal of infectious diseases. 1992 Sep;166(3):494–9. doi: 10.1093/infdis/166.3.494. [DOI] [PubMed] [Google Scholar]
- 34.Palu G, Benetti L, Calistri A. Molecular basis of the interactions between herpes simplex viruses and HIV-1. Herpes. 2001 Jul;8(2):50–5. [PubMed] [Google Scholar]
- 35.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011 Apr 6;3(77):77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 37.Duffus WA, Mermin J, Bunnell R, Byers RH, Odongo G, Ekwaru P, et al. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS. 2005 Nov;16(11):733–5. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- 38.Gray RH, Li X, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis. 2004 Apr 1;189(7):1209–15. doi: 10.1086/382750. [DOI] [PubMed] [Google Scholar]
- 39.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997 Sep;176(3):766–70. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 40.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003 Nov 15;188(10):1492–7. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 41.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002 Dec 15;186(12):1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 42.Mellors JW, Kingsley LA, Rinaldo CR, Jr, Todd JA, Hoo BS, Kokka RP, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995 Apr 15;122(8):573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 43.Barnabas RV, Webb EL, Weiss HA, Wasserheit JN. The role of coinfections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS. 2011 Aug 24;25(13):1559–73. doi: 10.1097/QAD.0b013e3283491e3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayaud P, Legoff J, Weiss HA, Gresenguet G, Nzambi K, Bouhlal H, et al. Impact of acyclovir on genital and plasma HIV-1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV-1-infected African women with herpes ulcers: a randomized placebo-controlled trial. J Infect Dis. 2009 Jul 15;200(2):216–26. doi: 10.1086/599991. [DOI] [PubMed] [Google Scholar]
- 45.Paz-Bailey G, Sternberg M, Puren AJ, Markowitz LE, Ballard R, Delany S, et al. Improvement in healing and reduction in HIV shedding with episodic acyclovir therapy as part of syndromic management among men: a randomized, controlled trial. J Infect Dis. 2009 Oct 1;200(7):1039–49. doi: 10.1086/605647. [DOI] [PubMed] [Google Scholar]
- 46.Phiri S, Hoffman IF, Weiss HA, Martinson F, Nyirenda N, Kamwendo D, et al. Impact of aciclovir on ulcer healing, lesional, genital and plasma HIV-1 RNA among patients with genital ulcer disease in Malawi. Sex Transm Infect. 2010 Oct;86(5):345–52. doi: 10.1136/sti.2009.041814. [DOI] [PubMed] [Google Scholar]
- 47.Weiss HA, Paz Bailey G, Phiri S, Gresenguet G, LeGoff J, Pepin J, et al. Episodic therapy for genital herpes in sub-saharan Africa: a pooled analysis from three randomized controlled trials. PloS one. 2011;6(7):e22601. doi: 10.1371/journal.pone.0022601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001 Apr 14;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 49.Chen KT, Segu M, Lumey LH, Kuhn L, Carter RJ, Bulterys M, et al. Genital herpes simplex virus infection and perinatal transmission of human immunodeficiency virus. Obstetrics and gynecology. 2005 Dec;106(6):1341–8. doi: 10.1097/01.AOG.0000185917.90004.7c. [DOI] [PubMed] [Google Scholar]
- 50.Drake AL, John-Stewart GC, Wald A, Mbori-Ngacha DA, Bosire R, Wamalwa DC, et al. Herpes simplex virus type 2 and risk of intrapartum human immunodeficiency virus transmission. Obstet Gynecol. 2007 Feb;109(2 Pt 1):403–9. doi: 10.1097/01.AOG.0000251511.27725.5c. [DOI] [PubMed] [Google Scholar]
- 51.Bollen LJ, Whitehead SJ, Mock PA, Leelawiwat W, Asavapiriyanont S, Chalermchockchareonkit A, et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: possibility to further decrease transmission? AIDS. 2008 Jun 19;22(10):1169–76. doi: 10.1097/QAD.0b013e3282fec42a. [DOI] [PubMed] [Google Scholar]
- 52.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Jun 21;371(9630):2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010 Feb 4;362(5):427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008 Apr 10;358(15):1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baeten JM, Reid SE, Delany-Moretlwe S, Hughes JP, Wang RS, Wilcox E, et al. Clinical and Virologic Response to Episodic Acyclovir for Genital Ulcers Among HIV-1 Seronegative, Herpes Simplex Virus Type 2 Seropositive African Women: A Randomized, Placebo-Controlled Trial. Sexually transmitted diseases. 2012 Jan;39(1):21–4. doi: 10.1097/OLQ.0b013e31823b50c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, Hendrix C, Celum C. Acyclovir achieves lower concentration in African HIV-, HSV21 women compared to non- African populations, possibly explaining lower herpes suppression. Sex Transm Infect. 2011;87(Suppl_1):A79. [Google Scholar]
- 57.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. The New England journal of medicine. 2002 Nov 21;347(21):1652–61. doi: 10.1056/NEJMoa011915. [Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 58.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. New England Journal of Medicine. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PloS One. 2011;6(3):e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen J. Immunology. Painful failure of promising genital herpes vaccine. Science. 2010 Oct 15;330(6002):304. doi: 10.1126/science.330.6002.304. [News] [DOI] [PubMed] [Google Scholar]
- 61.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrei G, Lisco A, Vanpouille C, Introini A, Balestra E, van den Oord J, et al. Topical Tenofovir, a Microbicide Effective against HIV, Inhibits Herpes Simplex Virus-2 Replication. Cell host & microbe. 2011 Oct 4;10(4):379–89. doi: 10.1016/j.chom.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Microbicide Trials Network. MTN Statement on Decision to Discontinue Use of Tenofovir Gel in VOICE, a Major HIV Prevention Study in Women. 2011 [updated 12/01/2011]; Available from: http://www.mtnstopshiv.org/node/3909.
- 64.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997 Jun 15;126(12):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 65.Stein DS, Graham NM, Park LP, Hoover DR, Phair JP, Detels R, et al. The effect of the interaction of acyclovir with zidovudine on progression to AIDS and survival. Analysis of data in the Multicenter AIDS Cohort Study. Ann Intern Med. 1994 Jul 15;121(2):100–8. doi: 10.7326/0003-4819-121-2-199407150-00004. [DOI] [PubMed] [Google Scholar]
- 66.Ioannidis JP, Collier AC, Cooper DA, Corey L, Fiddian AP, Gazzard BG, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998 Aug;178(2):349–59. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 67.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008 Oct 18;22(16):2179–85. doi: 10.1097/QAD.0b013e328312c756. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010 Mar 6;375(9717):824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reynolds SJ, Makumbi F, Kiwanuka N, Newell K, Ssebbowa P, Ssempijja V, et al. In: Impact of HSV-2 suppressive therapy with daily acyclovir on HIV-1 disease progression: a randomized placebo-controlled trial in Rakai, Uganda. Barnabas RV, editor. Seattle: 2011. Abstract for presentation at IAS. [Google Scholar]
- 70.Roxby AC, Drake AL, Ongecha-Owuror F, Richardson BA, Kiarie JN, John-Stewart G, et al. Peripartum Valacyclovir Improves Markers of HIV-1 Disease Progression in Women Co-infected with HSV-2: A Randomized Trial. Vol. 2011 IAS; Rome: Jul, 2011. [Google Scholar]
- 71.Drake AL, Roxby AC, Ongecha-Owuor F, Kiarie J, John-Stewart G, Wald A, et al. Valacyclovir Suppressive Therapy Reduces Plasma and Breast Milk HIV-1 RNA Levels During Pregnancy and Postpartum: A Randomized Trial. The Journal of infectious diseases. 2011 Dec 6; doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perti T, Baeten J, Diem K, Ochbamichael N, Huang M, Selke S, et al. High-dose Valacyclovir Decreases Plasma HIV-1 Levels More than Standard Dose Acyclovir in HIV, HSV-2 positive persons: a Randomized, Crossover Trial. Vol. 2011. Montreal: Jul, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, Celum C. High-dose Valacyclovir HSV-2 Suppression Results in Greater Reduction in Plasma HIV-1 Levels Compared With Standard Dose Acyclovir Among HIV-1/HSV-2 Coinfected Persons: A Randomized, Crossover Trial. The Journal of infectious diseases. 2011 Oct 12; doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PloS One. 2010;5(9):e12598. doi: 10.1371/journal.pone.0012598. [Randomized Controlled Trial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanton C, Abu-Raddad LJ, Weiss HA. Time to Refocus on HSV Interventions for HIV Prevention? The Journal of infectious diseases. 2011 Dec;204(12):1822–6. doi: 10.1093/infdis/jir653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds SJ, Makumbi F, Kiwanuka N, Newell K, Ssebbowa P, Ssempijja V, et al. Impact of HSV-2 suppressive therapy with daily acyclovir on HIV-1 disease progression: a randomized placebo-controlled trial in Rakai, Uganda. IAS; Rome: 2011. [Google Scholar]
- 77.Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008 Dec 15;198(12):1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, Legoff J, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009 Feb 20;23(4):461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunne EF, Whitehead S, Sternberg M, Thepamnuay S, Leelawiwat W, McNicholl JM, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008 Sep 1;49(1):77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 80.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007 Feb 22;356(8):790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 81.Tanton C, Weiss HA, Rusizoka M, Legoff J, Changalucha J, Baisley K, et al. Long-term impact of acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: a randomized controlled trial. J Infect Dis. 2010 May 1;201(9):1285–97. doi: 10.1086/651696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007 Nov 15;196(10):1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 83.Cohen MS, Eron JJ., Jr Aciclovir Treatment for Human Immunodeficiency Virus-1: Is the “Juice Worth the Squeeze?”. Sexually Transmitted Diseases. 2011 May;38(5):410–2. doi: 10.1097/olq.0b013e318214b6e1. [DOI] [PubMed] [Google Scholar]
- 84.McMahon MA, Siliciano JD, Lai J, Liu JO, Stivers JT, Siliciano RF, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008 Nov 14;283(46):31289–93. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lisco A, Vanpouille C, Tchesnokov EP, Grivel JC, Biancotto A, Brichacek B, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008 Sep 11;4(3):260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baeten JM, Lingappa J, Beck I, Frenkel LM, Pepper G, Celum C, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2011 Jan 1;203(1):117–21. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LeGoff J, Tanton C, Delaugerre C, Weiss HA, Changalucha J, Ross DA, et al. No selection of nucleoside reverse transcriptase inhibitor resistance associated mutations by acyclovir suppressive therapy in herpes simplex virus-2/HIV-1 dually infected persons. AIDS. 2010 Oct 23;24(16):2595–6. doi: 10.1097/QAD.0b013e32833e5176. [DOI] [PubMed] [Google Scholar]
- 88.Vickerman P, Devine A, Foss AM, Delany-Moretlwe S, Mayaud P, Meyer-Rath G. The cost-effectiveness of herpes simplex virus-2 suppressive therapy with daily aciclovir for delaying HIV disease progression among HIV-1-infected women in South Africa. Sexually transmitted diseases. 2011 May;38(5):401–9. doi: 10.1097/OLQ.0b013e31820b8bc8. [DOI] [PubMed] [Google Scholar]
- 89.Corbell C, Stergachis A, Ndowa F, Ndase P, Barnes L, Celum C. Genital ulcer disease treatment policies and access to acyclovir in eight sub-Saharan African countries. Sex Transm Dis. 2010 Aug;37(8):488–93. doi: 10.1097/OLQ.0b013e3181e212e5. [DOI] [PubMed] [Google Scholar]
- 90.Kohler P, Chung M, Benki-Nugent S, McGrath C, Attwa M, Saki S, et al. Free CTX Substantially Improves Retention among ART-ineligible Clients in a Kenyan HIV Treatment Program. 2011. [Google Scholar]
- 91.UNAIDS/WHO. UNAIDS Report on the Global AIDS Epidemic: Joint United Nations Programme on HIV/AIDS. 2010. [Google Scholar]
- 92.Tan DH, Kaul R, Raboud JM, Walmsley SL. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS. 2011 Jan 14;25(2):207–10. doi: 10.1097/QAD.0b013e328341ddf7. [DOI] [PubMed] [Google Scholar]
- 93.Collins FS. Reengineering translational science: the time is right. Sci Transl Med. 2011 Jul 6;3(90):90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galarraga O, Colchero MA, Wamai RG, Bertozzi SM. HIV prevention cost-effectiveness: a systematic review. BMC Public Health. 2009;9( Suppl 1):S5. doi: 10.1186/1471-2458-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents. Geneva: WHO; 2010. Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed] [Google Scholar]