Abstract
Various noncanonical sentence constructions are derived from basic sentence structures by a phrase displacement called Movement. The moved phrase (filler) leaves a silent copy at the extracted position (gap) and is reactivated when the hearer/reader passes over the gap. Consequently, memory operations are assumed to occur to establish the filler–gap link. For languages that have a relatively free word order like German, a distinct linguistic operation called Scrambling is proposed. Although Movement and Scrambling are assumed to be different linguistic operations, they both involve memory prone filler–gap processes. To clarify whether filler–gap memory processes in Scrambling and Movement differ neuroanatomically, we designed a functional magnetic resonance imaging study and compared the effect of memory load parameterized by filler–gap distance in the 2 sentence types. Here, we show that processing of the 2 sentence types commonly relies on a left hemispheric network consisting of the inferior frontal gyrus, middle part of the middle temporal gyrus, and intraparietal sulcus. However, we found differences for the 2 sentence types in the linearity of filler–gap distance effect. Thus, the present results suggest that the same neural substrate supports the memory processes of sentences constructed by Movement and Scrambling, although differentially modulated by memory load.
Keywords: fMRI, language, movement, scrambling, syntax
Introduction
In modern linguistics, Movement is one of the key concepts for understanding the various noncanonical structures of sentences (Chomsky 1981, 1995). Taking this view, the members of the question–answer pair
(1) <Which student did John see ◂?, John saw the chemistry student>
are related by a formal order-changing operation (i.e., Movement) that derives the former sentence from the latter, whose word order is considered more basic (i.e., canonical). The underlined question expression (i.e., the moved “antecedent,” or “filler”) is related to its canonical postverbal position (◂ also known as a “trace” or “gap”) by a formally established link (Chomsky 1981, 1995). The establishment of the filler–gap link has been shown to be a real psychological process. As demonstrated through a priming effect at the gap position (Tanenhaus et al. 1985; Clifton and Frazier 1988; McElree and Bever 1989), the moved word (a filler) is maintained in working memory and reactivated at the gap. In the above sentence example (1), the filler “Which student” is reactivated at the sentence-final gap position. Early imaging work roughly localized Movement in Broca's region and the posterior superior temporal sulcus (Ben-Shachar et al. 2003). Later, more refined studies focused on the left pars triangularis (PTr) (Brodmann Area [BA] 45) (Santi and Grodzinsky 2007, 2010).
Movement is considered to be a universal syntactic operation that can account for various word orders across languages. It is not clear, however, whether it covers the relatively free word order changes observed in German and Japanese, for example. Members of the German pair <Der Mann zeigte dem Kind den Onkel, Der Mann zeigte den Onkel dem Kind ◂ —the man showed the uncle to the child> have a similar meaning, even though they differ in the order of the objects. Dubbed Scrambling (Ross 1967), the similarity between this relation and Movement has been a subject of controversy among linguists (Saito 1989; Webelhuth 1989; Fanselow 1990, 2001; Müller and Sternefeld 1993). This question makes no commitment to any particular theoretical framework (generative or others) but is a question that any theory must address. Some linguistic analyses show that Scrambling obeys similar linguistic constraints to Movement (Saito 1989; Webelhuth 1989), thereby suggesting Scrambling as an instance of Movement (Fanselow 1990). Others propose that scrambled sentences are not derived from canonical word order sentences via a Movement operation but are generated de novo as they are (Müller and Sternefeld 1993; Fanselow 2001). However, even if it is the case that Scrambling involves movement, the constraints on Scrambling seem to differ from other forms of Movement (i.e., topicalization and wh-movement) (Müller and Sternefeld 1993). For example, scrambling in German must occur within the clause, whereas wh-movement and topicalization can occur across clauses. Additionally, topicalization prevents wh-movement but scrambling does not. From a processing perspective, the explicit case marking system in the languages that allow Scrambling might enable thematic role assignment independent of the filler–gap binding. Psycholinguistic studies, however, point to a similarity of the mechanisms underlying the processing of word order changes in German and Japanese sentences to those in English (Clahsen and Featherston 1999; Nakano et al. 2002). Imaging results suggest that the supporting neural tissues for Scrambling may differ from those for Movement; the former is reported to activate the pars opercularis (PO, BA 44), the presupplementary motor area, superior temporal gyrus, superior frontal gyrus, and the cingulate gyrus (Roder et al. 2002; Grewe et al. 2005; Friederici et al. 2006; Obleser et al. 2011), whereas the latter is shown to activate the PTr, posterior middle temporal sulcus, and parahippocampal gyrus (Ben-Shachar et al. 2003; Santi and Grodzinsky 2007, 2010). These divergent results may merely be due to crosslinguistic differences or limitations in anatomical localization across methods; alternatively, Movement and Scrambling may truly be different operations. Carefully controlled experiments using a single language and a consistent method are therefore needed in order to clarify the issue. German is an ideal candidate for this as it has both.
The present functional magnetic resonance imaging (fMRI) study examined neural correlates for the processing of German sentences constructed by Movement and Scrambling. To overcome problems that arise when very different structures are compared directly, we parameterized memory load by nesting a distance parameter that equally increased the number of noun phrases (NPs) that intervened between the filler and the gap in each construction. In this way, each construction type is its own control. This resulted in a within-subject 2 × 3 factorial design, with factors TYPE (Movement/Scrambling) and DISTANCE (3 levels of memory load), with 240 sentences, some with “basic” word order (canonical) and others in which word order is derived (noncanonical) (Fig. 1). Significant interactions between the factors would indicate a difference in the neural basis for the 2 sentence types.
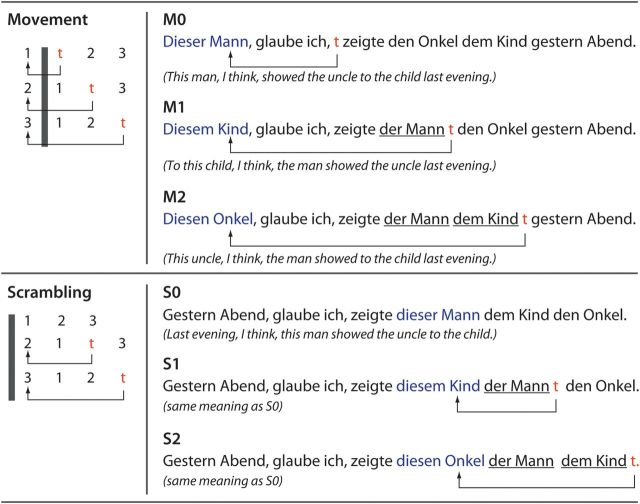
Figure 1.
Schematic representation of Movement and Scrambling, and sentence examples. Left panel: Sentences are derived by a displacement of one of the NPs of the canonical word order sentence. 1, 2, and 3 stand for NPNOM, NPDAT, and NPACC, and 1–2–3 is the canonical word order. In the MOV conditions, when NPDAT (2) moves to immediately before the NPNOM (1), one NP (NPNOM) exists between the moved NP (2) and the trace (t). When NPACC (3) moves to immediately before the NPNOM (1), 2 NPs (NPNOM (1) and NPDAT (2)) intervene between the filler (3) and the trace (t). The distance is measured by the number of intervening NPs between the moved NP and the trace (t). The displacement of NPs and the definition of the distance are the same in the SCR condition, but NPs move within the clause boundary (the gray bars). Right panel: Sentence examples. The distance was denoted as 0, 1, and 2. In the MOV condition, the NPs travel over the clause boundary “glaube ich.” The traces (t) are not pronounced, but the dislocated NPs (blue, connected with t by arrows) are reactivated at the position of t (the gap).
Materials and Methods
Participants
Twenty-two young, right-handed healthy participants were examined (11 females). Handedness was assessed with the Edinburgh Inventory (Oldfield 1971) (mean 93.9, range 80–100). The mean age was 25 years old, and the range was 20–33 years old. All of the participants were native German speakers. Reading span was measured by a German version of the Daneman and Carpenter reading span test (Daneman and Carpenter 1980) (mean 4.0, range 3–5.5). All had no history of neurological disorders. The experimental procedures were approved by the Research Ethics Committees of the University of Leipzig. Written informed consent was given by all participants. The participants were paid 7 euros per hour.
Experimental Design
Stimuli
Our goal was to investigate the relationship between Movement (MOV) and Scrambling (SCR). Sentences (all in German) were divided accordingly into 2 conditions. In both instances, a displaced filler may or may not be separated from its gap by one or more NP interveners. Exploiting this fact, we introduced a 3-valued DISTANCE parameter, which was nested within each condition. DISTANCE took the value of the number of NPs that intervened in the linear sequence between the filler and its gap (Fig. 1).
The 3 levels of the MOV condition were created as follows: at the “canonical” were sentences that can be described schematically as “I think that NPNOM V NPDAT NPACC Temporal Adverb” (where NPNOM, NPDAT, and NPACC denote NPs with case-marked definite articles for nominative, dative, and accusative), for example:
(2)Ich glaube, der Mann zeigte dem Kind den Onkel gestern Abend.
I think, theNOM man showed theDAT boy theACC uncle yesterday night.
Sentences for all 3 levels of Movement were derived from the canonical form by applying Movement to one of the 3 NPs, which moved it to the sentence-initial position, with the result that there were 0, 1, or 2 NP interveners between the filler and its gap (levels M0, M1, M2, respectively, Fig. 1, top). The execution of this operation had several consequences: 1) the moved (or topicalized) NP became the focus of the sentence, which forced the definite article (der/dem/den) to become a demonstrative (dieser/diesem/diesen = thisCASE), similar to English (cf. this man, I think, presented the uncle to the boy). 2) Movement left a trace behind. 3) The main verb glaube was forced into the second position in the sentence (a specific rule in German).
In the Scrambling condition (SCR), which featured a syntactic rule that does not manifest in English, the displacement operation was similarly applied, and 3 levels—S0, S1, and S2—were also created, with 0, 1, and 2 intervener NPs, respectively. The canonical form (which was S0 here) was similar to the canonical of MOV, except for the position of the temporal adverb, which was sentence-initial (Fig. 1, bottom):
(3)Gestern Abend, glaube ich, zeigte dieser Mann dem Kind den Onkel.
yesterday night, think I, showed thisNOM man theDAT boy theACC uncle.
Note that the leftmost NP is focused and appears with a demonstrative. Note also that SCR leaves a trace, and that the verb is in second position, similar to MOV.
In both MOV and SCR, all nouns were masculine and animate and were case-marked unambiguously (i.e., der, dem, and den, for nominative, dative, and accusative, respectively). All verbs (V) were ditransitive, obligatorily taking 2 objects, which were all human (beschreiben = describe; empfehlen = recommend; nennen = name; vermitteln = mediate; zeigen = show). The temporal adverbs were heute / gestern and Morgen / Mittag / Abend (today/yesterday and morning/noon/evening).
In sum, the experiment comprised of 6 conditions derived from a 2 × 3 factorial design with factors TYPE (Movement/Scrambling) and DISTANCE (0/1/2 NPs between the filler and the trace).
Stimuli Presentation
The stimuli presentation was programmed with Presentation 10.3 software (Neurobehavioral Systems, Inc., Albany, California) on a Windows PC. Stimuli were projected through an LCD projector (PLC-XP50L, SANYO, Tokyo, Japan) onto the back of a screen. Participants viewed the images on the screen above their heads through a mirror attached to the head coil.
Procedure
Several days or weeks prior to scanning, the candidate participants performed the same comprehension task with the all sentences used in the fMRI session. Only participants who performed the initial task with a mean accuracy of more than 75% took part in the fMRI experiment. An event-related design was adopted, and the sentences of the 6 conditions were presented in a pseudorandom order. In a trial, a sentence was visually presented word by word with a duration of 500 ms and an interword-interval of 100 ms, so that one sentence was presented with 9 frames (and 8 blanks) in 5.3 s. This allowed us to keep the timing of the input constant across the conditions. Fixed expression of the matrix subject and verb “glaube ich” (I think) and temporal adverbial such as “gestern Abend” (yesterday evening) were presented in 1 frame (e.g., a sentence was segmented as “Gestern Abend,| glaube ich,| zeigte| dieser |Mann| den| Onkel | dem | Kind.”). The beginning of the presentation of the first word/phrase was jittered against the scanning with 0 and 800 ms. Mean sentence onset asynchrony was 11.2 s (for details, see Supplementary Fig. S1). Forty distinct sentences per condition were presented, resulting in a total of 240 trials, which were performed in one session that lasted approximately 45 min.
In order to make the participants actually parse the sentences, we gave comprehension questions after the stimulus sentences. In 20% of the trials (i.e., 8 trials per condition), short probe sentences followed 100 ms after the end of the final word of the sentence and remained on the screen for 3 s. The presentation of trials with a probe was pseudorandomly distributed in each session, so that the participants were not able to predict when the probe would appear, thus requiring them to process all the sentences presented to understand thematic relations. Assignment of probes to sentences was different across participants. The probes assessed thematic role assignment to the NPs, for which correct syntactic processing of the given sentence is indispensable. Half of the probe sentences restated part of the content of the sentence presented previously. They were constructed with the subject, the verb (V), and the object of the correct combination, namely either NPNOM V NPDAT or NPNOM V NPACC. The other half was similarly made with a subject, the verb, and an object but did not match the sentence previously given. For example, we swapped the case marking from dative to accusative or vice versa. Alternatively, one of the objects was presented as the subject. The participants were requested to judge whether the probe sentence expressed the same content or not and to report it as soon as possible by pressing MRI-compatible response buttons using the index and the middle finger of the right hand.
The probes were given in only 20% of trials because we wanted to estimate the hemodynamic responses to the stimulus sentences without overlapping of the hemodynamic responses of the probe sentences. In the present design, the hemodynamic responses to the stimulus sentence were estimated from the trials without probes (80%) and with probes (20%). Since the trials without probes were dominant in number, the hemodynamic response to the sentence was robustly estimated. If all trials had been followed by probes (i.e., 100%), we could not have observed the hemodynamic response to the stimulus sentences without overlapping with the response to the probes, thus compromising the separate estimation of the responses to the stimulus and to the probe. Since the performance was sampled only in 20% of trials in each condition, the performance estimation for a session may be less reliable. However, we reasoned that the performance estimation from 8 probes per condition would allow us to infer how well participants were able to process the sentences in each condition.
Image Acquisition
Functional MRI data were acquired with a whole-body 3 T Magnetom TRIO operating at 3 T (Siemens Medical Solution, Erlangen, Germany) with a gradient-echo echoplanar imaging (EPI) sequence. The brain was covered with 2.5 mm thick 24 axial images with 0.5 mm gaps (repetition time [TR] = 1.6 s, echo time [TE] = 30 ms, flip angle = 90°, field of view [FOV] = 19.2 × 19.2 cm2, 64 × 64 matrix). The resulting voxel size was 3 × 3 × 3 mm3. The slices were aligned to the AC-PC plane and placed to cover the whole of Broca’s and Wernicke’s area. The field map data was also acquired. The same slices as in the EPI were scanned with a T1-weighted MDEFT sequence (TR = 1300 ms, TE = 7.4 ms, 256 × 256 matrix) for the spatial coregistration of EPI images to high-resolution anatomical images. The participants had one session of fMRI scanning with 1682 volumes per session in about 45 min. Structural high-resolution images of the participants were collected on a different day with a three-dimensional MDEFT sequence (TR = 1300 ms, TE = 3.93 ms, time to inversionf = 650 ms, flip angle = 10°, FOV = 25.6 × 24 cm2, 256 × 240 matrix, sagittal 128 slices, 1 mm thick, 2 NEX). During scanning, a stabilization cushion was laid under and to the sides of the head to reduce head motion.
Analysis
Behavioral Data
Mean reaction times (RTs) and accuracy rates were calculated for each condition of each participant and were analyzed using a two-way within-subject analysis of variance (ANOVA) with factors TYPE and DISTANCE. Lack of response was counted as a non-correct response. Mean RT was computed using RTs of correct trials.
Imaging Data
Preprocessing of Structural and Functional MRI Data
The first 5 volumes of the fMRI session were discarded to eliminate magnetic saturation effects, and a total of 1682 volumes were used. The data analysis was carried out using SPM8 (available at http://www.fil.ion.ucl.ac.uk/spm/) on Linux PC workstations. Structural images were co-registered to individuals’ functional images and normalized using the DARTEL procedure (Ashburner 2007), in which individual structural images are segmented into gray and white matter, and mean images of all individuals’ images serve as templates. The DARTEL normalization proceeds in 6 steps with increasing spatial resolution, with the final step for the linear transformation into the Montreal Neurological Institute space. For functional data preprocessing, EPI images were realigned to the first image and resliced with correction for geometrical warping using a deformation field map scan. Subsequently, the difference in the slice acquisition time was corrected, and all volumes were resliced again. The first-level statistics were computed with the unnormalized and unsmoothed images, and the resulting statistical images were normalized using the DARTEL parameters with voxel resampling at 3 × 3 × 3 mm3. We also normalized individual structural and functional data using the individual structural images normalized by DARTEL as the target images.
fMRI Data Analysis
Each participant's hemodynamic responses induced by the trials were modeled with a boxcar function with the duration of 5.3 s for sentences and 3.0 s for probe short sentences (comprehension task) and convolved with a hemodynamic function that reached a peak 6.0 s after the stimuli onset. We model the sentences and probes separately, so that the design matrix has a total of 12 conditions (the sentences of M0, M1, M2, S0, S1, and S2 and the probes of the 6 conditions) instead of 6 conditions (M0, M1, M2, S0, S1, and S2). Six motion parameters were included as covariates of noninterest in the design matrix. The global mean intensity of each session was normalized to 100. Confounds by global signal changes were removed by applying a high-pass filter with a cutoff cycle of 128 s. Signal increase relative to the baseline in each condition of each participant was estimated according to the general linear model. The resulting individual contrast images were normalized using the DARTEL parameters, smoothed with 6-mm full-width at half-maximum Gaussian kernel, and submitted to the second-level (group) analysis, a 2 × 3 within-subject ANOVA with factors TYPE (MOV/SCR) and DISTANCE (3 levels) with correction for nonsphericity. Main effects were tested with t-tests since we were interested in a positive linear effect by DISTANCE. The interactions were examined with an F-test. Statistical inferences were drawn at P < 0.05 at cluster level: the statistical maps (SPM{T}) were thresholded at P < 0.001 (not corrected) for intensity and then thresholded by the cluster size (50 contiguous voxels). A similar threshold was applied to SPM{F}, but cluster level inference is not available.
Since we had a critical interest in the PO and PTr, we also performed the 2 × 3 within-subject ANOVAs within the PO, PTr, and the union of the PO and PTr (hereafter denoted as PO + PTr). In these analyses, the sensitivity of the statistical tests increases because the search volumes are much smaller than the whole brain. We used cytoarchitectonic probabilistic maps to build anatomical masks for the PO and PTr. The cytoarchitectonic map is a digitized 3-dimensional population map of cytoarchitectonic areas. It is based on the analysis of cell body stained sections of 10 postmortem human brains in which borders of areas were determined by statistically significant changes in laminar density patterns of neuronal cell bodies (Roland and Zilles 1998; Amunts et al. 1999; Eickhoff et al. 2005). This is the only anatomical map presently available that considers intersubject variability in space and localization of areas; which is an important prerequisite for comparison with foci of activation. We thresholded the population maps of area 44 and 45 of the left hemisphere at 50% (i.e., we selected the areas that are labeled as 44 or 45 in 5 of 10 brains) and created mask images. Such thresholding enabled to exclude regions with high intersubject variability, that is, regions with a low probability of an area to be present. Since cytoarchitectonic probabilistic maps are not yet available for the whole cortex, we considered in the following macroscopically defined regions of interest.
Trial Time Course Plot
To inspect the main effects in detail, trial time courses (TTCs) of the activated foci were plotted. First, the volumes of interest (VOIs) were defined as 6 mm radius spheres with the individual local maxima nearest to the group maxima for the PO, inferior frontal sulcus (IFS), middle part of the middle temporal gyrus (mMTG), and intraparietal sulcus (IPS) for the main effect of DISTANCE and inferior and superior occipital gyri (IOGs and SOGs) for the main effect of TYPE (see Table 2). All VOIs were in the left hemisphere. The selection of maxima was performed in each participant using his/her own statistical map. Second, VOI time series data were extracted as eigenvariates (without adjustment) from the voxels that showed activation in the F-contrast for the effect of interest contrast with a threshold of P < 0.05 (not corrected) (the null hypothesis for this contrast is no activation in all conditions), and the TTCs were estimated for each participant’s preprocessed (upsampled to have data points at every 0.8 s by a piecewise cubic Hermite interpolation, high-pass filtered [128 s], and linear trend removed) time series data. The TTCs for each condition were estimated as follows: The TTC of sentence and probe in each condition in a VOI were modeled with 21 variables representing the BOLD (blood oxygen level–dependent) signal every 0.8 s from 0 to 16.0 s after the stimuli or probe onset. No assumption was made for the shape of the hemodynamic response functions. We assumed that the TTC holds the same shape throughout the scanning (i.e., assumption for the linear time invariant system). Then, we made a general linear model:
Table 2.
Activation revealed by the within-subject ANOVA
| Anatomical region | Cluster P | Cluster size | Peak Z | Coordinates |
| Main effect of DISTANCE (as the linear effect S/M0 < S/M2) | ||||
| Left | ||||
| IFS | 0.000 | 653a | 5.50 | −36, 6, 33 |
| PO | 0.000 | 653a | 5.47 | −51, 15, 18 |
| IPS | 0.000 | 171 | 5.21 | −33, −51, 36 |
| Precuneus | 0.020 | 64 | 4.41 | 9, −66, 42 |
| mMTG | 0.031 | 57 | 4.30 | −54, −36, −6 |
| Globus pallidus | 0.033 | 56 | 3.86 | −21, −15, 6 |
| Right | ||||
| IPS | 0.031 | 57 | 4.18 | 33, −45, 39 |
| PO | 0.001 | 127 | 4.17 | 45, 21, 21 |
| Main effect of TYPE | ||||
| MOV > SCR | ||||
| Left | ||||
| IOG | 0.000 | 813 | 5.77 | −18, −93, −6 |
| SOG | 0.017 | 67 | 4.15 | −33, −78, 21 |
| SCR > MOV | ||||
| n.s. |
Note: The unit for the cluster size is voxel, which is 27 mm3. Statistical inferences were drawn at P < 0.05 corrected for multiple comparisons (The statistical images were thresholded at P < 0.001 for peak height in each voxel, then the cluster size threshold <50 voxels was applied). n.s. = not significant.
The same cluster.
where Y stands for the preprocessed time series data of a VOI, X design matrix, β the estimates for hemodynamic response, and e the error term. The design matrix X was created as follows. If a trial of ith condition was given at time t, we add a 21 × 21 identity matrix I from the ith column and tth row of an m (= length of upsampled time series, 3364) × n (number of conditions = 12, namely the sentences and the probes for M0, M1, m2, S0, S1, and S2) zero matrix. X was obtained by creating this matrix for each trial of each condition and summing all of them up. The β is a column vector consisting of the estimates for the 12 conditions in order and is estimated by applying a Moore–Penrose pseudoinverse of X to Y. This analysis is a variation of a finite impulse response analysis applied to whole-brain data with parametric modulation by RT (Weissman et al. 2006).
Results
Behavioral Results
Accuracy and RTs to the probe sentence are summarized in Table 1. One participant, who performed at 50% (chance level) on S2, M1, and M2 conditions, was excluded from the behavioral and fMRI analyses. A 2 × 3 within-subject ANOVA with factors TYPE (MOV/SCR) and DISTANCE (3 levels) was performed on accuracy and RT. For accuracy, the interaction was significant (F2,40 = 5.06 [P < 0.05]). An analysis of simple main effect revealed a significant effect of TYPE at DISTANCE 2 (F1,20 = 14.01 [P < 0.01]) and a significant effect of DISTANCE at SCR (F2,40 = 11.47 [P < 0.01]). These results indicate that S2 is significantly more difficult than the other conditions. A 2 × 3 within-subject ANOVA for RT did not show any significant main effects or interactions.
Table 1.
Accuracy and RT of the probe sentence judgment
| M0 | M1 | M2 | S0 | S1 | S2 | |
| Accuracy (%) | 94.5 (9.53) | 92.8 (10.2) | 90.9 (11.4) | 90.8 (SD 9.96) | 91.5 (10.0) | 77.2 (17.9) |
| RT (ms) | 2021 (341) | 2051 (404) | 2051 (412) | 2173 (320) | 2060 (382) | 2044 (403) |
Note: The results are for 21 participants. One participant was excluded because of poor performance.
Imaging Results
Whole-Brain Analysis
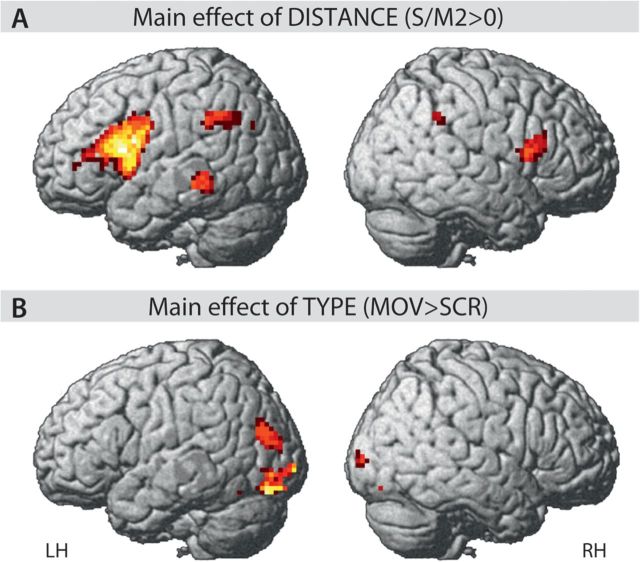
A 2 × 3 within-subject ANOVA with factors TYPE (MOV/SCR) and DISTANCE (3 levels) was performed on the whole-brain fMRI data (Figs 2, 3, and 4 and Supplementary Figs S2, S3, and S4). Firstly, no significant interaction was found. Secondly, a main effect of TYPE (as t contrast) was only found in the bilateral occipital regions, including IOGs and SOGs (Figs 2 and 4 and Table 2). Crucially, a main effect of DISTANCE (as a t contrast for S/M0 < S/M2) involves the left inferior frontal gyrus (IFG), including the PO (BA 44), the posterior portion of the PTr (BA 45), and the IFS. The total volume of the left interior frontal gyrus (LIFG) activation cluster in the cytoarchitectonic areas 44 and 45 is 3.67 cm3, which consist of areas 44 (69%) and 45 (31%) (Supplementary Fig. S2). Moreover, activation was found in the mMTG in the left temporal cortex, as well as the left globus pallidus, the right pars opercularis (RPO), and the bilateral intraparietal sulci (IPS/RIPS) (Figs 2 and 3 and Table 2). Although the activation is identified as a main effect of DISTANCE for both sentence types, a linear increase of activation with the level of DISTANCE is found only for the SCR conditions (Fig. 3 and Supplementary S2, S3, and S4). Especially, the BOLD signal in M1 and M2 is almost same in all 4 VOIs (Fig. 3 and Supplementary Fig. S4). Moreover, all 3 levels showed a similar activation in the mMTG (Fig. 3). The TTC plots for the occipital activation in the SOG and the IOG reflect the difference in the TYPE of sentences (Fig. 4). Of note are the 2 peaks of the TTC plots of the occipital activation for the MOV conditions.
Figure 2.
ANOVA results (A). Main effect of DISTANCE as a t contrast of S/M2 > S/M0. (B). Main effect of TYPE as a t contrast of MOV > SCR. Statistical inferences were drawn at P < 0.05 (corrected).
Figure 3.
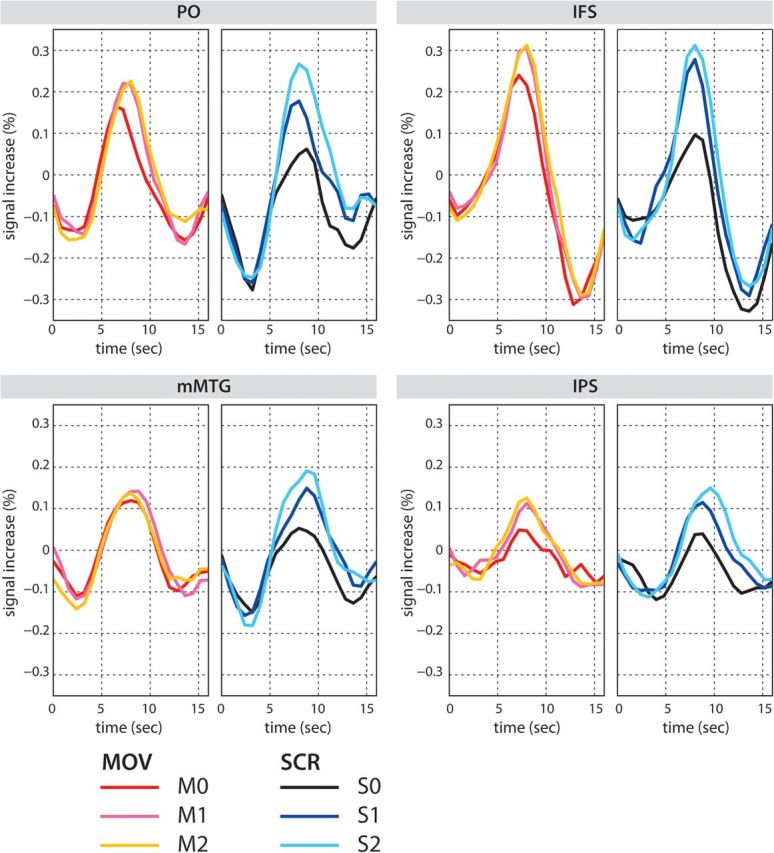
TTC plots for the activated regions revealed by the main effect of DISTANCE. The TTCs in the PO, IFS, mMTG, and IPS are shown. Note that a linear increase of activation is seen in the SCR condition, while the TTCs for M1 and M2 are almost same in the PO, IFS, and IPS. In the mMTG, MOV conditions show almost the same level of activation. For the statistical tests on the peak signals, see Supplementary Analysis.
Figure 4.
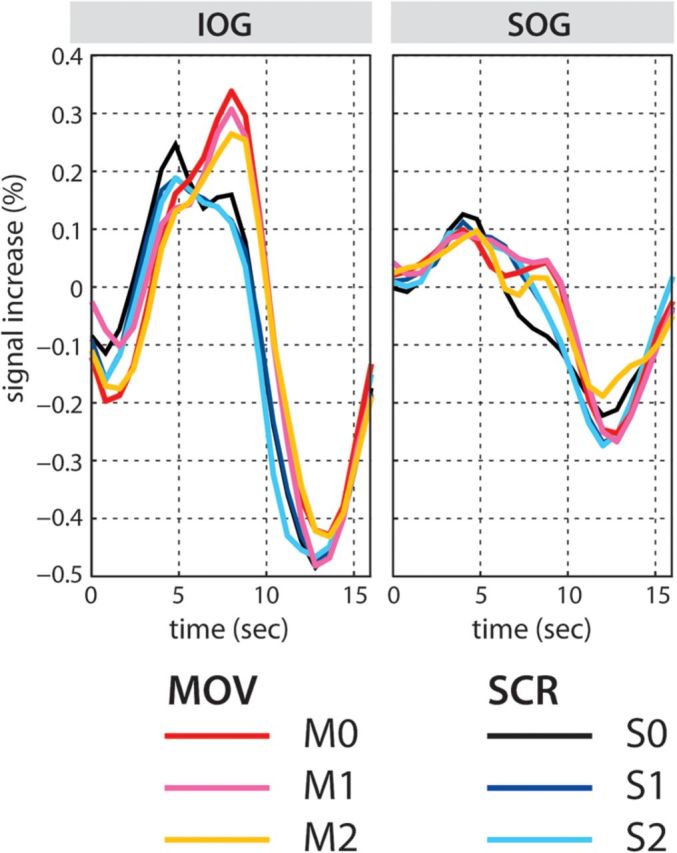
TTC plots for the activated regions revealed by the main effect of TYPE In the IOG and SOG. The TTCs for MOV conditions show later peaks compared to those for SCR conditions.
Individual Peak Analysis in VOIs
To qualitatively evaluate the difference in the effect of DISTANCE in MOV and SCR, we performed similar 2 × 3 within-subject ANOVAs using the individual peak signals at the time of the peaks in the group mean plots of the TTCs (Fig. 3). In this analysis, we found significant interactions in the PO (F2,40 = 6.39 **P < 0.01), IFS (F2,40 = 3.37 *P < 0.05), and mMTG (F2,40 = 6.47 **P < 0.01) but not in the left IPS (F2,40 = 0.14 ns). For more details, see Supplementary Analysis.
ANOVAs were also performed in the cytoarchitectonically defined masks of PO, PTr, and their union. Similar ANOVAs in the anatomical masks (PO, PTr, and conjoined PO + PTr) showed a main effect of DISTANCE (as a t contrast for S/M0 < S/M2) in the PO mask (cluster P = 0.00, cluster size 133, peak z = 5.03 [−51, 15, 18]), PTr mask (cluster P = 0.00, cluster size 176, peak z = 5.69 [−51, 27, 21]), and the PO + PTr mask (cluster P = 0.00, cluster size 550, peak z = 5.49 [−51, 27, 21]). The interaction and the main effect of TYPE were not significant in all analyses.
Discussion
Brain Reflections of Movement Distance in Sentence Processing
The present fMRI study used a 2 × 3 factorial design to compare the neural activity caused by memory operation at the 3 levels of memory load (factor DISTANCE) in 2 sentence constructions, namely Movement and Scrambling (factor TYPE). A main effect of DISTANCE was found in the left inferior frontal gyrus (IFG) encompassing the PO, posterior portion of PTr, and IFS, and in the mMTG and IPS. The IFG and the mMTG in the left hemisphere are often coactivated in sentence processing and comprise a core language network to compute linguistic information (Roder et al. 2002; Bornkessel et al. 2005; Demonet et al. 2005; Hoen et al. 2006; Vigneau et al. 2006; Newman et al. 2010; Price 2010; Friederici et al. 2011). Of these 2 regions, the IFG has more reliably been activated by filler–gap distance (Friederici et al. 2006; Santi and Grodzinsky 2010), and generally, the activation observed in the left temporal lobe during sentence processing is more superior than that observed in the current study. The location in the mMTG found in the current study is more often associated with conceptual and/or lexical processing (Dronkers et al. 2004; Martin 2007). However, there is quite a bit of variability across studies with lexical processes which also demonstrate activation in superior temporal regions (Wise et al. 1991), thus it is likely that both middle and superior temporal cortex are engaged in semantic processes at the lexical and syntactic level. Given the single word presentation used in the current study, it is possible that it biased activation to the lexicon and that the longer the filler–gap distance, the greater the difficulty in reaccessing the filler from the lexicon. Both syntactic and semantic processing demands increase as the distance between the filler and the gap increases, suggesting that the filler–gap dependency is solved by the maintenance of syntactic and semantic features of the filler until the gap is met.
Crucially, the interaction between the 2 factors in the different regions is not significant, not even when the analysis is confined to cytoarchitectonically defined PO, PTr, or the conjoined PO and PTr (PO + PTr). Hence, these results do not indicate a differential involvement of PTr and PO for the 2 constructions but rather suggest that the same brain region supports the processing of the filler–gap dependency relation in both Movement and Scrambling constructions. This finding is in line with the behavioral results reporting similarities across different studies which are conducted in English (Movement) and in German and Japanese (Scrambling) (Tanenhaus et al. 1985; Clifton and Frazier 1988; McElree and Bever 1989; Clahsen and Featherston 1999; Nakano et al. 2002).
According to a recent receptoarchitectonic study of Broca's area, the PTr has anterior/posterior subdivisions (45a and 45p) (Amunts et al. 2010). The LIFG cluster is located mainly in the PO and extends anteriorly to the posterior part of the PTr (Fig. 2 and Supplementary Fig. S2). Thus, the present activation in the PTr seems to be in the 45p (Amunts et al. 2010) and may point to functional difference between the 45a and the 45p in the sentence processing.
With respect to linguistic theory, the present study supports the view that Scrambling is an instance of Movement (Fanselow 1990, 2001; Müller and Sternefeld 1993). By comparing the 2 constructions within a single language, the present study dissolves the apparent difference between the finding that the processing of Movement constructions in English maximally activates the PTr (Santi and Grodzinsky 2007, 2010), while Scrambling constructions in German maximally activate the PO (Friederici et al. 2006). The results from the present within-language and within-subject design indicate that, in principle, filler–gap processing in the 2 constructions involve both the PO and the posterior portion of PTr as parts of Broca's area.
Difference between Processing Movement and Scrambling Constructions
A closer look at the TTC plots of the brain activations (Fig. 3), however, reveals an interesting difference between Movement and Scrambling. Activation in the left PO increased systematically (i.e., S0 < S1 < S2) as a function of the number of intervening NPs between the filler and the gap for Scrambling sentences, whereas the difference between the 2 noncanonical sentences in the Movement conditions did not (i.e., M0 < M1 ≈ M2). The ANOVA using the individual peak values taken from the reconstructed TTCs revealed a significant interaction between the factors DISTANCE and TYPE (for details, see Supplementary Analysis), confirming this difference between Movement and Scrambling. The differential outcomes between the anatomically defined analyses (i.e., whole brain as well as the cytoarchitectonic mask analyses), on the one hand, and the functionally defined VOI analyses, on the other, may be due to the increased sensitivity in the latter analysis and/or the interindividual spatial variability of the functional loci.
While the finding of a linear increase of activation in Scrambling replicates the results previously reported for scrambled German sentences (Friederici et al. 2006), the nonlinear increase in the Movement construction in German is a novel observation. Note that in the present Movement constructions, one of the case-marked NPs moves out from the subordinate clause to the sentence-initial position, which is a special position to make the constituent salient. This sentence-initial position is advantageous in terms of verbal short-term memory demands, since short-term memory for items in a sequence is most robust at start and end positions but deteriorates at the middle positions (Henson 1999). For sentence processing, this might mean that a filler in a topicalized position is encoded robustly in memory, and the parser may therefore be able to establish a strong link between the filler and the gap such that the link is not disturbed by 1 or 2 intervening NPs. As a result of this strong link, the difference between M1 and M2 might shrink. In contrast, Scrambling in the present study moves the constituent into the middle position of the sentence (Fig. 1), which is an unfavorable region in terms of short-term memory encoding (at least without prosodic cue). The latter may make the processing of filler–gap dependency more sensitive to the number of interveners, resulting in the linear increase of activation with distance.
Another cognitive aspect that may have a differential impact on processing of the 2 sentence types is the syntactic predictability of the sentence structure. A case-marked object (i.e., nonsubject) NP at the sentence-initial position in the Movement construction may allow the parser to effectively assign a thematic role to this NP. This may in turn allow predictions of the forthcoming 2 thematic roles. For example, when the sentence-initial NP is the indirect object, the subject and direct object NPs are predicted. The prediction of the forthcoming elements in visually presented sentences may drive visual attention and elicit activation in the occipital regions (Fig. 2B) as documented in a previous study using a syntactic violation paradigm (Dikker et al. 2009). This syntactic prediction may facilitate filler–gap processing in the Movement sentences. By contrast, topicalization of a temporal adverb, as in the present Scrambling sentences, does not allow such predictions. Since the temporal information is orthogonal to thematic role assignment, robustly encoded temporal information in topicalized position is not relevant for thematic role assignment. Note that this does not represent the general difference between Movement and Scrambling but rather provides a possible explanation for the processing difference observed for the sentences used in the present study. We thus consider the occipital activation observed in the MOV > SCR contrast to be due to the visual attention caused by syntactic prediction driven by the sentence-initial case-marked object NP.
Memory Load Effect in the IPS
The bilateral IPS also revealed a main effect of DISTANCE. The IPS has been identified as a neural correlate of short-term memory of visual objects (Todd and Marois 2004; Klingberg 2006; Xu and Chun 2006; Champod and Petrides 2007), as well as verbal items (Champod and Petrides 2010; Majerus et al. 2010). Since this region was not activated in the previous Scrambling (Roder et al. 2002; Friederici et al. 2006; Kinno et al. 2008; Obleser et al. 2011) and Movement (Ben-Shachar et al. 2003; Fiebach et al. 2005; Santi and Grodzinsky 2007, 2010) studies, the activation in the present study might reflect memory processes required specifically in the current task. We speculate that the special memory demand in the present study could be associated with the handling of the 3 NPs (which all contain animate nouns, i.e., persons). Thematic role assignment of 3 persons in the noncanonical sentence will demand more verbal working memory resources than in the canonical sentences, thereby increasing the activation in the IPS.
Conclusion
The present study demonstrates that memory processes involved in the processing of filler–gap relations in noncanonical sentences constructed either by Movement or Scrambling operations are supported by a neural network consisting of the PO, IFS, mMTG, and IPS. Analyses on the MRI signal taken from the individual activation peak reveal an interaction between the type of sentence construction and the memory due to the filler–gap distance. Taken together, these results suggest that Movement and Scrambling employ the same neural basis for the filler–gap processing, with slight quantitative difference in the linear effect of distance.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Partial support for this project was provided by an Alexander von Humboldt Foundation Research Award (Y.G.) and by National Institute of Health (grant #00094), Social Sciences and Humanities Research Council (standard grant #410-2009-0431), Canada Research Chairs (Y.G.), and the German Ministry of Education and Research (BMBF; grant 01 GW0773 A.F. and 01 GW0771 K.A. and A.F.).
Supplementary Material
Acknowledgments
We are grateful to Annet Wiedemann, Wipper Simone, and Anke Kummer for MRI data acquisition, to Kerstin Flake and Andrea Gast-Sandmann for the graphics, and to Rosie Wallis for English correction. Conflict of Interest: None declared.
References
- Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, Zilles K. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8:e1000489. doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, von Cramon DY, Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007;104:14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: a parametric functional magnetic resonance imaging study. J Neurosci. 2010;30:3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N. Lectures on government and binding: the pisa lectures. Dordrecht, Holland: Foris Publications; 1981. [Google Scholar]
- Chomsky N. The minimalist program. Cambridge (MA): MIT Press; 1995. [Google Scholar]
- Clahsen H, Featherston S. Antecedent priming at trace positions: evidence from German scrambling. J Psycholinguistic Res. 1999;28:415–437. [Google Scholar]
- Clifton C, Jr, Frazier L. Comprehending sentences with long-distance dependencies. In: Tanenhaus M, Carlson G, editors. Linguistic structure in language processing. Dordrecht (The Netherlands): Reidel; 1988. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verb Learn Verb Behav. 1980;19:450–466. [Google Scholar]
- Demonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Dikker S, Rabagliati H, Pylkkanen L. Sensitivity to syntax in visual cortex. Cognition. 2009;110:293–321. doi: 10.1016/j.cognition.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fanselow G. Scrambling as NP-movement. In: Grewendorf G, Wolfgang S, editors. Scrambling and barriers. Amsterdam: Benjamins; 1990. [Google Scholar]
- Fanselow G. Features, θ-roles, and free constituent order. Linguist Inq. 2001;32:405–437. [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G. Maturation of the language network: from inter- to intrahemispheric connectivities. PLoS One. 2011;6:e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel ID, von Cramon DY. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M. The emergence of the unmarked: a new perspective on the language-specific function of Broca's area. Hum Brain Mapp. 2005;26:178–190. doi: 10.1002/hbm.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN. Positional information in short-term memory: relative or absolute? Mem Cognit. 1999;27:915–927. doi: 10.3758/bf03198544. [DOI] [PubMed] [Google Scholar]
- Hoen M, Pachot-Clouard M, Segebarth C. When Broca experiences the Janus syndrome: an ER-fMRI study comparing sentence comprehension and cognitive sequence processing. Cortex. 2006;42(4):605–623. doi: 10.1016/s0010-9452(08)70398-8. [DOI] [PubMed] [Google Scholar]
- Kinno R, Kawamura M, Shioda S, Sakai KL. Neural correlates of noncanonical syntactic processing revealed by a picture-sentence matching task. Hum Brain Mapp. 2008;29:1015–1027. doi: 10.1002/hbm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Müller G, Sternefeld W. Improper movement and unambiguous binding. Linguist Inq. 1993;24:461–507. [Google Scholar]
- Majerus S, D'Argembeau A, Martinez Perez T, Belayachi S, Van der Linden M, Collette F, Salmon E, Seurinck R, Fias W, Maquet P. The commonality of neural networks for verbal and visual short-term memory. J Cogn Neurosci. 2010;22:2570–2593. doi: 10.1162/jocn.2009.21378. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McElree B, Bever TG. The psychological reality of linguistically defined gaps. J Psycholinguist Res. 1989;18:21–35. [Google Scholar]
- Nakano Y, Felser C, Clahsen H. Antecedent priming at trace positions in Japanese long-distance scrambling. J Psycholinguist Res. 2002;31:531–571. doi: 10.1023/a:1021260920232. [DOI] [PubMed] [Google Scholar]
- Newman SD, Ikuta T, Burns T. The effect of semantic relatedness on syntactic analysis: an fMRI study. Brain Lang. 2010;113:51–58. doi: 10.1016/j.bandl.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Meyer L, Friederici AD. Dynamic assignment of neural resources in auditory comprehension of complex sentences. Neuroimage. 2011;56(4):2310–2320. doi: 10.1016/j.neuroimage.2011.03.035. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Roder B, Stock O, Neville H, Bien S, Rosler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage. 2002;15:1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Roland PE, Zilles K. Structural divisions and functional fields in the human cerebral cortex. Brain Res Brain Res Rev. 1998;26:87–105. doi: 10.1016/s0165-0173(97)00058-1. [DOI] [PubMed] [Google Scholar]
- Ross JR. Constraints on variables in syntax. Cambridge: MIT; 1967. [Google Scholar]
- Saito M. Scrambling as semantically vacuous A′-movement. In: Baltin M, Kroch A, editors. Alternative conceptions of phrase structure. Chicago (IL): University of Chicago Press; 1989. [Google Scholar]
- Santi A, Grodzinsky Y. Working memory and syntax interact in Broca's area. Neuroimage. 2007;37:8–17. doi: 10.1016/j.neuroimage.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Santi A, Grodzinsky Y. fMRI adaptation dissociates syntactic complexity dimensions. Neuroimage. 2010;51:1285–1293. doi: 10.1016/j.neuroimage.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenhaus MK, Carlson GN, Seidenberg MS. Do listeners compute linguistic representations? In: Dowty DR, Karttunen L, Zwicky AM, editors. Natural language parsing: psychological, computational, and theoretical perspectives. New York: Cambridge University Press; 1985. [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Webelhuth G. Syntactic saturation phenomena and the modern Germanic languages [PhD dissertation] [Amherst (MA)]: University of Massachusetts; 1989. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114(Pt 4):1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.