Abstract
Purpose
To report a case of acyclovir-resistant herpetic keratitis in a solid-organ lung transplant recipient that was effectively treated with topical trifluridine.
Methods
A case of a 35-year-old female with herpetic epithelial keratitis resistant to acyclovir is described. The patient presented following treatment for 4 weeks with topical acyclovir ointment five times per day and oral valacyclovir 1 g three times per day for herpetic keratitis with no resolution of the epithelial defect or symptoms. Corneal scrapes and swabs were taken for confirmation of the diagnosis and resistance testing. The results were positive for herpes simplex virus 1 and showed acyclovir resistance (inhibitor concentration 90 = 200 μg/mL) and foscarnet sensitivity (inhibitor concentration 90 = 200 μg/mL). The patient was treated with topical trifluridine 2-hourly for 3 weeks and weaned off the drops over the following week.
Results
The patient showed resolution of the epithelial defect, but did have significant corneal toxicity associated with the use of the trifluridine. At 8 weeks, the patient had some stromal shadowing associated with the recent active infection, but symptoms had settled.
Conclusion
This case documents the effective use of topical trifluridine in proven acyclovir-resistant herpetic keratitis. It highlights three things: (1) the importance of considering topical trifluridine as an alternative to topical acyclovir in unresponsive disease; (2) the need to consider solid-organ transplant recipients in the immunocompromised population with resistant herpetic disease, and (3) the need to look for alternatives to treatment of resistant herpetic disease.
Keywords: acyclovir resistance, herpetic keratitis, trifluridine
Introduction
Topical trifluridine has known efficacy in the treatment of herpetic keratitis, but its usefulness in acyclovir-resistant disease is not well known. Furthermore, within Australia, where trifluridine is not readily available and the only topical treatment available is acyclovir, the clinician needs to be mindful of the possibility of resistance in unresponsive proven herpetic keratitis. Acyclovir is a potent and highly selective antiviral nucleoside analogue, which remains one of the standard treatments for herpes simplex virus (HSV) infections in both normal and immunocompromised patients. The triphosphorylated form of acyclovir serves as both a substrate and inhibitor of the herpes DNA polymerase. Acyclovir requires viral thymidine kinase to undergo phosphorylation to its active form prior to being able to inhibit DNA polymerase and subsequent replication. The same is the case for acyclovir’s valyl ester prodrug valacyclovir. Valacyclovir is converted to acyclovir by intestinal and hepatic metabolism. Acyclovir resistance is relatively uncommon, but needs to be considered in herpetic disease, which is unresponsive to both oral and topical therapy. Resistance typically occurs via mutations to viral thymidine kinase, resulting in complete deficiency or decreased production, which prevents the conversion of acyclovir and its analogues to their active form.1 Acyclovir-resistant herpes simplex virus has been shown to be uncommon in the immunocompetent population (0.1%–0.98%).2,3 Resistant viral isolates are more commonly found in the immunocompromised population (3.92%–14.3%), including those with acquired immunodeficiency virus and following stem cell transplantation.2,3 Within this article, we report a case from another group of immunocompromised patients who may be at risk of acyclovir-resistant ophthalmic herpes and the effective use of topical trifluridine. This is a case report of a bilateral lung-transplant recipient with proven acyclovir-resistant herpetic keratitis successfully treated with topical trifluridine.
Case presentation
A 35-year-old female presented to the Princess Alexandra Hospital Eye Casualty in 2010, as a referral from a private ophthalmologist for ongoing management of her left epithelial herpetic keratitis. She had a history of bilateral lung transplantation performed in 2002 for cystic fibrosis and was currently on an immunosuppressive regimen of tacrolimus 2 mg twice daily and prednisolone 5 mg once daily, which she had been on for the duration of her transplantation. Prior to this episode of herpetic keratitis, she had been on a suppressive dose of valacyclovir (Valtrex) 500 mg twice per day since February 2008 for previous episodes of herpetic keratitis. On initial presentation at Eye Casualty, she was currently being treated with valacyclovir (Valtrex) 1 g three times per day and acyclovir (Zovirax) 3% ointment 5 times per day for a total duration of 4 weeks, with little resolution of signs or symptoms. The patient had been compliant with therapy. Prednisolone acetate 1% (Prednefrin Forte) 1 drop in the morning was subsequently commenced, which the patient had been taking for approximately 1 week.
On initial examination at Eye Casualty, the left eye had an uncorrected visual acuity of 6/36 and a pinhole acuity of 6/12. A large dendritiform lesion, with its leading edge extending from the 9 o’clock corneolimbal border horizontally across, with branches extending superiorly and inferiorly, covered an approximate surface area of 3 × 3 mm. There was an associated anterior stromal haze underlying the epithelial defect of the dendrite. There was no deep stromal reaction, neovascularization to the cornea or anterior chamber reaction. At presentation to Eye Casualty, it was suspected that the patient may have acyclovir-resistant disease due to the duration of therapy and the apparent lack of clinical response. The prednisolone acetate was immediately ceased, as its reason for use was not readily apparent. A corneal scrape (inoculating corneal tissue into viral support media) and dry viral swab for polymerase chain reaction was subsequently taken to confirm the clinical diagnosis and to assess acyclovir sensitivity. The scraping also acted as a form of mechanical debridement. Polymerase chain reaction was positive for HSV-1. Sensitivities were determined by measuring the concentration of antiviral required to inhibit 90% of viral replication (IC90). This confirmed acyclovir resistance (IC90 = 200 μg/mL, with 200 μg/mL not being sufficient to inhibit 90% of viral replication) and sensitivity to foscarnet (IC90 = 200 μg/mL). Sensitivity testing to trifluridine was not performed, as this facility was not available at the lab that conducted the test. The patient was subsequently maintained on valacyclovir (Valtrex) 1 g three times per day and trifluridine (Viroptic) 1% eyedrops were commenced every 2 hours in the left eye for a period of approximately 3 weeks, whilst awaiting sensitivity testing. The patient was slow to respond, but showed resolution of the epithelial defect over a 3-week period with significant corneal toxicity secondary to the trifluridine in the form of conjunctival hyperaemia and punctate epitheliopathy (Figure 1). On final review at 8 weeks, the patient had a final visual acuity of 6/24 uncorrected and a pinhole acuity of 6/12. The corneal epithelial defect had completely resolved, leaving an underlying anterior stromal haze in the footprint of the original dendrite. The patient has not had recurrence of disease to our knowledge since this episode.
Figure 1.
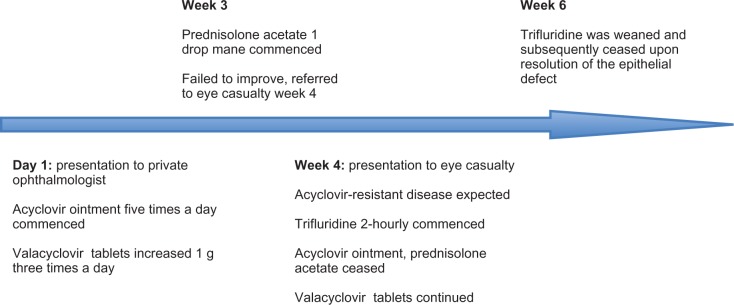
Timeline of treatment.
Discussion
This case highlights the successful use of trifluridine eyedrops in a proven case of acyclovir-resistant herpetic keratitis. Trifluridine, although not used in Australia, has a long history of use in other parts of the world, including the United States, where topical acyclovir is not readily available. It has a different mechanism of action to acyclovir, and is therefore a reasonable alternative when acyclovir-resistant disease is expected. Specifically, trifluridine interferes with viral replication by incorporating into viral DNA in place of thymidine, inhibiting thymidylate synthetase, resulting in the formation of defective proteins. There is only one other existing report within the literature highlighting the effectiveness of trifluridine in acyclovir-resistant proven corneal disease.4 That case highlighted the use of trifluridine in HSV epithelial keratitis caused by an acyclovir-resistant HSV frameshift mutation in a 7Gs homopolymer region.4 Our case further highlights the difficulty with treating acyclovir-resistant disease, with limited topical options available. Discussion was had with the patient about the possibility of intravenous foscarnet being required for treatment of her herpetic eye disease prior to the return of sensitivities, but the patient was extremely reluctant to have inpatient treatment for her infection. This case highlights three important points: (1) the need to consider solid-organ transplant recipients in the immuncompromised group who may be at an increased risk of acyclovir-resistant herpes infections, (2) the limited options available for topical and oral treatment for acyclovir-resistant herpetic infections, and (3) the need to consider topical trifluridine in cases where acyclovir resistance is suspected.
A number of cases of acyclovir-resistant herpetic eye disease have been reported in the literature, with the majority of identified cases having to be treated with intravenous foscarnet in order to produce resolution of the infection. This includes one article that reported two cases of proven acyclovir-resistant herpetic keratitis and one suspected case of herpetic keratitis that were unresponsive to topical and oral acyclovir/valacyclovir and subsequently required intravenous foscarnet.5 A further two articles, one by Pratuangtham et al, reported the case of a patient with Wiskott–Aldrich syndrome who developed cutaneous and corneal herpetic infections that were resistant to acyclovir (intravenous acyclovir 30 mg/kg), also requiring intravenous foscarnet.6 The second article, being a case report by Bodaghi et al, reported the case of a 31-year-old female with AIDS who developed bilateral dendritic corneal ulcers associated with severe disciform keratitis that was unresponsive to acyclovir treatment (800 mg 5 times a day; topical 5 times a day) and subsequently required intravenous foscarnet.7 These case reports emphasize the difficulty with treating acyclovir-resistant herpetic eye disease, with limited topical and oral options. The majority of reported cases within the literature have required intravenous foscarnet. We were only able to identify one other case of proven acyclovir-resistant disease that was treated successfully with trifluridine alone.4
Options for treatment for acyclovir-resistant herpes in the literature are cited as being foscarnet, cidofovir, trifluridine, vidarabine, and interferon. The first two options, which are readily available within Australia, are typically only found in an intravenous preparation. Reports in the literature for the topical use of these agents do exist, but there is limited availability of these drugs in topical preparation and not much experience with their use.8,9 Trifluridine has been reported as being an option for the treatment of acyclovir-resistant herpetic keratitis, but again its availability within Australia is limited and its efficacy is not proven.9 The same is the case for vidarabine. It is no longer available and requires special formulation by compounding pharmacists.
This case highlights the need to consider acyclovir-resistant herpetic disease in immunosuppressed individuals, primarily solid-organ transplant recipients. It also highlights the necessity to consider trifluridine and the importance of conducting studies on the treatment of topical foscarnet in herpetic eye disease and for a commercially available preparation to be made.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Duan R, de Vries RD, van Dun JM, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 2009;200:1402–1414. doi: 10.1086/606028. [DOI] [PubMed] [Google Scholar]
- 2.Christophers J, Clayton J, Craske J, et al. Survery of the resistance of herpes simplex virus to aciclovir in northwest England. Antimicrob Agents Chemother. 1998;42:868–872. doi: 10.1128/aac.42.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to aciclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Suzuki T, Shiraishi A, Shimamura I, Inoue Y, Ohashi Y. Dendritic keratitis caused by an acyclovir-resistant herpes simplex virus with frameshift mutation. Cornea. 2007;26:105–106. doi: 10.1097/01.ico.0000240081.19635.db. [DOI] [PubMed] [Google Scholar]
- 5.Choong K, Walker NJ, Apel AJ, Whitby M. Aciclovir-resistant herpes keratitis. Clin Experiment Ophthalmol. 2010;38:309–313. doi: 10.1111/j.1442-9071.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- 6.Pratuangtham S, Bornstein SM, Boyer KM, McAuley JB, Deutsch TA, Gotoff S P. Treatment of acyclovir-resistant herpes simplex virus keratitis in a patient with Wiskott-Aldrich syndrome. Clin Infect Dis. 1997;25(5):1257–1258. doi: 10.1086/516968. [DOI] [PubMed] [Google Scholar]
- 7.Bodaghi B, Mougin C, Michelson S, et al. Acyclovir-resistant bilateral keratitis associated with mutations in the HSV-1 thymidine kinase gene. Exp Eye Res. 2000;71(4):353–359. doi: 10.1006/exer.2000.0886. [DOI] [PubMed] [Google Scholar]
- 8.Levin MJ, Bacon TH, Leary JJ. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin Infect Dis. 2004;39( Suppl 5):S248–S257. doi: 10.1086/422364. [DOI] [PubMed] [Google Scholar]
- 9.Behrens-Baumann W. Phosphonoformate versus trifluorthymidine in the treatment of keratitis dendritica in the human. Acta Ophthalmol (Copenh) 1992;70(5):690–692. doi: 10.1111/j.1755-3768.1992.tb02154.x. [DOI] [PubMed] [Google Scholar]


