Abstract
Invasive fungal disease is associated with increased morbidity and mortality in hematologic malignancy patients and hematopoietic stem cell transplant recipients. Timely recognition and treatment of invasive fungal diseases in these patients are essential and decrease mortality. However, conventional definitive diagnostic methods are difficult and time consuming. While conventional microbiological and histopathological methods are still needed for a definitive diagnosis of invasive fungal disease, new noninvasive diagnostic methods including serologic and molecular biomarkers are now available. These new diagnostic methods facilitate an early diagnosis of invasive fungal disease and allow for utilization of a pre-emptive treatment approach, which may ultimately lead to improved treatment outcomes and reduced toxicity.
Keywords: Aspergillus, Candida, empiric antifungal therapy, galactomannan assay, glucan assay, hematologic malignancies, hematopoietic stem cell transplant, invasive fungal disease, PCR, pre-emptive antifungal therapy
Invasive fungal disease (IFD) is a major cause of morbidity and mortality in patients receiving therapy for hematologic malignancies and in hematopoietic stem cell transplant (HSCT) recipients. In this group of patients, Candida and Aspergillus species are the major invasive fungal pathogens [1–4]. Although historically invasive candidiasis was the most common fungal pathogen, today with routine fluconazole prophylaxis, invasive aspergillosis (IA) has a higher incidence than invasive candidiasis in this patient population [1,2,4]. The increased prophylactic use of antifungal agents in these patients is contributing to the increase in incidence of resistant and previously rare fungal species. As a result, epidemiologic shifts are observed with an increasing incidence of nonalbicans Candida species such as Candida tropicalis, Candida krusei and Candida glabrata and nonfumigatus Aspergillus species such as Aspergillus terreus [1,5–8]. Moreover, highly fatal IFD caused by formerly rare fungal organisms such as the agents of mucormycosis, Fusarium and Trichosporonare are more common in HSCT recipients and patients receiving treatment for hematologic malignancies [1,3,5,9]. All together, the association of IFD, with high morbidity and mortality and the evolving epidemiologic characteristics of fungal pathogens, necessitates early diagnosis and treatment of IFD in this high-risk group of patients.
Timely diagnosis of IFD is essential as delaying treatment initiation is associated with an increased mortality [10,11]. However, diagnosis of IFD in hematologic malignancy patients and HSCT recipients remains difficult. Contributors that make the diagnosis of IFD challenging in this immunocompromised patient population include the fact that the clinical symptoms are often difficult to distinguish from symptoms of bacterial or viral infections. Moreover, conventional diagnostic methods for IFD are each associated with shortcomings. As most recently revised by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG), IFD is defined as proven, probable or possible based on diagnostic certainty [12]. According to these definitions, the proven diagnosis of IFD requires a microbiological and/or histopathological diagnostic method. However, these more conventional diagnostic methods for IFD have limited sensitivity and specificity, are time consuming, and frequently are not practical since the invasive means needed to obtain tissue are fraught with risks of morbidity or mortality in this patient population. Noninvasive diagnostic tools including imaging with CT and MRI scans, serological testing including serum galactomannan assay for Aspergillus and the serum (1,3)-β-d-glucan (BDG) antigen test, and molecular techniques including PCR-based assays may facilitate earlier initiation of antifungal treatment despite having their own limitations [13,14]. The serum galactomannan assay for Aspergillus and the serum BDG antigen test are now incorporated in the diagnosis of probable IFD in patients with appropriate host and clinical factors as defined by the revised EORTC/MSG criteria. Moreover, these assays have also been found to be applicable for testing other body fluids, especially bronchoalveolar lavage (BAL) fluid. These more easily performed assays provide mycological supportive criteria that in conjunction with suitable imaging scan findings in the appropriate clinical setting can be used to support the diagnosis of probable IFD [12]. The remainder of this article focuses on a review of diagnostic methods for IFD in patients with hematologic malignancies and HSCT recipients with emphasis on the newer noninvasive methods and their clinical impact.
Limitations of microbiological & histopathological diagnostic methods
According to the revised EORTC/MSG criteria, the diagnosis of proven IFD can only be made by cultures obtained by sterile procedure or histopathologic, cytopathologic or direct microscopic evaluation of tissue specimens [12]. However, tissue specimens are sometimes hard to obtain in patients receiving treatment for hematologic malignancies and HSCT patients due to thrombocytopenia and the potential risk of bleeding complications [15]. Moreover, cultural-based methods have low sensitivity and specificity and are time consuming. Blood cultures identify only 50–60% of disseminated candidiasis cases, and typically require a 2- to 5-day period of incubation and are rarely successful in identifying hematogenous aspergillosis [13,16–18]. Therefore, negative blood cultures cannot readily exclude IFD. Moreover, when blood cultures detect Aspergillus species, they are mostly associated with contaminated specimens [19]. Thus, while microbiological and histopathological diagnostic tools will always remain important to pursue a specific definitive diagnosis, newer diagnostic tools with higher specificity and sensitivity that can identify and treat IFD earlier have largely replaced tissue diagnosis of IFD in HSCT recipients and patients receiving treatment for hematologic malignancies.
Radiologic methods
Using CT and MRI scans for early identification and treatment of pulmonary IA, extrapulmonary IA and other IFD is of very important clinical value [20]. Chest CT scan findings can identify pulmonary IA and other invasive molds at an earlier stage of the disease. Halo sign or macronodules on chest CT scans are usually found earlier in the course of pulmonary IA while cavitations and air-crescent sign are found later in the course of the disease [21]. Moreover, sinus CT scan findings of fluid or bony erosions are highly suggestive of IFD [11,15,22]. In addition, initiation of antifungal therapy with the presence of an early chest CT scan finding of a halo sign has been associated with improved mortality in invasive pulmonary aspergillosis [23]. Chest CT scan findings of dense well-circumscribed lesions with or without a halo sign, air-crescent sign or cavity are now incorporated in the criteria of clinical factors that support the diagnosis of probable or possible IFD as defined by the revised EORTC/MSG criteria [12]. Furthermore, there are other radiologic findings that are recognized as suggestive of fungal causes of CNS infections, liver or spleen abscesses, as well as fungal sinusitis; such findings, in conjunction with other clinical, host and microbiologic factors support the diagnosis of probable or possible IFD [12]. Even though CT and MRI scans help in the early identification and treatment of presumed IFD, pursuing a specific microbiological or histological definitive diagnosis even after initiation of antifungal treatment using techniques, such as bronchoscopic biopsy, BAL, invasive lung biopsy, sinus endoscopic exams with aspiration and biopsy, biopsy of skin or subcutaneous lesions, or other invasive biopsy techniques for extrapulmonary and extrasinus sites, is still recommended to help guide treatment decisions, since other pathogens can sometimes give rise to similar radiologic findings. However, even in the absence of diagnostic confirmation with such techniques, strong clinical suspicion and CT scan findings may alone justify presumptive antifungal treatment in high-risk patients receiving treatment for hematologic malignancies and HSCT recipients.
Serologic methods
Aspergillus galactomannan (GM) antigen detection test
The use of the double-sandwich ELISA for GM antigen detection is one of the major recent advances in IFD diagnosis. Detection of GM antigen in plasma, serum, BAL fluid or cerebrospinal fluid (CSF) is included in the mycological criteria that support the diagnosis of probable IA as defined by the revised EORTC/MSG criteria [12]. GM is a polysaccharide cell wall component of Aspergillus species that is released during hyphal growth in tissues in invasive infections. GM may also be found in the cell walls of other filamentous fungi such as Paecilomyces and Penicillium species. However, GM is found in smaller amounts in the cell walls of these species when compared with the amounts found in the cell walls of Aspergillus species. These smaller amounts of GM are mostly undetectable on GM antigen test. Thus, GM antigen tests mostly detect IA caused by Aspergillus species [20].
In hematologic malignancy patients and HSCT recipients, the cutoff value of the serum GM antigen index found to correlate with sufficient sensitivity and specificity is 0.5. GM antigen may be detected as long as a median of 5–8 days before patients present with clinical or radiologic signs of IA [24]. Sensitivity and specificity of serum GM antigen assay varies in different patient populations. In a meta-analysis of 27 studies from 1995 to 2005, the sensitivity and specificity of the serum GM antigen assay was 70 and 92%, respectively, in hematologic malignancy patients and 82 and 86%, respectively, in HSCT recipients. By contrast, sensitivity was dramatically lower at 22% and the specificity was 84% in solid organ transplant patients [25]. The positive-predictive value (PPV) of the GM antigen assay in this meta-analysis was shown to increase as prevalence increased. The PPV was 0.7 in studies limited to patients with hematolgic malignancies and 0.82 in studies limited to patients undergoing bone marrow transplant versus 0.22 in studies limited to solid organ transplant recipients. This indicates that GM antigen assay is a more effective screening test in patient populations in whom the pretest probability of IA is high, and therefore it is a useful test in HSCT recipients and hematologic malignancy patients. It is important to note that the sensitivity and specificity noted above is not for a single test result but rather for a series of tests performed twice-weekly during the period at risk. Nevertheless, the sensitivity of the GM assay is still only moderate in this group of patients. The negative predictive value (NPV) of the GM antigen assay with a cutoff value of 0.5 is higher than 95% [24–27]. Therefore, a negative GM antigen assay is a good test for ruling out the diagnosis of IA. The GM antigen assay can also be used for prognostic purposes in monitoring response to treatment, as GM antigen concentrations may correlate to fungal burden. Some studies found the GM assay cutoff value higher than 1 to be predictive of treatment failure while other studies found that predictive value to be higher than 2 [28–30]. Persistently elevated GM values over the first 2 weeks of therapy also is associated with treatment failure. In patients with hematologic malignancies and HSCT recipients, the BAL GM assay has been shown to add value to serum GM assay in diagnosing invasive pulmonary aspergillosis [31,32]. In our own experience, BAL GM added diagnostic sensitivity to serum GM and to other diagnostic methods for invasive pulmonary aspergillosis and was not affected by short courses of antimold therapy that ranged from 1 to 3 days [33]. Using a cutoff value of 1 rather than 0.5 increases the sensitivity and specificity of the BAL GM assay in diagnosing pulmonary IA [32,34,35]. A study using BAL GM assay with a cutoff value of 1 had sensitivity of 90% and PPV of >75 % [36]. A positive GM antigen assay in CSF has been shown to support the diagnosis of probable cerebral aspergillosis in HSCT recipients [37].
Several factors can affect GM antigen assay testing leading to false-positive and false-negative results. False-negative results have been associated with antecedent antimold prophylaxis [27,38]. False-positive GM antigen assay results have also been associated with the use of antibiotics including β-lactams such as piperacillin–tazobactam and amoxicillin–clavulanate and also with the use of immunosuppressive drugs such as cyclophosphamide [20, 39–42]. Recent data suggest that pipercillin may no longer be associated with false-positive GM tests [36]. False-positive results have also been reported in patients with IgG multiple myeloma [43]. Moreover, false-positive GM antigen assay results have been associated with the use of plasmalyte in BAL collection solution and with respiratory colonization with other molds such as Penicillium that may have detectable GM amounts in their cell walls [44]. Some crossreactivity with Histoplasma and Blastomycetes has been reported. Repeat testing of GM antigen assay has been shown to resolve false-positive results. Moreover, two consecutive positive GM antigen assays have been shown to improve the diagnostic performance of the test for IA [20,45,46]. The repeat testing can be from a separate sample or from the same sample. Although a separate sample is preferred, in practice, most laboratories will repeat the assay on the same sample on the same day before reporting the results to the clinician, thus resolving diagnostic uncertainty quickly.
In conclusion, the authors recommend checking serum GM antigen assay serially twice-weekly in high-risk patients undergoing treatment for hematologic malignancies and HSCT recipients. The results of serial GM antigen assays, if positive, can be used to support the EORTC/MSG diagnosis of probable fungal infection in patients who also meet the host factors and clinical criteria. The use of GM antigen assay positivity alone as a screening test for IFD that would necessitate initiation of antimold treatment in patients with persistent neutropenic fever in the absence of other clinical or radiologic findings is controversial. However, one reasonable approach would be to initiate antimold treatment in such patients, while repeating testing, performing imaging and continuing attempts at obtaining a definitive diagnosis. Negative results of serial GM antigen assays in patients with persistent neutropenic fever without clinical or radiologic findings are more helpful in supporting the absence of IA and avoiding empiric antimold treatment.
BDG antigen detection test
The use of ELISA for detection of 1,3-β-d-glucan (BDG) antigen is another recent advancement in the diagnosis of IFD. Similar to GM, BDG is a polysaccharide cell wall component. However, in contrast to GM, BDG is found in most fungal species except Cryptococcus and the agents of mucormycosis. Therefore, a shortcoming of the BDG antigen detection test is that it is not specific for IA; however, a strength of the assay is that it detects a broader spectrum of IFD. Nevertheless, like the GM antigen detection test, the BDG antigen detection test will not distinguish between different fungal genera or species [6,20,47]. According to the revised EORTC/MSG criteria, serum BDG antigen detection is used to support the diagnosis of probable IFD other than cryptococcosis and mucormycosis [12]. However, clinical research experience with the BDG antigen assay is limited compared with the GM antigen assay and therefore the BDG antigen assay is less utilized than the GM antigen assay despite its inclusion in the EORTC/MSG probable IFD diagnostic criteria.
There are multiple commercial assays available for BDG antigen detection. The Fungitell® assay (Beacon Diagnostics, MA, USA) is the most utilized in the USA. With this assay, at least two consecutive results greater than 60 pg/ml are optimal for diagnosis of invasive fungal infection [46]. Although studies are limited, BDG antigen assay may have higher sensitivity than GM antigen assay in diagnosing IA. BDG antigen assay may be detected as long as a median of 5–10 days before patients present with clinical or radiologic signs of IFD [45]. A study in acute myeloid leukemia and myelodysplastic syndrome patients found the BDG antigen assay to be highly sensitive and highly specific in diagnosing early IFD due to a variety of yeasts and molds. In that study, two sequential positive BDG antigen assay results increased the specificity of the test. The NPV in that study was 100%, suggesting that, like the GM antigen assay, the BDG antigen assay is a useful test in ruling out IFD [48]. A recent meta-analysis of cohort studies in hematologic malignancy patients from the Third European Conference on Infections in Leukemia (Juan-les Pins, France, 25–26 September, 2009) showed that two consecutive positive BDG antigen assays were better than one positive assay in diagnosing proven or probable IFD due to a variety of yeasts and molds. Two consecutive tests had high specificity (98.9%), high PPV (83.5% for an IFD prevalence of 10%) and high NPV (94.6% for an IFD prevalence of 10%). However, in that study, sensitivity was only 49.6%. Therefore, it was concluded that the BDG antigen assay may be used only as an adjunct to clinical, radiological and microbiological findings to diagnose IFD [49]. There are limited data of BDG antigen assay in HSCT recipients and more studies are needed. Like the GM antigen assay, many factors may affect the BDG antigen assay causing false-positive results. False-positive results of the BDG antigen assay have been identified in patients who have concurrent bacterial infections with bacterial pathogens that can produce glucan, in patients receiving immunoglobulin or albumin products that are contaminated with fungal components, and in patients receiving certain antibiotics, such as amoxicillin–clavulanate [20,50–52]. A study in patients with hematologic malignancies showed that using BDG antigen assay along with GM antigen assay serially improved the specificity and PPV of diagnosing IA to 100% without affecting the sensitivity or NPV results. These findings suggest that the use of both serologic tests serially in hematologic malignancy patients at high risk of developing IA may overcome the false-positive results associated with each of the tests when used alone [53].
Considering the above, the authors recommend consideration of checking the BDG antigen detection test serially as an alternative for checking the GM antigen test in high-risk patients with prolonged neutropenia undergoing treatment for hematologic malignancies or HSCT recipients. However, the use of the GM antigen assay has been studied more extensively in HSCT patients than the BDG antigen assay. Therefore, the authors use a strategy that uses serial testing of GM antigen assay rather than BDG antigen assay in these patients. Positive BDG antigen assay results can be used to support the diagnosis of probable IFD in the setting of appropriate host and clinical criteria as defined by the revised EORTC/MSG criteria [12]. Again, the use of either the GM antigen or BDG antigen test as screening tools is controversial, but the authors recommend using positive results for early identification and treatment of IFD in high-risk patients. Similar to a negative GM antigen assay, a negative BDG antigen assay in the setting of persistent neutropenic fever alone and absence of clinical or radiologic findings is helpful in ruling out IFD and withholding empiric treatment, given the high NPV of this test.
Molecular methods
Due to the limitations of conventional diagnostic methods in diagnosing IFD, there has been more focus on the development of fungal nucleic acid detection techniques in hopes of increasing sensitivity and speed of IFD diagnosis. Recent studies of different Aspergillus PCR assays in the diagnosis of IA have shown promising results. Positive Aspergillus PCR assays in IA have been shown to precede radiological and clinical findings as well as microbiological and pathological diagnosis of IA [54–56]. A recent meta-analysis of 16 studies between 2000 and 2008 in 1618 patients using different Aspergillus; PCR assay techniques for the screening of IA was conducted. This meta-analysis showed that a single positive Aspergillus PCR assay had a sensitivity of 88% and a specificity of 75%, while two consecutive positive assays decreased the sensitivity to 75% and improved the specificity to 87%. The conclusion from the study was that a single negative Aspergillus PCR assay can exclude IA and two positive assays can diagnose IA [57].
Currently, a wide variety of nucleic acid detection techniques for IFD are available that include the use of PCR to detect fungal DNA and the use of nucleic acid sequence-based amplification to detect fungal mRNA, but PCR-based assays are the most widely used. These PCR-based assays can range from detecting a broad spectrum of fungal pathogens to detecting genus-specific and even species-specific pathogens. However, most of the available PCR assays focus on detecting Aspergillus and Candida species. PCR assays can be used for fungal DNA detection in different types of specimens, including whole blood, serum, plasma, BAL, CSF and biopsy tissue from different sites. Some laboratories are now using internally developed PCR methods for the diagnosis of IFD. However, the use of these PCR methods and other nucleic acid detection techniques in IFD diagnosis is still not widely adopted due to the lack of standardization. In fact, most published studies utilize different nucleic acid detection techniques making available data hard to interpret. Moreover, molecular methods are expensive and laborious procedures to conduct. In addition, many factors can affect the molecular methods used to detect IFD, and therefore lead to false-positive results. Those factors include specimen handling, which may lead to contamination with fungal DNA and difficulty in extracting intact fungal DNA [20,58–60].
Molecular methods are not a part of the current revised EORTC/MSG criteria for diagnosing proven, probable or possible IFD and are still considered investigational due to the lack of standardized methods [12]. To standardize fungal molecular methods, the European Aspergillus PCR Initiative was formed in 2006, which developed recommendations for protocols for Aspergillus PCR that are now being used in multiple centers [61]. In conclusion, the use of molecular techniques for IFD diagnosis is still investigational; however, results are very promising. Standardizing molecular methods for IFD diagnosis and validation of those methods in clinical studies are needed.
Impact of evolving diagnostic methods on treatment approaches
Given the current limitations of the individual diagnostic methods for IFD, it is widely recognized that initiation of antifungal treatment in hematologic malignancy patients and HSCT recipients in whom IFD is clinically suspected should not be delayed until a definitive diagnosis is made [62]. Historically, physicians treated all persistently febrile neutropenic patients with empirical antifungal treatment [63–65]. However, such an empiric treatment approach is now in question due to multiple shortcomings including overtreatment with potentially toxic antifungal agents of many patients, and inadequate course of treatment in some patients who truly have undiagnosed IFD. An inadequate course of treatment may occur as empiric antifungal therapy is usually stopped with neutrophil count recovery as no laboratory or clinical diagnostic signs are guiding empiric antifungal therapy. This could place the truly infected patient receiving subsequent courses of chemotherapy at risk for recurrence since multiple studies have demonstrated a high risk for recurrence during subsequent chemo-therapy or HSCT unless continued antifungal therapy is given. Moreover, an empiric treatment approach does not utilize recent diagnostic developments [66,67].
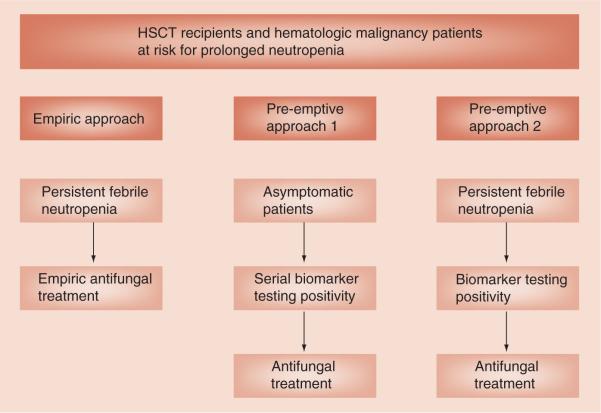
As a result of the shortcomings in the empiric treatment approach, recently, several studies incorporated the various new noninvasive diagnostic tools including imaging studies, serological testing and molecular techniques to trigger initiation of therapy in a pre-emptive or targeted treatment approach. Such a pre-emptive approach monitors serological or molecular biomarkers at regular intervals and uses the results of biomarker testing to trigger mold-active antifungal therapy either with or without clinical symptoms and imaging findings suggestive of IFD. This approach typically uses fluconazole prophylaxis to eliminate the concern for Candida infections. This would allow withholding antimold treatment in patients with persistent neutropenic fever with no evidence of IFD [68]. There are two subtle but important uses of the term pre-emptive therapy (Figure 1). In one definition of pre-emptive therapy, the goal is to reduce IFD by detecting a positive biomarker in an asymptomatic patient, well before onset of symptoms. In the other definition of pre-emptive therapy, the goal is to target antifungal therapy to those who are probably infected and to reduce needless antifungal therapy to those not needing it by finding the subset of febrile patients with a positive biomarker or radiologic findings highly suggestive of IFD. Several studies have examined pre-emptive antifungal treatment approaches and some have compared pre-emptive with empiric approaches with mixed results. Therefore, choosing a pre-emptive versus empiric antifungal treatment approach for IFD in high-risk patients with prolonged neutropenia is currently controversial [69–71]. In a pilot study, Maertens et al. examined the feasibility of a GM antigen assay and high resolution CT scan based on pre-emptive antifungal treatment approach. With this pre-emptive approach, there were no undetected cases of IA, one missed case of mucormycosis and the 12-week survival rate for patients with IFD was 63.6% [71]. In another study, Cordonnier et al. compared empiric versus preemptive antifungal treatment strategy. In that trial, patients were treated pre-emptively if they had clinical signs of IFD or imaging findings indicative of IFD or GM antigen assay positivity. The rates of probable IFD and proven IFD incidence were higher in the pre-emptive treatment group. However, overall survival, which was the primary end point of this study, was similar in both the empiric and pre-emptive treatment groups [69]. Shortcomings of the study include: lack of blinding; the use of a much higher level of GM positivity (1.5, rather than 0.5), which delayed the initiation of pre-emptive therapy; and the lack of standardized use of fluconazole resulting in an excess of Candida infections in the pre-emptive arm, which accounted for the excess numbers of IFD in the pre-emptive arm. Of note, patients could be enrolled as late as onset of neutropenic fever and overall initiation of the preemptive therapy occurred much later than in the empiric therapy approach (13 vs 7 days). Clearly, the trial was testing the second sense of the term pre-emptive therapy. In a third trial, Hebart et al. compared an empiric versus PCR-based pre-emptive antifungal treatment strategy [70]. There was no difference between the two treatment groups in the incidence of probable or proven IFD at day 100, which was the primary end point of the study. Moreover, there was no difference in overall survival at day 100. However, the mortality at day 30 was significantly lower in the PCR-based pre-emptive treatment group than in the empiric treatment group, with a strong trend to fewer fatal IFD. That trial was a test of the first sense of the term pre-emptive with a goal to reduce IFD from occurring or detecting IFDs early. One shortcoming of that trial was that compliance with the PCR testing was poor: only 24% compliance during the first 5 weeks and compliance was much worse later. Thus, the early benefit suggested at 30 days may have disappeared later at 100 days due to poor compliance with the monitoring schedule. Based on the results of these studies, the third European Conference on Infections in Leukaemia guidelines for antifungal management in leukemia and HSCT recipients deemed pre-emptive therapy for IFD to be experimental while the Infectious Diseases Society of America clinical practice guidelines for antimicrobial use in neutropenic patients deemed it to be an acceptable alternative to empirical therapy in high-risk neutropenic patients [72,73]. Therefore, the authors believe further future studies of the use of different new diagnostic techniques in pre-emptive treatment approaches are needed to standardize screening tools for IFD.
Figure 1. The use of biomarker testing positivity in pre-emptive treatment approaches.
HSCT: Hematopoietic stem cell transplant.
In conclusion, the authors recommend utilizing new diagnostic methods in a pre-emptive rather than empiric antifungal treatment strategy when possible to avoid over- and under-treatment. This is particularly useful in high-risk patients with prolonged neutropenia undergoing treatment for hematologic malignancies and in HSCT recipients. Given the high NPV for IFD diagnosis of the serological and molecular methods, it is reasonable to withhold antifungal treatment in patients with persistent febrile neutropenia who lack biomarker positivity in the absence of other clinical signs or imaging indicative of IFD. However, in view of the occasional false-negative test, any patient with a nodular pulmonary infiltrate should be presumptively treated for mold IFD while diagnostic assessment proceeds.
Expert commentary
We recommend using the new serologic diagnostic methods in conjunction with clinical and radiologic findings for supporting an early diagnosis of probable IFD based on the revised EORTC/MSG criteria. We would continue to pursue a definitive diagnosis of IFD with bronchoscopic examination when possible to identify additional copathogens since pulmonary fungal infections are sometimes mixed infections with viruses or bacteria. In addition, we would proceed to bronchoscopy in patients with nodular pulmonary infiltrates even if the serum fungal biomarkers are negative since some patients have false-negative serum assays but testing of BAL samples for GM can confirm IFD. However, we would not delay antifungal treatment for IFD until such a definitive diagnosis is made. We recommend utilizing these serologic tests to identify patients with incipient IFD to initiate pre-emptive antifungal treatment based on serial biomarker screening with either GM antigen test, BDG antigen test or molecular tests in high-risk patients with prolonged neutropenia undergoing treatment for hematologic malignancies and in HSCT recipients. The presence of these biomarkers can be used alone or in conjunction with radiological and clinical signs for early initiation of pre-emptive antifungal treatment in patients with or without persistent febrile neutropenia. We would withhold empiric antifungal therapy in patients with persistent febrile neutropenia and negative biomarkers in the absence of clinical and radiologic signs of IFD.
Five-year view
We predict that over the next 5 years, diagnostic methods for IFD will improve further by standardization of methodology and greater availability of molecular methods and further validating their role in IFD diagnosis. This will in turn lead to the inclusion of molecular methods in diagnostic guidelines for IFD. We also predict that the role of screening for IFD in high-risk patients with serial serologic and molecular methods will become more defined and standardized in terms of optimal timing and preferred methods.
Key issues
Invasive fungal disease is associated with epidemiologic shifts and high morbidity and mortality in both patients receiving therapy for hematologic malignancies and in hematopoietic stem cell transplant recipients.
Timely diagnosis and treatment of invasive fungal disease is essential in improving survival.
Definitive diagnosis of invasive fungal disease relies on microbiological and histological methods.
New serologic methods including Aspergillus galactomannan antigen detection test and 1,3-β-d-glucan antigen detection test are now utilized in supporting the early diagnosis of probable invasive fungal disease.
The use of molecular methods for early diagnosis of invasive fungal disease is promising but standardization and validation of molecular methods is still underway.
Empiric antifungal treatment approach is widely used in high-risk populations but has shortcomings including both over- and under-treatment.
Using serial serologic and molecular biomarkers for screening high-risk populations is effective in ruling out invasive fungal disease and utilizing a pre-emptive antifungal treatment approach that can be used as an alternative to empiric therapy.
Footnotes
Financial & competing interests disclosure
JR Wingard has received consulting fees from Merck, Athersys, Gamida and Astellas and lecture fees from Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 2006;43(1):S3–S14. [Google Scholar]
- 2.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075. [PubMed] [Google Scholar]
- 3.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 4.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin. Infect. Dis. 2007;45(9):1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 5.Lass-Flörl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52(3):197–205. doi: 10.1111/j.1439-0507.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 6.Pasqualotto AC, Rosa DD, Medeiros LR, Severo LC. Candidaemia and cancer: patients are not all the same. BMC Infect. Dis. 2006;6:50. doi: 10.1186/1471-2334-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 1991;325(18):1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 8.Lionakis MS, Lewis RE, Torres HA, Albert ND, Raad II, Kontoyiannis DP. Increased frequency of non-fumigatus Aspergillus species in amphotericin B- or triazole-pre-exposed cancer patients with positive cultures for aspergilli. Diagn. Microbiol. Infect. Dis. 2005;52(1):15–20. doi: 10.1016/j.diagmicrobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Richardson M, Lass-Flörl C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008;14(Suppl. 4):5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 10.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 2005;49(9):3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heussel CP, Kauczor HU, Heussel GE, et al. Pneumonia in febrile neutropenic patients and in bone marrow and blood stem-cell transplant recipients: use of high-resolution computed tomography. J. Clin. Oncol. 1999;17(3):796–805. doi: 10.1200/JCO.1999.17.3.796. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Article about the revised EORTC/MSG criteria for invasive fungal disease (IFD) diagnosis.
- 13.Preuner S, Lion T. Towards molecular diagnostics of invasive fungal infections. Expert Rev. Mol. Diagn. 2009;9(5):397–401. doi: 10.1586/erm.09.27. [DOI] [PubMed] [Google Scholar]
- 14.Lease ED, Alexander BD. Fungal diagnostics in pneumonia. Semin. Respir. Crit. Care Med. 2011;32(6):663–672. doi: 10.1055/s-0031-1295714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirk B, Wingard JR. Current approaches in antifungal prophylaxis in high risk hematologic malignancy and hematopoietic stem cell transplant patients. Mycopathologia. 2009;168(6):299–311. doi: 10.1007/s11046-009-9188-6. [DOI] [PubMed] [Google Scholar]
- 16.Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 1997;10(3):444–465. doi: 10.1128/cmr.10.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 2005;5(10):609–622. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 18.Ellepola AN, Morrison CJ. Laboratory diagnosis of invasive candidiasis. J. Microbiol. 2005;43:65–84. [PubMed] [Google Scholar]
- 19.Simoneau E, Kelly M, Labbe AC, Roy J, Laverdière M. What is the clinical significance of positive blood cultures with Aspergillus sp in hematopoietic stem cell transplant recipients? A 23 year experience. Bone Marrow Transplant. 2005;35(3):303–306. doi: 10.1038/sj.bmt.1704793. [DOI] [PubMed] [Google Scholar]
- 20.Cuenca-Estrella M, Bassetti M, Lass-Flörl C, Rácil Z, Richardson M, Rogers TR. Detection and investigation of invasive mould disease. J. Antimicrob. Chemother. 2011;66(Suppl. 1):i15–i24. doi: 10.1093/jac/dkq438. [DOI] [PubMed] [Google Scholar]
- 21.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J. Clin. Oncol. 2001;19(1):253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 22.Barnes RA. Early diagnosis of fungal infection in immunocompromised patients. J. Antimicrob. Chemother. 2008;61(Suppl. 1):i3–i6. doi: 10.1093/jac/dkm424. [DOI] [PubMed] [Google Scholar]
- 23.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin. Infect. Dis. 2007;44(3):373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]; • Data supporting the use of the halo sign on chest computed tomography for the early diagnosis and treatment of invasive pulmonary aspergillosis.
- 24.Maertens JA, Klont R, Masson C, et al. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin. Infect. Dis. 2007;44(10):1329–1336. doi: 10.1086/514349. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin. Infect. Dis. 2006;42(10):1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]; •• A meta-analysis providing data for the use of the galactomannan assay for the early diagnosis of invasive aspergillosis in patients with hematologic malignancies and hematopoietic stem cell transplant recipients.
- 26.Herbrecht R, Letscher-Bru V, Oprea C, et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 2002;20(7):1898–1906. doi: 10.1200/JCO.2002.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J. Infect. Dis. 2004;190(3):641–649. doi: 10.1086/422009. [DOI] [PubMed] [Google Scholar]
- 28.Koo S, Bryar JM, Baden LR, Marty FM. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J. Clin. Microbiol. 2010;48(4):1255–1260. doi: 10.1128/JCM.02281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maertens J, Buvé K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009;115(2):355–362. doi: 10.1002/cncr.24022. [DOI] [PubMed] [Google Scholar]
- 30.Miceli MH, Grazziutti ML, Woods G, et al. Strong correlation between serum aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin. Infect. Dis. 2008;46(9):1412–1422. doi: 10.1086/528714. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron A, Belle A, Sulahian A, et al. Contribution of galactomannan antigen detection in BAL to the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies. Chest. 2010;137(2):410–415. doi: 10.1378/chest.09-0701. [DOI] [PubMed] [Google Scholar]
- 32.Musher B, Fredricks D, Leisenring W, Balajee SA, Smith C, Marr KA. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J. Clin. Microbiol. 2004;42(12):5517–5522. doi: 10.1128/JCM.42.12.5517-5522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen MH, Leather H, Clancy CJ, et al. Galactomannan testing in bronchoalveolar lavage fluid facilitates the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies and stem cell transplant recipients. Biol. Blood Marrow Transplant. 2011;17(7):1043–1050. doi: 10.1016/j.bbmt.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Maertens J, Maertens V, Theunissen K, et al. Bronchoalveolar lavage fluid galactomannan for the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Clin. Infect. Dis. 2009;49(11):1688–1693. doi: 10.1086/647935. [DOI] [PubMed] [Google Scholar]
- 35.Becker MJ, Lugtenburg EJ, Cornelissen JJ, Van Der Schee C, Hoogsteden HC, De Marie S. Galactomannan detection in computerized tomography-based bronchoalveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. Br. J. Haematol. 2003;121(3):448–457. doi: 10.1046/j.1365-2141.2003.04308.x. [DOI] [PubMed] [Google Scholar]
- 36.Mikulska M, Furfaro E, Del Bono V, et al. Piperacillin/tazobactam (Tazocin™) seems to be no longer responsible for false-positive results of the galactomannan assay. J. Antimicrob. Chemother. 2012;67(7):1746–1748. doi: 10.1093/jac/dks111. [DOI] [PubMed] [Google Scholar]
- 37.Viscoli C, Machetti M, Gazzola P, et al. Aspergillus galactomannan antigen in the cerebrospinal fluid of bone marrow transplant recipients with probable cerebral aspergillosis. J. Clin. Microbiol. 2002;40(4):1496–1499. doi: 10.1128/JCM.40.4.1496-1499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 2005;40(12):1762–1769. doi: 10.1086/429921. [DOI] [PubMed] [Google Scholar]
- 39.Viscoli C, Machetti M, Cappellano P, et al. False-positive galactomannan platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 2004;38(6):913–916. doi: 10.1086/382224. [DOI] [PubMed] [Google Scholar]
- 40.Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 2004;4(6):349–357. doi: 10.1016/S1473-3099(04)01045-X. [DOI] [PubMed] [Google Scholar]
- 41.Boonsarngsuk V, Niyompattama A, Teosirimongkol C, Sriwanichrak K. False-positive serum and bronchoalveolar lavage Aspergillus galactomannan assays caused by different antibiotics. Scand. J. Infect. Dis. 2010;42(6–7):461–468. doi: 10.3109/00365541003602064. [DOI] [PubMed] [Google Scholar]
- 42.Zandijk E, Mewis A, Magerman K, Cartuyvels R. False-positive results by the platelia Aspergillus galactomannan antigen test for patients treated with amoxicillin-clavulanate. Clin. Vaccine Immunol. 2008;15(7):1132–1133. doi: 10.1128/CVI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori Y, Nagasaki Y, Kamezaki K, et al. High incidence of false-positive Aspergillus galactomannan test in multiple myeloma. Am. J. Hematol. 2010;85(6):449–451. doi: 10.1002/ajh.21697. [DOI] [PubMed] [Google Scholar]
- 44.Hage CA, Reynolds JM, Durkin M, Wheat LJ, Knox KS. Plasmalyte as a cause of false-positive results for Aspergillus galactomannan in bronchoalveolar lavage fluid. J. Clin. Microbiol. 2007;45(2):676–677. doi: 10.1128/JCM.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen SC, Kontoyiannis DP. New molecular and surrogate biomarker-based tests in the diagnosis of bacterial and fungal infection in febrile neutropenic patients. Curr. Opin. Infect. Dis. 2010;23(6):567–577. doi: 10.1097/QCO.0b013e32833ef7d1. [DOI] [PubMed] [Google Scholar]
- 46.Mulanovich VE, Kontoyiannis DP. Fungal pneumonia in patients with hematologic malignancies: current approach and management. Curr. Opin. Infect. Dis. 2011;24:323–332. doi: 10.1097/QCO.0b013e3283486d1d. [DOI] [PubMed] [Google Scholar]
- 47.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. Diagnostic performance of the (1→3)-β-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 2009;49(11):1650–1659. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]
- 48.Odabasi Z, Mattiuzzi G, Estey E, et al. β-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 2004;39(2):199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]; •• Data supporting the use of 1,3-β-d-glucan antigen assay in the early diagnosis of IFD in patients with acute myeloid leukemia and myelodysplastic syndrome.
- 49.Lamoth F, Cruciani M, Mengoli C, et al. Third European Conference on Infections in Leukemia (ECIL-3). β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3) Clin. Infect. Dis. 2012;54(5):633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 50.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. Evaluation of a (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005;43(12):5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marty FM, Lowry CM, Lempitski SJ, Kubiak DW, Finkelman MA, Baden LR. Reactivity of (1→3)-β-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob. Agents Chemother. 2006;50(10):3450–3453. doi: 10.1128/AAC.00658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mennink-Kersten MA, Ruegebrink D, Verweij PE. Pseudomonas aeruginosa as a cause of 1,3-β-d-glucan assay reactivity. Clin. Infect. Dis. 2008;46(12):1930–1931. doi: 10.1086/588563. [DOI] [PubMed] [Google Scholar]
- 53.Pazos C, Pontón J, Del Palacio A. Contribution of (1→3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 2005;43(1):299–305. doi: 10.1128/JCM.43.1.299-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuenca-Estrella M, Meije Y, Diaz-Pedroche C, et al. Value of serial quantification of fungal DNA by a real-time PCR-based technique for early diagnosis of invasive Aspergillosis in patients with febrile neutropenia. J. Clin. Microbiol. 2009;47(2):379–384. doi: 10.1128/JCM.01716-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Florent M, Katsahian S, Vekhoff A, et al. Prospective evaluation of a polymerase chain reaction-ELISA targeted to Aspergillus fumigatus and Aspergillus flavus for the early diagnosis of invasive aspergillosis in patients with hematological malignancies. J. Infect. Dis. 2006;193(5):741–747. doi: 10.1086/500466. [DOI] [PubMed] [Google Scholar]
- 56.Kami M, Fukui T, Ogawa S, et al. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 2001;33(9):1504–1512. doi: 10.1086/323337. [DOI] [PubMed] [Google Scholar]
- 57.Mengoli C, Cruciani M, Barnes RA, Loeffler J, Donnelly JP. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect. Dis. 2009;9(2):89–96. doi: 10.1016/S1473-3099(09)70019-2. [DOI] [PubMed] [Google Scholar]; • A meta-analysis providing data for the use of Aspergillus PCR for the early diagnosis of invasive aspergillosis.
- 58.Lau A, Chen S, Sorrell T, et al. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J. Clin. Microbiol. 2007;45(2):380–385. doi: 10.1128/JCM.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiess B, Seifarth W, Hummel M, et al. DNA microarray-based detection and identification of fungal pathogens in clinical samples from neutropenic patients. J. Clin. Microbiol. 2007;45(11):3743–3753. doi: 10.1128/JCM.00942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison E, Stalhberger T, Whelan R, et al. Aspergillus Technology Consortium (AsTeC). Aspergillus DNA contamination in blood collection tubes. Diagn. Microbiol. Infect. Dis. 2010;67(4):392–394. doi: 10.1016/j.diagmicrobio.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White PL, Bretagne S, Klingspor L, et al. European Aspergillus PCR Initiative. Aspergillus PCR: one step closer to standardization. J. Clin. Microbiol. 2010;48(4):1231–1240. doi: 10.1128/JCM.01767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wingard JR. New approaches to invasive fungal infections in acute leukemia and hematopoietic stem cell transplant patients. Best Pract. Res. Clin. Haematol. 2007;20(1):99–107. doi: 10.1016/j.beha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin. Infect. Dis. 2002;34(6):730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 64.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011;52(4):427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 65.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 2004;351(14):1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 66.de Pauw BE. Between over- and under-treatment of invasive fungal disease. Clin. Infect. Dis. 2005;41(9):1251–1253. doi: 10.1086/496933. [DOI] [PubMed] [Google Scholar]
- 67.Bennett JE, Powers J, Walsh T, et al. Forum report: issues in clinical trials of empirical antifungal therapy in treating febrile neutropenic patients. Clin. Infect. Dis. 2003;36(Suppl. 3):117–122. doi: 10.1086/367839. [DOI] [PubMed] [Google Scholar]
- 68.Mikolajewska A, Schwartz S, Ruhnke M. Antifungal treatment strategies in patients with haematological diseases or cancer: from prophylaxis to empirical, pre-emptive and targeted therapy. Mycoses. 2012;55(1):2–16. doi: 10.1111/j.1439-0507.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- 69.Cordonnier C, Pautas C, Maury S, et al. Empirical versus pre-emptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin. Infect. Dis. 2009;48(8):1042–1051. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- 70.Hebart H, Klingspor L, Klingebiel T, et al. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 2009;43(7):553–561. doi: 10.1038/bmt.2008.355. [DOI] [PubMed] [Google Scholar]; •• A randomized trial comparing empiric versus PCR-based pre-emptive treatments for IFD in allogeneic hematopoietic stem cell transplant recipients.
- 71.Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based pre-emptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin. Infect. Dis. 2005;41(9):1242–1250. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]; •• A study providing data for the use of a pre-emptive treatment approach for IFD utilizing galactomannan antigen assay and high resolution CT scan.
- 72.Maertens J, Marchetti O, Herbrecht R, et al. Third European Conference on Infections in Leukemia. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3-2009 update. Bone Marrow Transplant. 2011;46(5):709–718. doi: 10.1038/bmt.2010.175. [DOI] [PubMed] [Google Scholar]
- 73.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]