Abstract
Tactile co-activation, i.e., synchronous stimulation of a region of skin, has been reported to improve tactile spatial acuity and expand the corresponding somatosensory cortical representation. The current study aimed to clarify the nature of the changes resulting from tactile co-activation, using three measures of tactile sensitivity obtained with controlled mechanical stimulation. One was the grating orientation (GR/OR) discrimination task, where acuity is indexed by the threshold groove width required for 75% correct discrimination between two orthogonal orientations of a grating on the fingerpad. Since this task may be susceptible to intensity cues due to tactile anisotropy, another acuity measure, the 3-dot task, was also used. In this task, the acuity threshold corresponds to 75% correct discrimination of the direction of offset of the central dot in a 3-dot array. In Experiment 1, co-activation failed to induce significant improvement in acuity with either of these measures. Experiment 2 employed both the GR/OR task and a third measure based on discriminating a grooved from a smooth surface (SM/GV). While the former task demands detailed spatial resolution, the latter requires only that spatial modulation in the afferent population be detected. This experiment also included a control group. GR/OR performance did not significantly improve for either the control or experimental groups. There was, however, a significant improvement in SM/GV performance following co-activation for the experimental but not the control group. These findings indicate that the SM/GV task may be better suited than the GR/OR or 3-dot tasks for measuring changes in tactile sensitivity following co-activation.
Keywords: tactile acuity, gratings, 3-dot, somatosensory plasticity, finger, hand
Recent studies have used a tactile co-activation paradigm to examine changes in the somatosensory cortical representation of the finger and concomitant changes in tactile spatial acuity, as measured using the two-point threshold and grating orientation (GR/OR) discrimination (Dinse et al., 2003, 2005, 2006; Godde et al., 2000; Hodzic et al., 2004; Pleger et al., 2001, 2003). The idea behind the co-activation paradigm is that it follows Hebbian principles. In these studies, subjects were fitted with a small vibrotactile device on the distal fingerpad to achieve synchronous stimulation of a region of skin. After several hours of vibrotactile stimulation, post-treatment acuity thresholds were significantly lower than pre-treatment thresholds, with improvements in performance linearly correlated with shifts in the somatosensory cortical representation of the stimulated skin area (Hodzic et al., 2004; Pleger et al., 2001). The current study was aimed at clarifying the nature of the changes resulting from tactile co-activation. To do this we used three measures of tactile sensitivity, including the GR/OR task.
In Experiment 1, we used the GR/OR task with the aim of replicating earlier findings. In this task, subjects are asked to discriminate between two orthogonal orientations of gratings on the fingerpad (Van Boven and Johnson, 1994a, b). Subjects have to be able to resolve the grating elements in order to perform the orientation discrimination task. Performance on the task is a monotonically increasing function of grating groove width, and the minimal groove width corresponding to reliable (75% correct) performance is taken as the acuity threshold, which is closely related to the spacing between peripheral receptors of the Merkel (slowly adapting type I or SAI) afferent class (Craig, 1999; Gibson & Craig, 2002, 2005, 2006; Johnson & Phillips, 1981; Phillips & Johnson, 1981). Although the GR/OR task is a widely accepted measure of tactile spatial acuity, it is potentially susceptible to anisotropy: that is, greater sensitivity to one orientation as compared to the other orientation (Gibson & Craig, 2005). Such anisotropy could theoretically allow intensity cues to influence performance of the task. For this reason, we also used a second measure of tactile spatial acuity, one that is free of intensity cues. This measure was the 3-dot task (Stilla et al., 2007), where the stimulus consists of a row of three dots with the center dot offset either to the left or right. The subject’s task is to resolve the direction of offset. Performance on this task increases with offset magnitude, and the offset magnitude corresponding to reliable (75% correct) discrimination of offset direction is a measure of the acuity threshold that is close to that in the GR/OR task (Stilla et al., 2007), but is not subject to confounding by anisotropy.
Contrary to our expectations, neither of these tasks yielded significant effects of co-activation in Experiment 1. We therefore introduced a third task in Experiment 2, the smooth/grooved (SM/GV) task. The SM/GV task uses the same contactors as the GR/OR task, with an additional smooth contactor (no grooves). In this task, subjects are asked to indicate on a given trial whether the contactor is “smooth” or “grooved.” Like the GR/OR task, performance is measured as a function of the groove width of the contactor. Performance on this task depends on simply detecting spatial modulation in the afferent fibers (Craig et al., 2008), whereas performance on the GR/OR and 3-dot tasks requires more detailed evaluation of the spatial pattern of firing in the afferent population. Thus, the SM/GV task might be more sensitive to changes induced by co-activation. A preliminary report of the present study has appeared in abstract form (Gibson et al., Sathian, 2008).
Experiment 1
Methods
Subjects
Subjects were students at Emory University and were paid for their participation. All were right-handed, as assessed by the high-validity subset of the Edinburgh handedness inventory (Raczkowski et al., 1974), and were naïve to the tasks prior to participating. Ten subjects (7 female and 3 male) were tested on both the GR/OR and 3-dot tasks, before and again after co-activation. All procedures were approved by the Institutional Review Board of Emory University and the R&D Committee of the Atlanta VAMC, and subjects gave informed consent before participating.
Procedures
For the GR/OR task, commercially available JVP domes (Stoelting Co., Wood Dale, IL) were used. The hemispheric, 19 mm-diameter contactors have square wave gratings of equal groove and ridge width cut into them. The grooves are cut sufficiently deep so the skin does not touch the bottom of the groove. Seven gratings were used, with groove widths of 3, 2, 1.5, 1.2, 1, 0.75, and 0.5 mm. As described previously (Gibson & Craig, 2002), the gratings were applied to the immobile fingerpad using a counterweighted lever (Figure 1A) that allowed a precise contact force of 0.98 N, which is within the range of normal forces (0.49–1.96 N) used when making exploratory hand movements (Vega-Bermudez & Johnson, 2004). A precision air dashpot (Airpot, Norwalk, CT) was used to control and smooth the delivery of the contactor to the fingerpad at a controlled velocity of 20 mm/s. During testing for the GR/OR task the subject was seated with the right arm extended and the ulnar aspect of the arm and hand resting on a table. The subject’s right index finger was extended (as if pointing) and rested on a shelf so that the contactor could be brought into contact with the distal part of the fingerpad. The finger was held in place using padded double-sided adhesive tape applied to the fingernail. The subjects were instructed that the contactors would be presented in one of two orientations. The grooves of the contactor were aligned either along the proximal/distal axis of the finger or in the orthogonal orientation, across this axis. The subjects verbally reported the orientation of the contactor (i.e. “along” or “across”).
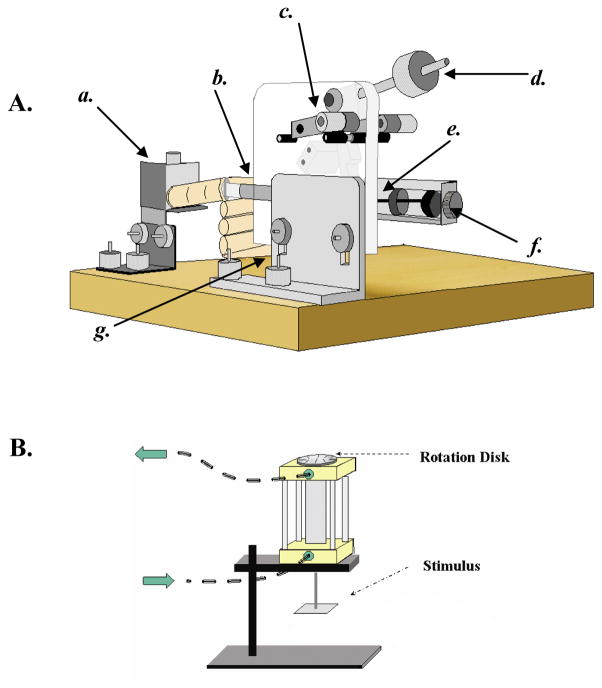
Figure 1.
A) Schematic drawing of the lever arm apparatus used in the grating orientation and smooth-grooved tasks. a) Adjustable 3-axis stage allowing the finger to be positioned precisely and supported. b) Contactor holder allowing rapid rotation or exchange of contactors between trails. c) Lever handle that was flipped to initiate each trial. d) Adjustable weight allowing for precise control of contact force. e) Push-rod that pushed the contactor into the finger. f) Precision air dashpot allowing precise control of contactor velocity. g) Adjustable base allowing lever arm to be precisely positioned. B) Schematic drawing of the pneumatic stimulator used to present the 3-dot stimuli. The arrows indicate direction of airflow. The stimuli were manually rotated 180° to change the direction of the center dot offset. Reproduced from Stilla et al. (2007).
As described previously (Sathian and Zangaladze 1998), the stimuli for the 3-dot task consisted of a row of three dots raised in relief (0.64 mm high) from a square base plate (20 mm square). The raised dots were 0.28 mm in diameter and had a center-to-center spacing of 2 mm. The center dot of the array was offset either to the right or to the left. Seven different offsets were tested: 1.94, 1.49, 1.19, 1.04, 0.8, 0.5, and 0.3 mm. A pneumatic stimulator (Figure 1B) was used to present the stimuli to the fingerpad with a contact force of ~0.6 N (Stilla, et al., 2007). The subject was seated with the right arm extended and supine, and the right index finger immobilized using a plastic finger mold and padded, double-sided adhesive tape. Care was taken that the stimulus was centered on the fingerpad. The center offset dot was either on the left or right side of the finger, achieved by rotating the stimulus 180°, and the subjects verbally reported the side that the offset dot was on (i.e. “left” or “right”).
Prior to testing, all participants were read a standardized description of the procedures. Each session began with practice trials during which feedback was provided; however, no feedback was provided during testing. The stimuli were presented in a block design from the largest groove width/dot offset to the smallest. Seven blocks of 20 trials each were used, for a total of 140 trials per session (i.e. 140 trials pre-treatment, 140 post-treatment). The grating orientation or direction of dot offset on any given trial was determined randomly, the two alternatives in each case being equally probable. Each trial began with the presentation of the stimulus and ended with the subject’s response (approximately 1–2 sec). If the subject took longer than 2 sec to respond the contactor was removed and the subject was prompted for a response. The task order (GR/OR or 3-dot) was counterbalanced across subjects. Subjects were blindfolded during testing. After the initial testing, the subjects were fitted with a small vibrotactile stimulator to the right index fingerpad. The vibrotactile stimulator, a mini-load speaker, was firmly attached using a single wrap of surgical tape around the finger, so that the device was maintained in a fixed position on the fingerpad, but without interfering with movement of the speaker membrane. A pseudo-random sequence of square-wave pulses, previously recorded onto an MP3 player, was played back through the vibrotactile stimulator. The inter-stimulus intervals of the pulses were 100–3000 ms; the average stimulation frequency was 1 Hz. The amplitude of the square-wave pulses was set at a level at which subjects reported they could clearly feel the pulses. Laser measurements indicate that the amplitude of skin displacement under such conditions is approximately 100 μm (H. Dinse, personal communication). The stimulator and skin mechanics impose a low-pass filter, tending to smooth out the high-frequency parts of the square-wave stimulation. This, together with the low average stimulation frequency, suggests that the co-activation stimulus was likely better at driving SAI afferents than rapidly adapting afferents (RAs). Subjects were instructed not to actively attend to the stimulus on their finger. The co-activation period lasted for 3 hours; during which subjects were allowed to read and/or use the computer (but only with the left hand). This is the same co-activation protocol that has been used in previous studies (Godde et al. 2000). Directly following the co-activation period, the GR/OR and 3-dot tasks were repeated to assess changes in tactile sensitivity. The order of tasks was the same in both pre- and post-treatment sessions.
In the current study, threshold was defined as 75% correct performance, which is halfway between perfect performance and chance performance. Thresholds were calculated using linear interpolation. For functions that crossed the threshold more than once, we took the first cross (i.e. the higher value) as the threshold estimate.
Results and Discussion
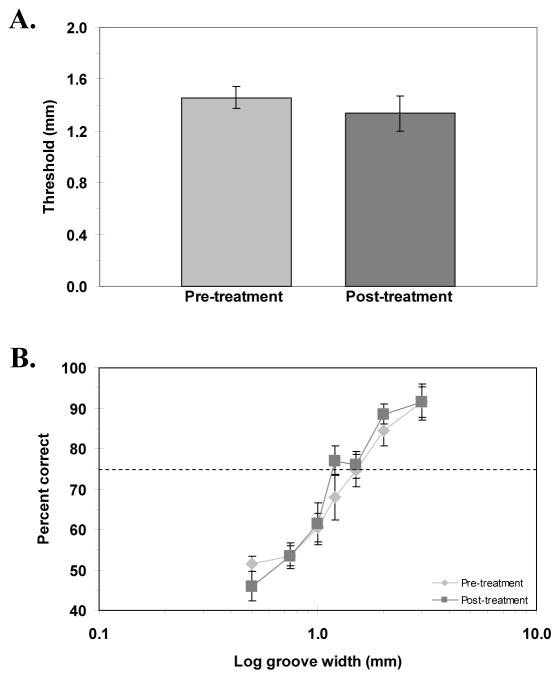
Figure 2A shows the pre- and post-treatment thresholds for the GR/OR task. The pre-and post-treatment thresholds (mean ± SEM) were 1.46 ± 0.08 mm, and 1.34 ± 0.13 mm, respectively. Figure 2A suggests that there was no effect of the co-activation treatment on GR/OR thresholds; the lack of a significant effect was confirmed by a paired samples t-test [t(9) = 0.79, p = .23]. Another way to investigate psychophysical performance is to examine the average psychometric function across subjects, illustrated in Figure 2B for the GR/OR task. The threshold is represented by the dashed line at 75% correct performance. Comparing the full psychometric functions pre- and post-treatment could reveal differences in performance other than threshold differences. For example, two functions might have identical thresholds but very different slopes. This did not, however, seem to be the case here (Figure 2B). A repeated-measures ANOVA confirmed that there was no significant difference in GR/OR performance between pre- and post-treatment sessions [F(1, 9) = 0.43, p = .53].
Figure 2.
A) Pre- and post-treatment grating orientation thresholds. B) Psychometric functions showing accuracy of grating orientation discrimination (percent correct) as a function of log groove width (mm), pre- and post-treatment. Horizontal dashed line represents 75% correct performance (threshold). Error bars: SEM.
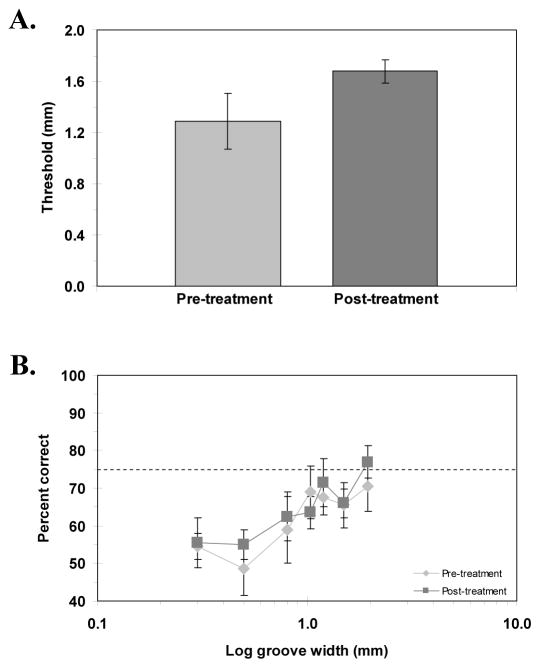
Figure 3A shows the pre- and post-treatment thresholds, and Figure 3B, the corresponding psychometric functions for the 3-dot task. Because we were unable to calculate thresholds for four of the ten subjects due to poor performance, only six subjects’ data were used in calculating the thresholds. As can be seen in Figure 3A, the effect of the treatment is in the opposite direction than predicted. The pre- and post-treatment thresholds (mean ± SEM) were 1.29 ± 0.17 mm and 1.68 ± 0.07 mm, respectively. A paired samples t-test showed a significant effect of the treatment [t(5) = −2.17, p < .05] but, as indicated, the effect was not in the predicted direction; moreover, this result is limited by the inability to compute thresholds for four subjects. When looking at the full psychometric function (Figure 3B), however, individual poor performance becomes less of a problem. In Figure 3B all of the subjects’ data have been included to generate the average psychometric functions. Examination of these functions indicates that they were very similar pre- and post-treatment, belying the apparent differences found for the thresholds based on a subset of subjects. A repeated-measures ANOVA confirmed that there was no significant difference in performance due to treatment [F(1, 9) = 1.19, p = .3]. Thus, performance on this task was also not improved by co-activation.
Figure 3.
A) Pre- and post-treatment 3-dot thresholds. B) Psychometric functions showing accuracy of offset discrimination (percent correct) as a function of log offset (mm), pre- and post-treatment. Horizontal dashed line represents 75% correct performance (threshold). Error bars: SEM.
We next examined the results of Experiment 1 for a task-by-session interaction using all of the data. A repeated measures ANOVA found a significant effect of task [F(1, 9) = 7.76, p < .05], but not of session [F(1, 9) = 1.41, p = .27], without a significant task-by-session interaction [F(1, 9) = 0.10, p = .76]. The significant effect of task is most likely due to the poor performance on the 3-dot task by several of the subjects. The lack of interaction is consistent with the lack of a significant effect of co-activation for either task. As previously mentioned, the GR/OR threshold has been reported to improve with co-activation (Hodzic et al., 2004). In Experiment 1, we were unable to replicate this finding with either of our measures of tactile acuity, the GR/OR task and the 3-dot task. This will be discussed further in the General Discussion.
Experiment 2
Since the results of Experiment 1 were at odds with the earlier study of Hodzic et al. (2004), we wanted to test their robustness. In Experiment 2, we examined the effects of co-activation on two tasks: GR/OR and SM/GV. As outlined above, the GR/OR task requires detailed resolution of stimulus-evoked spatial patterns in the SAI afferent population, whereas the SM/GV task can be performed by simply detecting the presence of afferent spatial modulation (Craig et al., 2008). For the GR/OR task, we wanted to verify the results of Experiment 1. The addition of the SM/GV task was to assess if the detection of spatial modulation might be a more sensitive way to quantify improvements in tactile sensitivity following tactile co-activation. In this experiment, we also incorporated a control group who underwent testing on each task twice, but without receiving co-activation in between. In general, the experimental methods were the same as those in Experiment 1, with the exception of the details noted below.
Methods
Subjects
Thirty naïve subjects participated in Experiment 2. Half of the subjects formed the experimental group (10 female and 5 male) and the other half, the control group (11 female and 4 male).
Stimuli and Procedures
JVP domes were used as in Experiment 1, with the addition of a smooth contactor lacking grooves for the SM/GV task. The GR/OR task was carried out as in Experiment 1. For the SM/GV task, subjects were instructed that one of two contactors would be presented, either smooth or grooved. The grooved contactor was always presented in the proximal/distal orientation, with the grooves aligned along the long axis of the finger. As with the GR/OR task, the first block used the largest groove width (3 mm) and each successive block used a smaller groove width. The same groove widths and number of trials were used for the SM/GV task as for the GR/OR task. The contactor presented on a given trial (smooth or grooved) was determined randomly; the two alternatives were equally probable. Previous work has shown that there is no significant anisotropy in SM/GV performance, despite a significant anisotropy for GR/OR performance (Gibson & Craig, 2005). Although Wheat and Goodwin (2000) reported anisotropic performance on a SM/GV task, they only used a single grooved contactor with a groove width of 0.75 mm, and the contactors were flat (as opposed to our dome-shaped contactors). On each trial the subject was to respond with either “smooth” or “grooved.” As in Experiment 1, the order of the tasks was counterbalanced across subjects. The experimental group received the tactile co-activation treatment as described in Experiment 1. The control group wore the vibrotactile stimulator but it was not plugged in and hence provided no phasic stimulation during the three-hour period. Other than this difference, the experimental and control group were treated exactly the same.
Results and Discussion
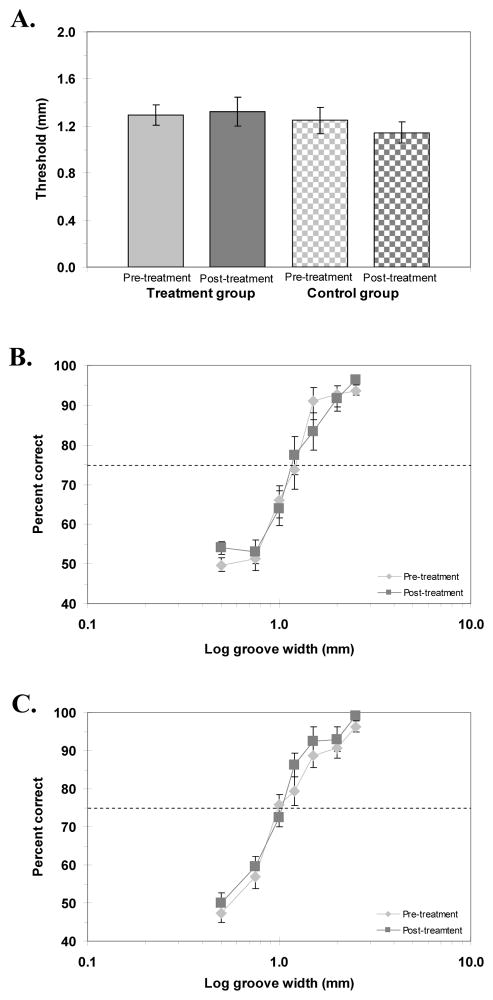
Experiment 2 replicated the GR/OR findings from Experiment 1. Figure 4A shows the pre- and post-treatment GR/OR thresholds for the experimental and control groups and Figure 4B and 4C, the psychometric functions for the experimental and control groups. Again there was no apparent change in GR/OR performance with the co-activation treatment. Pre- and post-treatment thresholds (mean ± SEM) were 1.29 ± 0.09 mm and 1.32 ± 0.12 mm for the experimental group; 1.25 ± 0.11 mm and 1.14 ± 0.09 mm for the control group. A paired samples t-test confirmed that there was no significant improvement in performance for either the experimental or control groups [t(14) = −0.25, p = .41 and t(14) = 1.03, p = .16, respectively]. A repeated-measures ANOVA on the data from the full psychometric functions confirmed the lack of a significant effect of the co-activation treatment on GR/OR performance for either the experimental or control groups [F(1, 14) = 0.07, p = .79 and F(1, 14) = 4.44, p = .054, respectively]. These results are in agreement with those of Experiment 1 but not with previous work, which did show an improvement in GR/OR performance following co-activation treatment on the fingerpad (Hodzic et al., 2004).
Figure 4.
A) Pre- and post-treatment grating orientation thresholds for experimental and control groups. B, C) Psychometric functions for grating orientation discrimination for experimental group (B) and control group (C), showing accuracy (percent correct) as a function of log groove width (mm), pre- and post-treatment. Horizontal dashed line represents 75% correct performance (threshold). Error bars: SEM
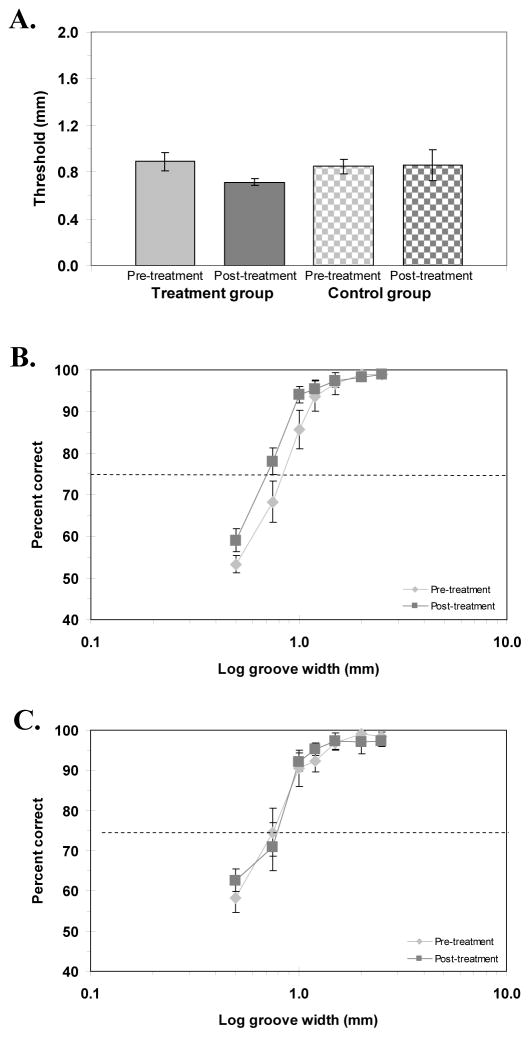
One aim of Experiment 2 was to see if detection of spatial modulation was a more sensitive metric of the psychophysical changes taking place following tactile co-activation. To test this hypothesis, we used the SM/GV task. Figure 5A shows the pre-and post-treatment SM/GV thresholds for the experimental and control groups; the corresponding psychometric functions for the experimental and control groups are shown in Figure 5B and 5C. The co-activation treatment improved performance in the SM/GV task for the experimental group but not the control group. Pre- and post-treatment thresholds (mean ± SEM) were 0.89 ± 0.07 mm and 0.71 ± 0.03 mm for the experimental group; 0.85 ± 0.05 mm and 0.86 ± 0.10 mm for the control group. A paired samples t-test indicated a significant effect of the co-activation treatment for the experimental group [t(14) = 2.91, p < .01] but not the control group [t(14) = −0.11, p = .46]. A repeated-measures ANOVA on the data from the full psychometric functions confirmed a significant effect of the treatment on SM/GV performance for the experimental group [F(1, 14) = 24.62, p < .01] but not the control group [F(1, 14) = 0.64, p = .44]. In fact, the linear portions of the functions (Figure 5B) are cleanly separated from 90% correct performance all the way down to chance performance. Compare these results with those seen in Figure 4B for the GR/OR task and in Figure 5C for the SM/GV task in the control group, where the functions are virtually identical.
Figure 5.
A) Pre- and post-treatment smooth-grooved thresholds for experimental and control groups. B, C) Psychometric functions for the smooth-grooved task for experimental group (B) and control group (C) showing accuracy (percent correct) as a function of log groove width (mm), pre- and post-treatment. Horizontal dashed line represents 75% correct performance (threshold). Error bars: SEM
We next examined the results of Experiment 2 for a task-by-session interaction using all of the data. A significant interaction, which is suggested by the analyses described to this point, would support the notion that the GR/OR and SM/GV tasks are differentially affected by co-activation. A repeated-measures ANOVA indicated a significant effect of task [F(1, 14) = 12.1, p < .01], and session [F(1, 14) = 12.51, p < .01] as well as a significant task-by-session interaction [F(1, 14) = 111.13, p < .01]. Taken together, these results indicate that the co-activation treatment had a significant effect on performance in the SM/GV task but not the GR/OR task.
These results differ from those previously found with tactile co-activation. As previously indicated, several studies using both the two-point limen and the GR/OR task have found improvements in performance following tactile co-activation, but here we did not find such improvement. We did, however, find an improvement in SM/GV performance following co-activation. In a recent study, Craig et al. (2008) showed that SM/GV performance probably depends on detecting spatial modulation in the SAI afferent population, whereas GR/OR performance requires spatial modulation in those same fibers to be adequate for a neural image of the stimulus to be resolved well enough to distinguish between two orthogonal orientations.
General Discussion
There are two main findings of the current study. First, we found no improvement in performance on the GR/OR task after co-activation in either Experiment 1 or 2, or on the 3-dot task in Experiment 1. This suggests that tactile spatial acuity, as indexed by tasks requiring detailed resolution of spatial patterns in the SAI afferent population, does not improve following tactile co-activation. The second finding was an improvement in SM/GV performance following the co-activation treatment in Experiment 2. Unlike the GR/OR and 3-dot tasks, performance on the SM/GV task is not dependent on subject’s ability to resolve a coherent neural image of the stimulus but rather, only requires detecting spatial modulation in the SAI afferent fibers (Craig et al., 2008).
As discussed previously, earlier studies of tactile co-activation have found an improvement in tactile acuity along with changes in somatosensory cortical representation of the fingerpad following treatment. The mechanisms underlying such changes are not fully understood, and the potential role of altered skin temperature and skin mechanics is unknown, but they are thought to arise from Hebbian co-activation of neurons with adjacent receptive fields. This improvement was found with the two-point threshold (Dinse et al. 2003, 2005, 2006; Godde et al. 2000; Pleger et al. 2001, 2003) as well as the GR/OR task (Hodzic et al. 2004). The results using the 3-dot and GR/OR tasks in the present study appear to be at odds with the reported improvements in tactile acuity following the co-activation treatment found in these earlier studies. In all but one of these earlier studies, the psychophysical changes in acuity following tactile co-activation were measured using the two-point threshold, which is a problematic measure of tactile spatial acuity (Boring, 1942; Friedline, 1918; Tawney, 1895; Weber, 1834/1996; Van Boven & Johnson, 1994b; for reviews, see Craig & Johnson, 2000; Johnson, Van Boven, & Hsiao, 1994). However, a study using the GR/OR task to measure changes in acuity following co-activation also found improvements in performance (Hodzic et al. 2004). This discrepancy may arise from methodological differences.
One difference between our study and the prior study of Hodzic et al. (2004) was the number of testing sessions prior to co-activation treatment. In the prior study, subjects were tested for four sessions with the goal of deriving stable thresholds prior to applying coactivation. In our study, subjects only had one pre-treatment testing session. Although the GR/OR threshold does not vary greatly with repeated testing (Sathian and Zangaladze, 1997; Van Boven and Johnson, 1994a), the greater number of pre-treatment sessions used by Hodzic et al. (2004) may have accounted for the decreased inter-subject variance in their study compare to ours. Thus, a treatment effect might have been masked in our data by a somewhat larger variance. While we cannot definitively prove this, it is worth investigating in future studies. Another methodological difference is that we used an apparatus that was designed to present the contactors with the same force, velocity, and contact location on each trial. Further, the aluminum push-rod (see Figure 1A) does not flex on contact, thereby eliminating all lateral shearing movements of the contactor as it travels into the skin. In the study of Hodzic et al. (2004), the contactors were presented manually (a common method), allowing potential variations in the force, velocity, and lateral movement of the contactor. Although it is generally held that such variations are unimportant in allowing grating spatial resolution, since they do not have much effect on SAI responses, such subtle variations could conceivably influence responses of other afferent types, e.g., shearing movements or micro-slip can engage rapidly adapting afferent fibers (Johansson and Westling, 1987, Westling and Johansson, 1987), and the co-activation treatment could possibly enhance such responsiveness. However, this is quite speculative at present. Finally, it is also possible that the constant force applied with the grating contactors in the present study was less than that achieved with manual application. This may also explain why the SM/GV task revealed effects that the GR/OR did not, in the present study.
With regard to the SM/GV task, one might expect it to be more sensitive to changes in tactile responsiveness than the GR/OR task. In the SM/GV task subjects are not asked to discriminate the orientation of the grating on the skin, which requires them to have a detailed neural representation of the grating (Phillips and Johnson, 1981). Rather, subjects are asked to detect the presence or absence of a grating, which only requires them to detect the presence of spatial modulation (Craig et al. 2008). Unlike the GR/OR task, performance on the SG/GV task is not linked to the spacing between receptors, because spatial modulation is still present in the afferent response, even for gratings whose stimulus elements are much finer than the spacing between the receptors (Bensmaia et al., 2006). For example, Gibson and Craig (2002) found that the SM/GV threshold on the palm was 0.96 mm, a location where the spacing between the SAI afferents is 3.5 mm (Johansson & Vallbo, 1979; see also Craig & Lyle, 2002). Thus, the SM/GV task appears to offer more sensitive metrics than the GR/OR task for measuring improvements in performance following tactile co-activation, at least when pre-treatment performance is assessed in a single session. If indeed the contact force applied with gratings and the smooth contactor, using the apparatus in the present study, was less than that achieved with manual application of gratings in previous studies, this could account for the differences between our findings and previous results. This could be tested in future work by experimentally varying contact force to test whether co-activation effects on GR/OR discrimination are sensitive to the overall contact force level.
Conclusions
The current study, using a single session each of pre-treatment and post-treatment evaluation, did not find that co-activation improved spatial acuity, as measured by either the GR/OR or 3-dot task. However, tactile co-activation did lead to improved performance on SM/GV task. The improvement in SM/GV performance may be associated with changes in the somatosensory cortical representation of the fingerpad (although this was not tested here), which could lead to improved detection of spatial modulation in the primary afferent fibers. These results also reinforce the notion that the GR/OR and SM/GV tasks tap different aspects of tactile spatial coding. We conclude that the SM/GV task is a more sensitive index of psychophysical changes induced by tactile co-activation than other currently available measures of tactile spatial acuity.
Acknowledgments
This work was supported by National Institutes of Health grants R01 EY012440 and K24 EY017332 to KS. Support to KS and GG by the Veterans Administration is also gratefully acknowledged. The authors wish to thank Jim Craig for loaning the lever arm apparatus and Hubert Dinse for his generosity in providing the co-activation apparatus and for very helpful discussions.
References
- Bensmaia SJ, Craig JC, Yoshioka T, Johnson KO. SA1 and RA afferent responses to static and vibrating gratings. J Neurophysiol. 2006;95:1771–1782. doi: 10.1152/jn.00877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring EG. Sensation and perception in the history of experimental psychology. D. Appleton-Century Company; New York, London: 1942. [Google Scholar]
- Craig JC. Grating orientation as a measure of tactile spatial acuity. Somatosens Mot Res. 1999;16:197–206. doi: 10.1080/08990229970456. [DOI] [PubMed] [Google Scholar]
- Craig JC, Johnson KO. The two-point threshold: Not a measure of tactile spatial resolution. Curr Dir Psycholog Sci. 2000;9:29–32. [Google Scholar]
- Craig JC, Lyle KB. A correction and a comment on Craig and Lyle (2001) Percept Psychophys. 2002;64:504–506. doi: 10.3758/bf03194721. [DOI] [PubMed] [Google Scholar]
- Craig JC, Rhodes RP, Gibson GO, Bensmaia SJ. Discriminating smooth from grooved surfaces: effects of random variations in skin penetration. Exp Brain Res. 2008;188:331–340. doi: 10.1007/s00221-008-1363-3. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Kalisch T, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Improving human haptic performance in normal and impaired human populations through unattended activation-based learning. ACM Trans Appl Percept. 2005;2:71–88. [Google Scholar]
- Dinse HR, Kleibel N, Kalisch T, Ragert P, Wilimzig C, Tegenthoff M. Tactile coactivation resets age-related decline of human tactile discrimination. Ann Neurol. 2006;60:88–94. doi: 10.1002/ana.20862. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- Friedline CL. The discrimination of cutaneous patterns below the two-point limen. Am J Psychol. 1918;29:400–419. [Google Scholar]
- Gibson GO, Craig JC. Relative roles of spatial and intensive cues in the discrimination of spatial tactile stimuli. Percept Psychophys. 2002;64:1095–1107. doi: 10.3758/bf03194759. [DOI] [PubMed] [Google Scholar]
- Gibson GO, Craig JC. Tactile spatial sensitivity and anisotropy. Percept Psychophys. 2005;67:1061–1079. doi: 10.3758/bf03193632. [DOI] [PubMed] [Google Scholar]
- Gibson GO, Craig JC. The effect of force and conformance on tactile intensive and spatial sensitivity. Exp Brain Res. 2006;170:172–181. doi: 10.1007/s00221-005-0200-1. [DOI] [PubMed] [Google Scholar]
- Gibson GO, Makinson CD, Dinse HR, Sathian K. Tactile co-activation improves detection of afferent spatial modulation. Soc Neurosci Abstr. 2008;38:177.x. doi: 10.1007/s00221-009-1717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 2000;20:1597–1604. doi: 10.1523/JNEUROSCI.20-04-01597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Ehrhardt J, Braun C. Behavioral significance of input-dependent plasticity of human somatosensory cortex. Neuroreport. 2003;14:543–546. doi: 10.1097/00001756-200303240-00002. [DOI] [PubMed] [Google Scholar]
- Hodzic A, Veit R, Karim AA, Erb M, Godde B. Improvement and decline in tactile discrimination behavior after cortical plasticity induced by passive tactile coactivation. J Neurosci. 2004;24:442–446. doi: 10.1523/JNEUROSCI.3731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res. 1987;66:141–154. doi: 10.1007/BF00236210. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Phillips JR. Tactile spatial resolution. I. Two-point discrimination, gap detection, grating resolution, and letter recognition. J Neurophysiol. 1981;46:1177–1192. doi: 10.1152/jn.1981.46.6.1177. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Van Boven RW, Hsiao SS. The perception of two points is not the spatial resolution threshold. In: Boivie J, Hansson P, Lindblom U, editors. Touch, temperature, and pain in health and disease: mechanisms and assessments. IASP Press; Seattle: 1994. pp. 389–404. [Google Scholar]
- Phillips JR, Johnson KO. Tactile spatial resolution. II. Neural representation of Bars, edges, and gratings in monkey primary afferents. J Neurophysiol. 1981;46:1192–1203. doi: 10.1152/jn.1981.46.6.1192. [DOI] [PubMed] [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci U S A. 2001;98:12255–12260. doi: 10.1073/pnas.191176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 2003;40:643–653. doi: 10.1016/s0896-6273(03)00677-9. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A. Tactile learning is task specific but transfers between fingers. Percept Psychophys. 1997;59:119–128. doi: 10.3758/bf03206854. [DOI] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu X, Sathian K. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. J Neurosci. 2007;27:11091–11102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawney G. The perception of two points not the space-threshold. Psycholog Rev. 1895;2:585–593. [Google Scholar]
- Van Boven RW, Johnson KO. The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue, and finger. Neurology. 1994a;44:2361–2366. doi: 10.1212/wnl.44.12.2361. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO. A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain. 1994b;117:149–167. doi: 10.1093/brain/117.1.149. [DOI] [PubMed] [Google Scholar]
- Vega-Bermudez F, Johnson KO. Fingertip skin conformance accounts, in part, for differences in tactile spatial acuity in young subjects, but not for the decline in spatial acuity with aging. Percept Psychophys. 2004;66:60–67. doi: 10.3758/bf03194861. [DOI] [PubMed] [Google Scholar]
- Weber EH. EH Weber on the tactile senses. Erlbaum (UK): Taylor & Francis, Hove; 1834/1996. [Google Scholar]
- Westling G, Johansson RS. Responses in glabrous skin mechanoreceptors during precision grip in humans. Exp Brain Res. 1987;66:128–140. doi: 10.1007/BF00236209. [DOI] [PubMed] [Google Scholar]
- Wheat HE, Goodwin AW. Tactile discrimination of gaps by slowly adapting afferents: effects of population parameters and anisotropy in the fingerpad. J Neurophysiol. 2000;84:1430–1444. doi: 10.1152/jn.2000.84.3.1430. [DOI] [PubMed] [Google Scholar]