Abstract
Normobaric hyperoxia (NBO), which maintains penumbral oxygenation, reduces brain injury during cerebral ischemia, and minocycline, a tetracycline derivative, reduces reperfusion injury, including inflammation, apoptosis and matrix metalloproteinases (MMPs) activation. Since they have different mechanisms of action, we hypothesized that combining them would provide greater neuroprotection. To test the hypothesis, we evaluated the neuroprotective effects of the combination of NBO with minocycline. Male Sprague Dawley rats were exposed to NBO (95% O2) or normoxia (21% O2) during 90-min filament occlusion of the middle cerebral artery, followed by 48 hrs of reperfusion. Minocycline (3 mg/kg) or vehicle was intravenously administered to rats 15 min after reperfusion onset. Treatment with NBO and minocycline alone resulted in 36% and 30% reductions in infarction volume, respectively. When the two treatments were combined, there was a 68% reduction in infarction volume. The combination therapy also significantly reduced hemispheric swelling, which was absent with monotherapy. In agreement with its greater neuro-and vasoprotection, the combination therapy showed greater inhibitory effects on MMP-2/9 induction, occludin degradation, caspase-3 and -9 activation and apoptosis inducing factor (AIF) induction in ischemic brain tissue. Our results show that NBO plus minocycline effectively reduces brain injury in transient focal cerebral ischemia with protection due to inhibition on MMP-2/9-mediated occludin degradation and attenuation of caspase-dependent and independent apoptotic pathways.
Keywords: oxygen, minocycline, stroke, infarction, hemispheric swelling
Introduction
The primary goal of current treatment for acute-phase ischemic stroke is to salvage the ischemic penumbra (Ramos-Cabrer et al., 2011). The ischemic penumbra is a region of ischemic brain tissue with sufficient energy for short-term survival (Hakim, 1998). If ischemia persists, the penumbra will progress to irreversible damage, primarily driven by ischemia-induced hypoxia and the consequent bioenergetic failure (Dirnagl et al., 1999). When reperfusion is achieved, additional irreversible cellular damage can also develop due to a mechanism of reperfusion injury (Pan et al., 2007; Schaller and Graf, 2004). This multi-stage progression of ischemic brain damage has prompted the stroke community to seek combinational therapeutic approaches for treating acute ischemic stroke (O'Collins et al., 2012).
Improving tissue oxygenation has been studied for many years as a simplistic but plausible treatment strategy to reduce ischemic injury. Recent animal and human stroke studies showed that normobaric hyperoxia (NBO) when administered early after ischemia onset is neuroprotective (Chiu et al., 2006; Henninger et al., 2007; Shin et al., 2007; Singhal et al., 2005; Tang et al., 2010). This protection is attributed to NBO's ability to improve energy metabolism in the ischemic penumbra, as evidenced by elevated interstitial oxygen partial pressure (Liu et al., 2006), increased cerebral blood flow and O2 delivery (Shin et al., 2007), and reductions in acidosis and ATP deletion (Sun et al., 2011). As NBO is readily available, safe and can be initiated promptly after stroke onset by paramedics, it has been suggested as a practical acute-phase treatment to slow down the onset of irreversible damage of the penumbra and to expand the therapeutic time window of reperfusion therapy (Henninger and Fisher, 2006; Kim et al., 2005; Liu et al., 2009a).
Minocycline is a tetracycline antibiotic with demonstrated properties against reperfusion injury including its anti-inflammatory (Yrjanheikki et al., 1999), anti-apoptotic (Arvin et al., 2002; Friedlander, 2003), and blood-brain barrier (BBB)-protecting actions (Wang et al., 2002; Xu et al., 2004). Animal studies and early phase clinical trials showed that minocycline, even when administered at delayed time points, is neuroprotective (Fagan et al., 2010; Hayakawa et al., 2008; Hewlett and Corbett, 2006; Lampl et al., 2007; Liu et al., 2007; Xu et al., 2004).
Given that NBO can ameliorate metabolic disturbance in the acute ischemic phase and minocycline can interfere with reperfusion-associated injury, in this study, we tested the hypothesis that NBO in combination with minocycline results in greater neuroprotection than each individual treatment on a rat model of transient focal cerebral ischemia. Matrix metalloproteinase (MMP)-2 and -9 are important mediators of reperfusion-associated BBB damage and apoptotic cell death in rodent stroke models (Liu et al., 2011; Yang et al., 2010; Yang et al., 2007). Therefore, we examined the effects of combination therapy on the changes of MMP-2 and -9, tight junction protein occludin, caspase-3 and -9, as well as apoptosis-inducing factor (AIF) in the ischemic brain.
Materials and methods
Rat model of focal cerebral ischemia and reperfusion
The Laboratory Animal Care and Use Committee of the University of New Mexico approved all experimental protocols. Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 290 to 320 g were anesthetized with isoflurane (4% for induction, 1.75 % for maintenance) in N2O:O2 (70:30) during surgical procedures. Middle cerebral artery occlusion (MCAO) was induced by advancing a 4-0 silicone-coated monofilament suture along the internal carotid artery to block the ostium of the MCA, and reperfusion was produced by gently withdrawing the suture, as we previously described (Liu et al., 2006). Body temperature was maintained at 37.5 ± 0.5 °C with a heating pad during surgical procedures. All animals were subjected to 90-min MCAO with 48 hrs of reperfusion and successful MCAO was confirmed post mortem by 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma) staining as previously described (Liu et al., 2009b). Four rats were excluded from this study due to insufficient arterial occlusion reflected by not circling to the left at the end of ischemia. No rats died because of stroke or surgical complications.
Treatment protocol
After MCAO, animals were randomly assigned into different treatment groups: 1) air plus vehicle group; 2) NBO plus vehicle group; 3) air plus minocycline group; 4) NBO plus minocycline group. Each treatment was described in detail as follows: 1) NBO or air treatment: five min after the onset of MCAO, anesthesia was discontinued, and the rats were put into an air-tight box (the same box used for anesthesia induction), which was ventilated (3 L/min) with medical air (21% O2, air) or a gas mixture of 95% O2+5% CO2 (NBO) until the end of 90-min MCAO. Our previous studies demonstrated that with this treatment regimen, NBO maintained penumbral oxygenation and effectively reduced ischemic brain injury (Liu et al., 2006). 2) minocycline or vehicle treatment: minocycline (Sigma) was dissolved in dimethy sulfoxide (DMSO) and diluted (1:50 in soluntol) to the final concentration of 3 mg/ml, which reduced the injection amount of DMSO, avoiding neuroprotection while dissolving the minocycline (Rosenberg et al., 2007). Fifteen min after reperfusion onset, minocycline at 3 mg/kg body weight or 2% DMSO in soluntol (vehicle) was administered to rats via the left femoral vein. The dosage was selected because it was shown to result in serum levels compatible with human administration (Xu et al., 2004). Treatment protocol was shown in Figure 1.
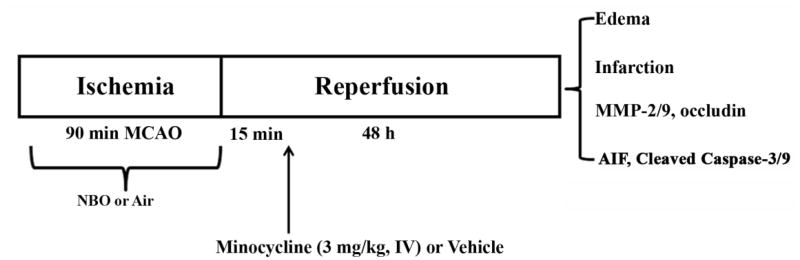
Fig. 1.
Outline of experimental design. Rats were subjected to 90-min MCAO with 48 hrs of reperfusion. NBO was initiated 5 min after MCAO onset and minocycline (3 mg/kg body weight) or vehicle (5% soluntol in saline) was intravenously administered 15 min after reperfusion onset. Outcome parameters including hemispheric swelling, infarction, MMP-2/9, tight junction protein occludin, AIF, and cleaved caspase-3 and -9 were determined at the end of reperfusion.
Measurement of hemispheric swelling and tissue infarction
At the end of 48 hrs of reperfusion, rats were transcardially perfused under deep anesthesia with cold phosphate buffered saline (PBS) to remove intravascular blood. Brains were quickly removed and 6 consecutive 2-mm-thick coronary slices were sectioned from a 12-mm-thick brain region which was 3 mm away from the tip of the frontal lobe. After digital photographing, hemispheric swelling was quantitated by measuring the hemispheric areas of each 2-mm thick brain slice and expressed as a relative increase of the brain area in the ischemic hemisphere versus the nonischemic hemisphere, as we described previously (Liu et al., 2009a). Infarct volume, brain swelling and outcome assessments were blinded to therapy.
To assess tissue infarction, all brain slices except the 4th one were stained with 2% TTC and digitally photographed. The 5th slice was reversed and photographed again, and used as a substitute for the 4th slice for calculating infarction volume. Infarction volume was quantitated and expressed as a percentage of infarcted tissue as compared to the total volume of all of the slices, as we described previously (Liu et al., 2006). The 4th brain slice was used for tissue collection to measure the molecules of interest by western blot and/or gel gelatin zymography, as described below.
Gel gelatin zymography analysis for MMP-2 and -9
Ischemic and nonischemic tissues were collected from the 4th coronal section, in which a longitudinal cut was made 2 mm away from the midline between two hemispheres to exclude tissue primarily supplied by the anterior cerebral artery. Tissue was homogenized with RIPA buffer (Santa Cruz Biotech.) and protein concentrations in the homogenates were determined using Bradford reagent (Bio-Rad). Aliquots of homogenates were subjected to zymography or western blot analysis (Abbruscato and Davis, 1999).
Zymography analysis was performed as we described previously (Yang et al., 2007). In brief, 2 mg of protein in 1 ml of homogenate was incubated with 50 μl Gelatin-Sepharose 4B beads (GE Healthcare) for 1 hr at 4°C with gentle rotation to pull down MMP-2 and -9. Then, MMP-2 and -9 were eluted out with 50 μl elution buffer (10% DMSO in PBS). Equal amounts of samples (20 μl) were electrophoretically separated on 10 % sodium dodecyl sulfate -polyacrylamide gels co-polymerized with 1 mg/ml gelatin (Sigma) under nonreducing conditions. After washing with 2.5 % Triton X-100, the gel was incubated for 48 hrs with a developing buffer containing 50 mmol/L Tris, pH 7.6, 5 mmol/L CaCl2, 0.2 mmol/L NaCl, and 0.02 % (w/v) Brij-35 at 37°C before staining with Coomassie blue R-250. Gels were destained and the band intensities for MMP-2 and -9 were quantitated using a Kodak 4000 imaging station (Carestream Molecular Imaging).
Western blot analysis for occludin, AIF, caspase-3 and -9
Homogenate aliquots (50 μg of total protein) were boiled and then electrophoresed in 12% SDS-PAGE acrylamide gels, transferred onto nitrocellulose membranes (Bio-Rad), and incubated for 1 hr in TBS-T (Tris-buffered saline and 0.1% Tween 20) containing 5% nonfat milk. Membranes were then incubated overnight at 4°C with primary antibodies against occludin (1:500, Invitrogen), caspase-9 (1:500, Cell Signaling, Danvers, MA), AIF (1:500, Cell Signaling), or cleaved caspase-3 (1:200, Cell Signaling), washed in TBS-T, and then incubated for 1 hr at room temperature with corresponding HRP-conjugated anti-rabbit or anti-mouse antibodies (Santa Cruz Biotechnology; 1:1000). The membranes were developed with the SuperSignal West Pico HRP substrate kit (Pierce) and photographed on a Kodak 4000 image station (Carestream Molecular Imaging). Protein band intensities were quantitated after normalization to Coomassie blue staining of the same membrane (Yang et al., 2010), as the commonly used control protein, β-actin, was found to be significantly reduced in ischemic brain tissue under our experimental condition.
Statistical analysis
The data are presented as means ± SEM. Statistical analysis was carried out with one-way ANOVA followed by Scheffe post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Effects of NBO and minocycline on infarction and hemispheric swelling
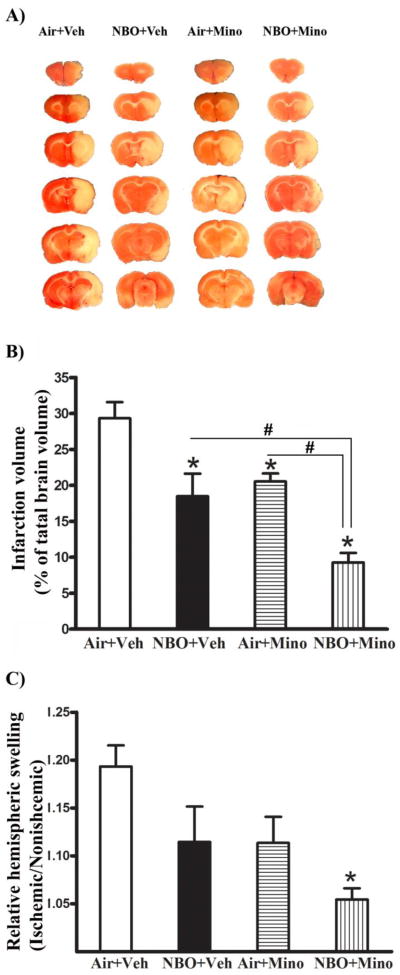
TTC staining of the 2-mm-thick brain sections showed that 90-min MCAO with 48 hrs of reperfusion induced significant infarction in the ischemic hemispheres (consistently seen in cortex and subcortex) of all rats (Fig. 2A). NBO or minocycline alone significantly reduced infarction volume compared to the control group that was treated with air plus vehicle (P < 0.05). Their combination resulted in a further reduction of infraction volume compared to each treatment alone (Fig. 2B, P < 0.05 versus NBO or minocycline).
Fig. 2.
Effects of NBO, minocycline and their combination on infarction and hemispheric swelling after 90-min MCAO and 48 hrs of reperfusion. (A) Representative TTC-stained coronal sections showed tissue infarction in the ischemic (right) hemisphere of each treatment group. Veh: vehicle; Mino: minocycline. (B) A greater neuroprotection was observed for the combination therapy compared to NBO or minocycline alone. Infarct volume was quantitated and expressed as a percentage of total brain volume. *P < 0.05 versus Air + Veh; #P < 0.05 versus NBO + Veh or Air + Mino. (C) Only the combination therapy significantly reduced hemispheric swelling. Hemispheric swelling was evaluated as hemispheric enlargement and expressed as the volume ratio between ischemic and nonischemic hemispheres. *P < 0.05 versus Air + Veh. Data are expressed as mean ± SEM, n = 8 for each group.
As expected, brain swelling was observed in the ischemic hemisphere of the control group (Fig. 2C). Different from their effects on tissue infarction, NBO or minocycline alone did not result in a significant reduction in hemispheric enlargement, though there was such a trend (P > 0.05 versus the air plus vehicle group). Remarkably, their combination led to a 71% reduction in hemispheric swelling compared to the control group (air plus vehicle) (P < 0.05).
Effects of NBO and minocycline on MMP-2, MMP-9 and occludin
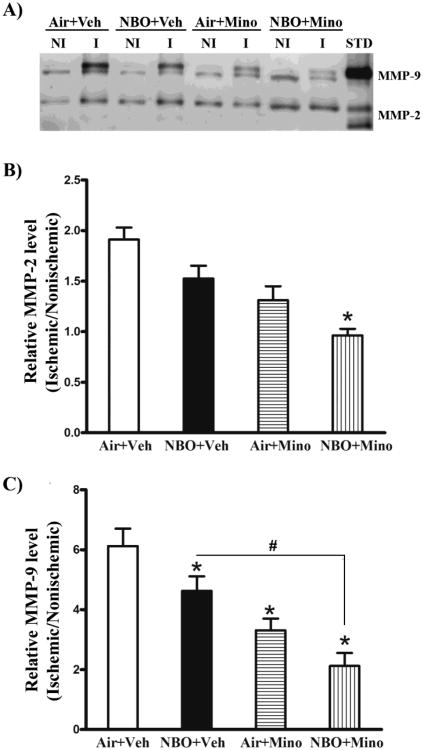
The gelatinases MMP-2 and -9 critically contribute to neuronal cell death and edema formation in ischemic stroke by degrading extracellular matrix substrates to interrupt cell–cell or cell–matrix homeostatic interactions necessary for cell survival (Lee and Lo, 2004) as well as BBB structural components for maintaining BBB integrity (Yang and Rosenberg, 2011). We next examined the effects of NBO, minocycline and their combination on MMP-2 and -9 inductions in ischemic brain using gel gelatin zymography (Fig. 3). In the control group (air plus vehicle), a low basal level of MMP-9 (∼ 88 kDa) was detected in contralateral tissue, which was drastically increased (6.1 folds) after 90-min MCAO with 48 hrs of reperfusion, which appeared as strong doublets (88 and 92 kDa) on zymogram gels (Figure 3A). According to the MMP-9 standard, no active MMP-9 band was detected in any samples. Compared to MMP-9, contralateral tissue expressed relatively higher basal levels of MMP-2 (72 kDa), which was also significantly increased after cerebral ischemia and reperfusion, but to a less extent (0.9-fold increase) (Fig. 3A). No active MMP-2 band was seen in the nonischemic samples, but was faintly visible in some ischemic samples. The band intensities of MMP-2 and -9 were quantitated and expressed as hemispheric MMP ratio (ischemic/nonischemic). Figure 3B showed that the combination therapy, but not NBO or minocycline alone, significantly reduced MMP-2 levels (P < 0.05). A more profound reduction in MMP-9 (Fig. 3C) was observed for minocycline alone or the combination therapy compared to NBO alone, but there was no significant difference between the minocycline group and combination group. As expected, all treatments had no significant effect on basal MMP-2 and -9 levels in the contralateral tissue
Fig. 3.
Effects of NBO, minocycline and their combination on MMP-2/9 induction in the ischemic brain after 90-min MCAO and 48 hrs of reperfusion. MMP-2 and -9 were analyzed by gel gelatin zymography. (A) A representative gelatin zymogram shows MMP-2/9 induction in the nonischemic (NI) and ischemic (I) hemispheric tissue of each group. According to MMP-2 and -9 standards (STD), the pro-MMP-2 and 9 were clearly seen in all samples, while their active forms were not or only barely detectable. Veh: vehicle; Mino: minocycline. The relative band intensity of MMP-2 (B) and MMP-9 (C) was quantitated and expressed as the hemispheric ratio (ischemic/nonischemic). Cerebral ischemia and reperfusion induced a 0.9-fold increase in MMP-2 and 6.1-fold increase in MMP-9 in the ischemic tissue. NBO, minocycline or their combination significantly attenuated MMP-2/9 induction (*P < 0.05 versus Air + Veh). Compared to NBO alone, a greater inhibition was observed for the combination therapy on both MMP-2 and -9 (#P < 0.05 versus NBO + Veh). Compared to minocycline alone, the combination led to a further reduction in MMP-2, but not in MMP-9 (&P < 0.05 versus Air + Mino). NBO, minocycline or their combination showed no significant effects on the basal levels of MMP-2 and -9 in the nonischemic hemisphere. Data are expressed as the mean ± SEM, n = 8 for each group.
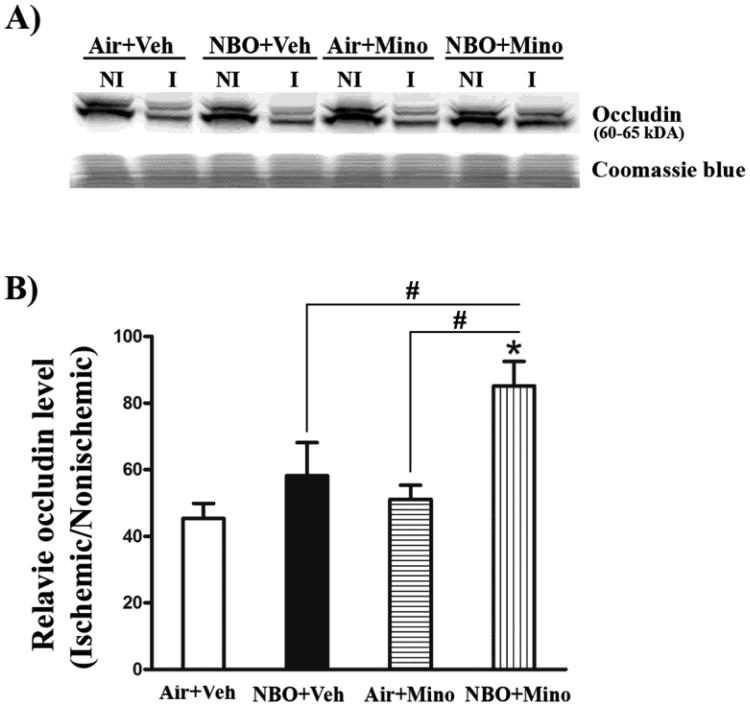
Occludin degradation is frequently seen in the ischemic brain to cause BBB disruption (ElAli et al., 2011; Zhang et al., 2008), and experimental evidence suggests that occludin is a direct substrate of MMP-2 and -9 (Giebel et al., 2005; Liu et al., 2009b). Fig. 4A shows that occludin exhibited as a doublet of 60 and 65 kDa on western blot, which represents two different isoforms of occludin monomers (McCaffrey et al., 2007). The band intensity of the occludin protein was quantitated and expressed as hemispheric ratio (ischemic/nonischemic) after normalizing to total protein (Coomassie blue staining). Fig. 4B shows that occludin protein levels were significantly reduced in ischemic hemispheric tissue after 90-min MCAO and 48 hrs of reperfusion, and this degradation was significantly inhibited by the combination therapy. While NBO and minocycline alone significantly inhibited expression of MMP-9, neither reduced occludin degradation (Fig. 4B). No significant difference was observed in occludin levels in the contralateral tissues across animal groups.
Fig. 4.
Effects of NBO, minocycline and their combination on occludin degradation in the ischemic brain after 90-min MCAO and 48 hrs of reperfusion. Western blot was conducted to detect occludin protein in the nonischemic (NI) and ischemic (I) hemispheric tissue. Veh: vehicle; Mino: minocycline. (A) A representative western blot revealed occludin protein a doublet of 60 and 65 kDa (upper panel). The same membrane was stained with Coomassie blue as a loading control (lower panel). (B) The band intensity of occludin protein was quantitated after normalization to the total protein reflected by Coomassie staining and expressed as hemispheric ratio (ischemic/nonischemic). Cerebral ischemia and reperfusion led to a 55% reduction in occludin protein in the ischemic tissue. The combination therapy significantly inhibited occludin reduction in the ischemic tissue (*P < 0.05 versus Air+Veh), while no significant effect was observed for NBO or minocycline alone. All three treatments did not affect occludin protein levels in the nonischemic hemisphere. Data are expressed as mean ± SEM, n = 8 for each group.
Effects of NBO and minocycline on AIF, cleaved caspase-3 and -9
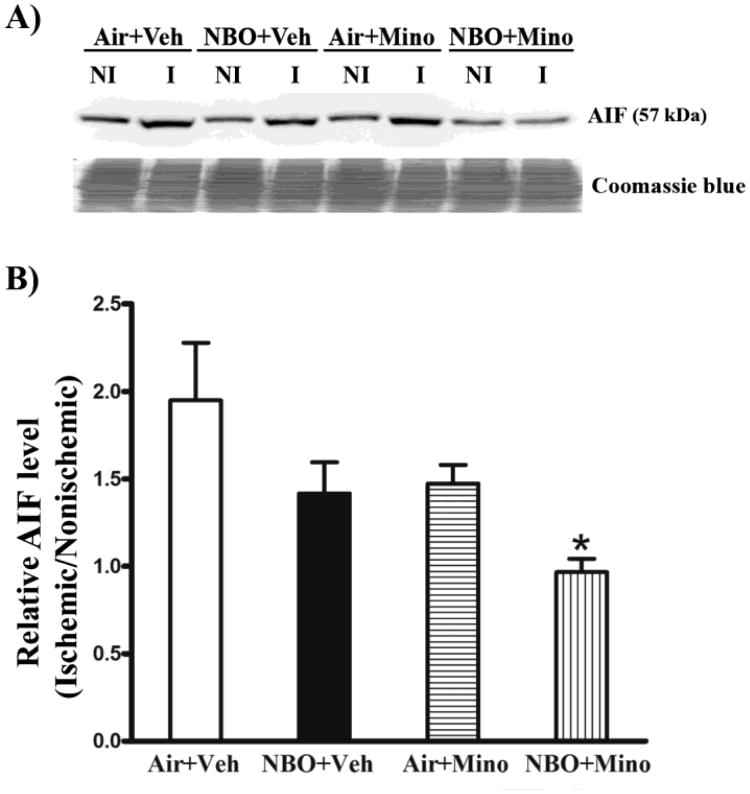
Both caspase-dependent and independent pathways critically contribute to neuronal death in ischemic stroke (Pallast et al., 2010; Van Hoecke et al., 2005). We next examined the effects of NBO, minocycline and their combination on the cleavage (or activation) of caspase-3 and -9 as well as the induction of AIF, a major caspase-independent mitochondrial cell death protein (Thal et al., 2011), to determine whether inhibition of caspase-dependent and independent death pathways accounts for their neuroprotective effects. Compared to its low basal level in the contralateral tissue, AIF protein was significantly increased in the ischemic tissue of the control group (air plus vehicle) (Fig. 5A). Interestingly, the ischemic AIF band appeared to run a little faster than its nonischemic counterpart on the gel. AIF bands were quantitated after normalizing to total protein (Coomassie blue staining). Fig. 5B shows that the combination therapy, but not each individual treatment alone, significantly inhibited AIF increase in the ischemic brain (P < 0.05). There were no detectable differences in AIF levels in the contralateral tissues across all animal groups.
Fig. 5.
Effects of NBO, minocycline and their combination on AIF induction in the ischemic brain after 90-min MCAO and 48 hrs of reperfusion. Western blot was conducted to detect AIF protein in the nonischemic (NI) and ischemic (I) hemispheric tissue. Veh: vehicle; Mino: minocycline. (A) A representative western blot revealed AIF changes in the ischemic brain of each group (upper panel). The membrane was stained with Coomassie blue as a loading control (lower panel). (B) AIF protein was quantitated after normalization to the intensity of Coomassie staining and expressed as hemispheric ratio (ischemic/nonischemic). Cerebral ischemia and reperfusion induced a 0.94-fold increase in AIF protein in the ischemic tissue, which was almost completely inhibited by the combination therapy (*P < 0.05 versus Air + Veh). NBO or minocycline alone resulted in a small but not significant reduction in AIF increase. All three treatments did not affect AIF level in the nonischemic hemisphere. Data are expressed as mean ± SEM, n = 8 for each group.
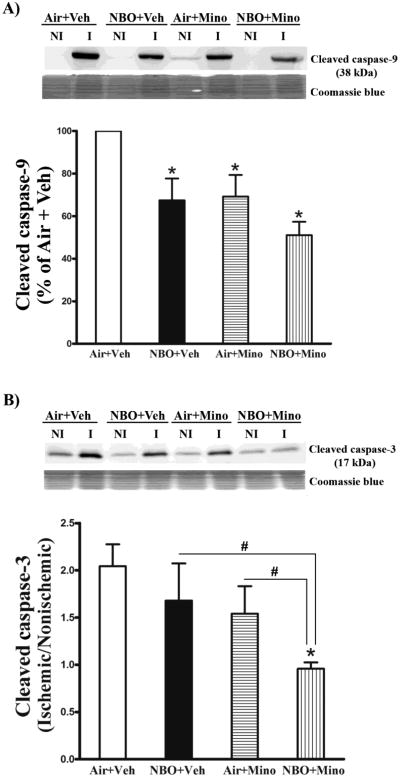
Caspase-3 and -9 are cleaved during their activation to cause apoptotic cell death (Chandra and Tang, 2003). Therefore, we measured their cleaved forms in brain tissue for all 4 groups. Cleaved caspase-9, a 38 kDa protein, was barely detectable in the contralateral tissue, a strong cleaved caspase-9 band was detected in the ischemic tissue of the control group (air plus vehicle) (Fig. 6A, upper panel). The cleaved caspase-9 band was quantitated after normalizing to total protein (Coomassie blue staining) and expressed as percentage of the control group. NBO or minocycline alone significantly reduced cleaved caspase-9 levels, and the combination therapy led to a further reduction (Fig. 6A, bottom panel). Different from caspase-9, there were detectable low levels of cleaved caspase-3 in the contralateral tissues of all animal groups (Fig. 6B, upper panel). The cleaved caspase-3 band was quantitated after normalizing to total protein (Coomassie blue staining) and expressed as a hemispheric ratio (ischemic/contralateral). In the ischemic hemispheric tissue of the control group, the level of cleaved caspase-3 was approximately one fold higher than that of the contralateral tissue. The combination therapy led to a significant reduction in cleaved caspase-3 in the ischemic tissue, while no significant effects, only trends toward reduction, were observed for each monotherapy (Fig. 6B, bottom panel). As expected, none of the treatments had significant effects on the levels of caspase-3 in the contralateral tissues.
Fig. 6.
Effects of NBO, minocycline and their combination on caspase-3 and -9 cleavage in the ischemic brain after 90-min MCAO and 48 hrs of reperfusion. Western blot was conducted to detect cleaved caspase-3 and -9 in the nonischemic (NI) and ischemic (I) hemispheric tissue. Veh: vehicle; Mino: minocycline. (A) The combination therapy led to a greater inhibition on caspase-9 cleavage than NBO or minocycline alone. A representative western blot showed that cleaved caspase-9 was barely seen in nonsichemic (NI) hemispheric tissue, but was significantly increased in ischemic (I) tissue (Upper panel). After normalization to the total intensity of Coomassie staining (Middle panel), cleaved caspase-9 was quantitated and expressed as a percentage of AIF level in the ischemic hemispheric tissue of the Air + Veh group (Bottom panel). NBO, minocycline or their combination significantly reduced the level of cleaved caspase-9 in ischemic tissue (*P < 0.05 versus Air + Veh), and a greater reduction was seen for the combination therapy (#P < 0.05 versus NBO + Veh or Air + Mino). (B) The combination therapy, but not NBO or minocycline alone, significantly reduced caspase-3 cleavage in ischemic hemispheric tissue. A representative western blot showed cleaved caspase-3 levels in the nonischemic and ischemic hemispheric tissue of each group (Upper panel). After normalizing to Coomassie staining (Middle panel), cleaved caspase-3 was quantitated and expressed as a hemispheric ratio (ischemic/nonischemic) (Bottom panel). Data are expressed as mean ± SEM. *P < 0.05 versus Air + Veh, n = 8 for each group.
Discussion
We showed the protective effects of combining NBO with minocycline in a rat model of transient focal cerebral ischemia. The combination therapy induces greater reductions in tissue infarction and hemispheric swelling compared to each individual treatment, and this greater neuro- and vaso-protection is associated with inhibition on MMP-2/9 induction, tight junction protein, occludin, degradation and the activation of caspase-dependent and independent apoptotic pathways.
NBO therapy is readily available, easy to administer, noninvasive, and can be initiated promptly after stroke onset by paramedics. When used as a monotherapy, NBO preserves the ischemic penumbra and reduces ischemic lesion volume (Chiu et al., 2006; Henninger et al., 2007; Liu et al., 2006; Shin et al., 2007; Singhal et al., 2005). Minocycline has neuroprotective effects in animal stroke models and has a delayed therapeutic time window (Fagan et al., 2011; Hayakawa et al., 2008; Hewlett and Corbett, 2006; Xu et al., 2004). Consistent with these previous studies, our present data show 36% and 30% reductions in infarction volume by NBO alone (given during ischemia) or minocycline alone (given during reperfusion), respectively. Importantly, when NBO is combined with minocycline, a significantly greater reduction in infarction volume of 68% is achieved. Our explanation for this greater protection is that combination treatment with NBO and minocycline targets different molecular events in transient cerebral ischemia; NBO reduces early ischemic neuronal death by improving penumbral tissue oxygenation (Liu et al., 2006; Liu et al., 2004), while minocycline attenuates secondary injury due to its anti-inflammation and anti-apoptotic actions (Fagan et al., 2011). It is conceivable that the greater neuroprotection afforded by the combination therapy may reduce stroke-associated neurological deficits. Further long-term studies are warranted to test this possibility in animal stroke models before moving this combination therapy into human studies.findings.
Traditionally, cell death after cerebral ischemia occurs by necrosis and caspase-mediated apoptosis (Broughton et al., 2009). Caspase-3, the key executive molecule in the apoptotic pathway, has been shown to be up-regulated and activated in ischemic brain tissue (Asahi et al., 1997; Namura et al., 1998; Rami et al., 2003), and genetic disruption or pharmacologic inhibition of caspase-3 leads to reduced neuronal death in the ischemic brain (Endres et al., 1998; Le et al., 2002). Caspase-9, which is upstream of caspase-3, initiates the cytochrome c-dependent cascade that leads to caspase-3 activation (Sugawara et al., 2004). Increased caspase-9 activation has been found in the ischemic brain (Gao et al., 2010), and intranasal delivery of caspase-9 inhibitor reduces ischemic neuronal death (Akpan et al., 2011). Our data show increased activation of caspase-3 and -9 in the ischemic brain tissue, and caspase-9, but not caspase-3, is significantly reduced after early treatment with either NBO or delayed administration of minocycline. Combination therapy results in more profound inhibition of both caspases than each individual treatment. Besides caspase-mediated apoptosis, mounting evidence implicates a significant role of caspase-independent apoptotic pathways in ischemic neuronal death, and AIF is the best understood among all the signal molecules in this cell death pathway (Cho and Toledo-Pereyra, 2008). Therefore, the combination treatment may also interfere with AIF-mediated apoptotic pathway to exert its neuroprotection. In support of this, AIF is induced in the ischemic brain tissue, and is almost completely inhibited by the combination of NBO and minocycline.
Besides neuroprotection, NBO can also protect the cerebrovascular bed against ischemic damage. Our earlier studies showed that NBO treatment during cerebral ischemia effectively reduces BBB disruption and edema formation within the first 24 hrs after ischemia onset (Liu et al., 2009b; Liu et al., 2008). These results have led to the investigation into the possibility of combining NBO with tPA to reduce thrombolytic reperfusion-associated neurovascular complications (Liu et al., 2009a; Sun et al., 2010). However, in this study, we found that NBO alone was not able to reduce hemispheric swelling following 48 hrs of reperfusion, which is different from what we observed earlier in the same MCAO model but with shorter (3 or 22.5 hrs) reperfusion durations (Liu et al., 2009b; Liu et al., 2008). The mechanism of reperfusion BBB injury may explain this discrepancy (Aronowski et al., 1997), in which NBO-afforded early cerebrovaso-protection could diminish over time during reperfusion. Minocycline alone has been shown to inhibit MMPs and inflammation and reduce BBB disruption in stroke models (Koistinaho et al., 2005; Machado et al., 2006; Nagel et al., 2008). However, to exert BBB protection, minocycline may need a relative large dose, supported by our observation that minocycline given in a single dose of 3 mg/kg body weight does not significantly affect edema formation, while Nagel et al (2008) observed a significant reduction in BBB disruption for minocycline given twice a day at a dose of 30 mg/kg body weight. When minocycline is combined with NBO, a dose of 3 mg/kg body weight leads to a 71% reduction in hemispheric swelling. In this context, NBO may serve as an adjunctive therapy to reduce minocycline dose and thus its side effects in treating ischemic stroke.
MMP-2 and -9 are induced in the ischemic brain and contribute to BBB disruption via proteolytic degradation of tight junction proteins (Yang et al., 2007). In animal stroke models, monotherapy with either NBO or minocycline has been shown to inhibit MMP-2 and -9 induction in the ischemic brain (Liu et al., 2006; Machado et al., 2006), but their mechanisms of action appear to be different. NBO attenuates MMP-9 induction through inhibiting NADPH oxidase (Liu et al., 2011), while minocycline's action is likely secondary to its inhibition on inflammatory cytokine production (Xing et al., 2012). Therefore, we have anticipated a greater inhibition of MMP-2 and -9 for the combination treatment. Surprisingly, our assumption is only partially correct because minocycline alone leads to a profound reduction in MMP-9, while no further reduction MMP-9 is shown for the combination. This may be explained by the fact that MMP-9 is an inflammatory molecule with NF-κB, AP-1, Sp-1, and an Ets site in the MMP-9 gene (Van den Steen et al., 2002), and its induction mainly occurs during reperfusion due to neuroinflammation. In further support of the inhibitory effects on MMP-9, the combination of NBO with minocycline significantly inhibits the degradation of tight junction protein occludin, a direct substrate of MMP-2/9 (Giebel et al., 2005; Liu et al., 2009b). A prior study showed that NBO alone inhibited MMP-mediated occludin degradation at 3 hrs of reperfusion (Liu et al., 2009b), but this is not seen here for each individual treatment alone. This discrepancy may be explained by the substantial amount of MMP-2/9 that remained in ischemic brain tissue of NBO- or minocycline-treated animals after 48 hrs of reperfusion (Fig. 3), which could be enough to cause the proteolytic degradation of occludin. Our results suggest that inhibition of MMP-mediated occludin may account for the vasoprotection by the combination treatment.
Conclusion and Clinical implications
NBO in combination with minocycline results in greater neuro- and vaso-protection than each monotherapy. Given the facts that 1) NBO has a superior advantage as an early stroke treatment to improve ischemia-induced metabolic disturbance, 2) minocycline can work as a delayed treatment strategy to reduce reperfusion injury, and 3) both agents have good safety profiles, this study provides important experimental data supporting the combination of NBO and minocycline as potential stroke therapy.
Highlight.
NBO combined with minocycline showed greater neuroprotection in ischemic stroke.
The combination therapy led to greater reduction in hemispheric swelling.
Inhibition on MMP-2/9, caspases and AIF accounts for the greater protection.
Acknowledgments
This work was supported by National Institutes of Health National Center for Research Resources (P20 RR15636 and P20 RR15636-09S1).
Abbreviations
- NBO
normobaric hyperoxia
- AIF
apoptosis-inducing factor
- BBB
blood brain barrier
- DMSO
dimethy sulfoxide
- ECA
external carotid artery
- FDA
Food and Drug Administration
- ICA
internal carotid artery
- MCAO
middle cerebral artery occlusion
- MMP
matrix metalloproteinase
- NBO
normobaric hyperoxia
- TTC
2,3,5 triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- Akpan N, Serrano-Saiz E, Zacharia BE, Otten ML, Ducruet AF, Snipas SJ, Liu W, Velloza J, Cohen G, Sosunov SA, Frey WH, 2nd, Salvesen GS, Connolly ES, Jr, Troy CM. Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J Neurosci. 2011;31:8894–8904. doi: 10.1523/JNEUROSCI.0698-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- Asahi M, Hoshimaru M, Uemura Y, Tokime T, Kojima M, Ohtsuka T, Matsuura N, Aoki T, Shibahara K, Kikuchi H. Expression of interleukin-1 beta converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17:11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Chandra D, Tang DG. Mitochondrially localized active caspase-9 and caspase-3 result mostly from translocation from the cytosol and partly from caspase-mediated activation in the organelle. Lack of evidence for Apaf-1-mediated procaspase-9 activation in the mitochondria. J Biol Chem. 2003;278:17408–17420. doi: 10.1074/jbc.M300750200. [DOI] [PubMed] [Google Scholar]
- Chiu EH, Liu CS, Tan TY, Chang KC. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Arch Neurol. 2006;63:741–744. doi: 10.1001/archneur.63.5.741. [DOI] [PubMed] [Google Scholar]
- Cho BB, Toledo-Pereyra LH. Caspase-independent programmed cell death following ischemic stroke. J Invest Surg. 2008;21:141–147. doi: 10.1080/08941930802029945. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- ElAli A, Doeppner TR, Zechariah A, Hermann DM. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke. 2011;42:3238–3244. doi: 10.1161/STROKEAHA.111.615559. [DOI] [PubMed] [Google Scholar]
- Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman BT, Moskowitz MA. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Cronic LE, Hess DC. Minocycline Development for Acute Ischemic Stroke. Transl Stroke Res. 2011;2:202–208. doi: 10.1007/s12975-011-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Gao Y, Liang W, Hu X, Zhang W, Stetler RA, Vosler P, Cao G, Chen J. Neuroprotection against hypoxic-ischemic brain injury by inhibiting the apoptotic protease activating factor-1 pathway. Stroke. 2010;41:166–172. doi: 10.1161/STROKEAHA.109.561852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- Hakim AM. Ischemic penumbra: the therapeutic window. Neurology. 1998;51:S44–46. doi: 10.1212/wnl.51.3_suppl_3.s44. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Iwasaki K, Fujiwara M. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke. 2008;39:951–958. doi: 10.1161/STROKEAHA.107.495820. [DOI] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- Henninger N, Fisher M. Normobaric hyperoxia - a promising approach to expand the time window for acute stroke treatment. Cerebrovasc Dis. 2006;21:134–136. doi: 10.1159/000090446. [DOI] [PubMed] [Google Scholar]
- Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience. 2006;141:27–33. doi: 10.1016/j.neuroscience.2006.03.071. [DOI] [PubMed] [Google Scholar]
- Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA. Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A. 2002;99:15188–15193. doi: 10.1073/pnas.232473399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Lo EH. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2004;24:720–727. doi: 10.1097/01.WCB.0000122747.72175.47. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Liu W, Chen Q, Liu J, Liu KJ. Normobaric hyperoxia protects the blood brain barrier through inhibiting Nox2 containing NADPH oxidase in ischemic stroke. Med Gas Res. 2011;1:22. doi: 10.1186/2045-9912-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009a;40:2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem. 2009b;108:811–820. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, Miyake M, Liu KJ. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. Journal of Neurochemistry. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- Nagel S, Su Y, Horstmann S, Heiland S, Gardner H, Koziol J, Martinez-Torres FJ, Wagner S. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008;1188:198–206. doi: 10.1016/j.brainres.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Howells DW. Evaluation of combination therapy in animal models of cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:585–597. doi: 10.1038/jcbfm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49:93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A, Sims J, Botez G, Winckler J. Spatial resolution of phospholipid scramblase 1 (PLSCR1), caspase-3 activation and DNA-fragmentation in the human hippocampus after cerebral ischemia. Neurochem Int. 2003;43:79–87. doi: 10.1016/s0197-0186(02)00194-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011;42:S7–11. doi: 10.1161/STROKEAHA.110.596684. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Mobashery S. Effect of synthetic matrix metalloproteinase inhibitors on lipopolysaccharide-induced blood-brain barrier opening in rodents: Differences in response based on strains and solvents. Brain Res. 2007;1133:186–192. doi: 10.1016/j.brainres.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–371. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, Narasimhan P, Maier CM, Chan PH. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRX. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Strelow H, Mies G, Veltkamp R. Oxygen therapy improves energy metabolism in focal cerebral ischemia. Brain Res. 2011;1415:103–108. doi: 10.1016/j.brainres.2011.07.064. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou W, Mueller C, Sommer C, Heiland S, Bauer AT, Marti HH, Veltkamp R. Oxygen therapy reduces secondary hemorrhage after thrombolysis in thromboembolic cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1651–1660. doi: 10.1038/jcbfm.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Liu KJ, Ramu J, Cheng Q, Li T, Liu W. Inhibition of gp91phox contributes towards normobaric hyperoxia afforded neuroprotection in focal cerebral ischemia. Brain Research. 2010;1348:174–180. doi: 10.1016/j.brainres.2010.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal SE, Zhu C, Thal SC, Blomgren K, Plesnila N. Role of apoptosis inducing factor (AIF) for hippocampal neuronal cell death following global cerebral ischemia in mice. Neurosci Lett. 2011;499:1–3. doi: 10.1016/j.neulet.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- Van Hoecke M, Prigent-Tessier A, Bertrand N, Prevotat L, Marie C, Beley A. Apoptotic cell death progression after photothrombotic focal cerebral schaemia: effects of the lipophilic iron chelator 2,2′-dipyridyl. Eur J Neurosci. 2005;22:1045–1056. doi: 10.1111/j.1460-9568.2005.04297.x. [DOI] [PubMed] [Google Scholar]
- Wang CX, Yang T, Noor R, Shuaib A. Delayed minocycline but not delayed mild hypothermia protects against embolic stroke. BMC Neurol. 2002;2:2. doi: 10.1186/1471-2377-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Levchenko T, Guo S, Stins M, Torchilin VP, Lo EH. Delivering minocycline into brain endothelial cells with liposome-based technology. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, Hill WD, Feuerstein G, Hess DC. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi: 10.1186/1471-2377-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Candelario-Jalil E, Thompson JF, Cuadrado E, Estrada EY, Rosell A, Montaner J, Rosenberg GA. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem. 2010;112:134–149. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol. 2011;762:333–345. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang C, Zhang L, Roberts C, Lu M, Kapke A, Cui Y, Ninomiya M, Nagafuji T, Albala B, Zhang ZG, Chopp M. Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke. Stroke. 2008;39:2830–2836. doi: 10.1161/STROKEAHA.108.515684. [DOI] [PMC free article] [PubMed] [Google Scholar]