A remarkable decline in nontyphoidal salmonellosis in the United Kingdom since the late 1990s coincides closely with the introduction of voluntary vaccination programs in broiler-breeder and laying flocks, demonstrating the success of this concerted, industry-led public health action.
Keywords: Salmonella, eggs, vaccination, food safety, public health
Abstract
Remarkable changes in the epidemiology of human nontyphoidal salmonellosis have occurred in the United Kingdom over the last century. Between 1981 and 1991, the incidence of nontyphoidal salmonellosis in the United Kingdom rose by >170%, driven primarily by an epidemic of Salmonella enterica subspecies enterica serovar Enteritidis phage type (PT) 4, which peaked in 1993. Measures introduced to control this epidemic included legislation, food safety advice, and an industry-led vaccination program in broiler-breeder and laying poultry flocks. The incidence of Salmonella Enteritidis has been falling since 1997, and levels of Salmonella Enteritidis PT4 have fallen to preepidemic levels and have stayed low. The temporal relationship between vaccination programs and the reduction in human disease is compelling and suggests that these programs have made a major contribution to improving public health.
Nontyphoidal Salmonella species are important foodborne pathogens worldwide [1], causing diarrhea, vomiting, nausea, fever, and abdominal pain. Illness has been linked to a wide range of food items including eggs, chicken, beef, pork, salad vegetables, and dairy products, and other risk factors including overseas travel [2–7]. Outbreaks are fairly common [5]. The burden of illness, defined as morbidity and mortality, is substantial. In the United States, nontyphoidal Salmonella species are estimated to cause 1 million foodborne illnesses [8] and are the leading cause of death among foodborne bacterial pathogens [9]. Across the 27 member states of the European Union (EU), there were estimated to be 6.2 million cases of salmonellosis in 2009 [10]. In a population-based study in the United Kingdom (UK) in 2008–2009, there were >38 600 estimated cases and nearly 11 300 patients presenting to a primary care physician [11]. This represented a marked reduction in incidence compared with a similar study conducted more than a decade earlier [12, 13]. The purpose of this article is to discuss the factors associated with a substantial decline in nontyphoidal salmonellosis in the United Kingdom since the mid-1990s.
A BRIEF HISTORY OF NONTYPHOIDAL SALMONELLOSIS IN THE UNITED KINGDOM
Remarkable changes in the epidemiology of human nontyphoidal salmonellosis have occurred in the United Kingdom over the last century. Prior to 1942, the dominant foodborne salmonellas causing disease were Salmonella enterica subspecies enterica serovar Typhimurium, Salmonella Enteritidis, Salmonella Thompson, Salmonella Newport, Salmonella Bovismorbificans, and Salmonella Choleraesuis [14]. Salmonella Typhimurium remained the dominant serovar causing human disease for much of the 20th century, although there were fluctuations in other salmonellas in the “top 10” over time. For example, Salmonella Agona emerged as an important serovar in the 1960s following its introduction into pigs and poultry through contaminated fish meal imported from Peru [15]. Salmonella Hadar became the second most commonly isolated cause of human nontyphoidal salmonellosis in the mid-1970s when particular genetic lines of turkeys became infected [15]. Against this background, the incidence of Salmonella Enteritidis increased fairly gradually from around 150 to approximately 900 laboratory-confirmed cases per year between 1961 and 1980 [16]. During this time, phage type (PT) 8 dominated and was responsible for several turkey-associated outbreaks in the late 1960s [16]. By 1975 Salmonella Enteritidis was consistently the second or third most frequently isolated serovar annually [17].
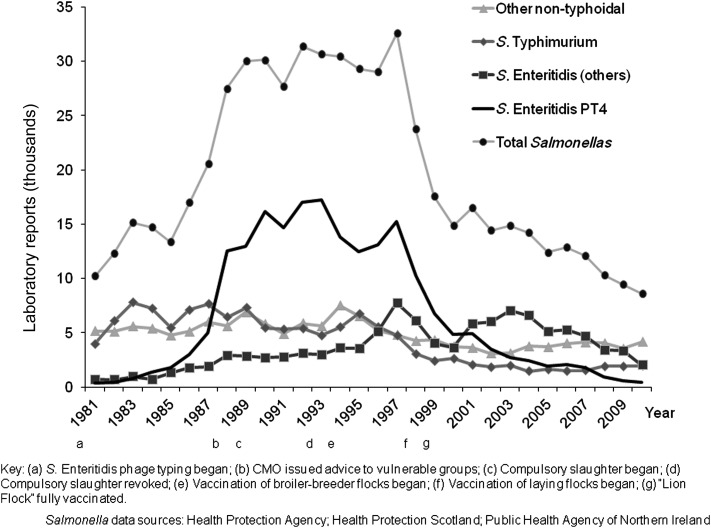
Between 1981 and 1991, the incidence of nontyphoidal salmonellosis in the United Kingdom rose by >170% [18], driven primarily by an epidemic of Salmonella Enteritidis PT4 [16, 18–20] (Figure 1). In 1981 Salmonella Enteritidis accounted for approximately 10% of human Salmonella illnesses, but by 1993 this proportion had risen to nearly 70% [20]. In the early 1980s, PT4 overtook PT8 to become the predominant phage type in 1983, comprising 46% of isolations that year. By 1988 PT4 had risen to account for 81% of Salmonella Enteritidis strains isolated [16] and had ended the political career of a prominent government minister [21]. The United Kingdom was not alone; analysis of data submitted to the World Health Organization's Salmonella surveillance system showed that Salmonella Enteritidis in the late 1980s was increasing on several continents, with North America, South America, and Europe appearing to bear the brunt [22].
Figure 1.
Laboratory reports of human Salmonella cases in the United Kingdom, 1981–2010. Abbreviations: CMO, Chief Medical Officer; PT, phage type.
EVIDENCE THAT THE DECLINE IN SALMONELLA IS REAL
Compelling evidence that the decline in Salmonella is real is derived from 3 sources. The first comprises 2 population-based prospective cohort studies of infectious intestinal disease (IID) in the community conducted more than a decade apart [11–13]. The primary outcome measures in both studies were estimates of the incidence of IID in the community, presenting to primary healthcare and reported to national surveillance. They were conducted using identical study designs and case definitions and employed similar microbiological methods, the exception being that molecular microbiological techniques were used alongside traditional microbiology in the second study of infectious intestinal disease (IID2). In the first study of infectious intestinal disease (IID1) in 1993–1996, the incidence of nontyphoidal Salmonella in the community in England was 2.2 cases per 1000 person-years (95% confidence interval [CI], 1.1–4.3) but by 2008–2009 this had fallen to 0.7 cases per 1000 person-years (95% CI, .2–3.0). For nontyphoidal Salmonella cases presenting to primary care in England, the incidence rate had fallen from 1.6 cases per 1000 person-years (95% CI, 1.2–2.1) in IID1 to 0.2 cases per 1000 person-years (95% CI, .1–.5) in IID2. The decline in incidence in the community was not statistically significant because in IID2 the study power was insufficient to detect statistically significant changes in organism-specific incidence—to do this would have required >100 000 person-years of follow-up, based on incidence rates in IID1. Nevertheless, the reduction in presentations to primary healthcare was statistically significant.
Second, there has been a substantial fall in laboratory-confirmed Salmonella cases reported to national surveillance (Figure 1). Phage typing of Salmonella Enteritidis was implemented from 1981 as an addition to the centralized, national service already in existence for confirmation and further typing [17], and all clinical diagnostic laboratories have continued to refer all Salmonella isolates to the national reference laboratories since that date. At the beginning of 1992, 2 separate national Salmonella databases were merged to form a single national dataset, which became patient-based rather than isolate-based, thus eliminating potential duplication if people were tested more than once [18]. Laboratory testing methods have remained constant since then and reporting algorithms have not changed [23], suggesting that the reduction in Salmonella is real. When Salmonella Enteritidis PT4 peaked in 1993 in the United Kingdom, >18 000 laboratory-confirmed cases of illness were recorded in national surveillance statistics, yet by 2010 PT4 isolations had fallen to just 459 [24]. Thus, the decline in nontyphoidal salmonellosis witnessed in the United Kingdom in recent years reflects this major contraction in reports of Salmonella Enteritidis PT4.
Finally, outbreaks of salmonellosis have declined. Standardized reporting of outbreaks of gastrointestinal infection was introduced in 1992 in England and Wales and in 1996 in Scotland partly in response to the increase in nontyphoidal salmonellosis. A foodborne outbreak is defined in European legislation as “an incidence, observed under given circumstances, of two of more human cases of the same disease and/or infection, or a situation in which the observed number of human cases exceeds the expected number and where the cases are linked, or are probably linked, to the same source” [25]. Between 1992 and 2008, foodborne Salmonella outbreaks reported to national surveillance fell from nearly 150 per year to just over 20 annually, and the pattern of decline closely mirrors that of laboratory-confirmed cases [25].
EPIDEMIOLOGY OF SALMONELLA ENTERITIDIS IN THE UNITED KINGDOM
Epidemiologic investigations of outbreaks and sporadic cases repeatedly showed that Salmonella Enteritidis PT4 infection in humans was frequently associated with consumption of poultry meat and hens' eggs on both sides of the Atlantic [25–31]. In nearly 2500 foodborne disease outbreaks reported to the UK Health Protection Agency between 1992 and 2008, Salmonella species accounted for 47% of all outbreaks, 46% of cases, 70% of hospital admissions, and 76% of deaths [25]. Salmonella Enteritidis PT4 was the causative organism in 51% of all the Salmonella outbreaks throughout the surveillance period but the percentage of outbreaks caused by Salmonella Enteritidis PT4 declined from the late 1990s onward. At least one food vehicle was identified in 75% of outbreaks reported, and poultry meat was the vehicle most often implicated (19% of outbreaks). Desserts were also implicated commonly (11% of outbreaks), and raw shell eggs were used as an ingredient in 70% of these desserts. Eggs were implicated separately in an additional 6% of outbreaks. Analysis of outbreak data also showed that nearly 50% of foodborne Salmonella outbreaks occurred in the food service/catering sector.
Salmonella Gallinarum and Salmonella Pullorum had been the dominant Salmonella serovars in UK poultry until the early 1970s. These strains both caused clinical disease in the birds and were virtually eradicated by a combination of slaughtering of seropositive hens and vaccination [20]. However, the ecological niche left by these 2 serovars was filled by Salmonella Enteritidis. Complete genome sequencing of a host-promiscuous Salmonella Enteritidis PT4 isolate (P125109) and a chicken-restricted Salmonella Gallinarum isolate (287/91) has indicated that Salmonella Gallinarum 287/91 is a recently evolved descendent of Salmonella Enteritidis [32]. Importantly, Salmonella Enteritidis infects poultry without causing overt disease, which probably facilitated its rapid spread internationally [20]. Another key feature of Salmonella Enteritidis is colonization of the reproductive tissues leading to the production of eggs with Salmonella-positive contents [20, 33] and, in some eggs, the numbers of organisms can be very high [34].
CONTROLLING SALMONELLOSIS AND OTHER FOODBORNE ILLNESSES
In August 1988, as evidence of a link between Salmonella Enteritidis PT4 and raw shell eggs strengthened, the Chief Medical Officer issued advice to consumers to avoid eating raw eggs or uncooked foods in which raw eggs were an ingredient. In December of the same year, he issued further advice to vulnerable people such as the elderly, individuals with chronic illness, infants, and pregnant women. They were counseled only to eat eggs that had been cooked until the yolks and whites were solid [18]. Caterers were encouraged to use pasteurized eggs, especially where foodstuffs were not going to be cooked further (eg, mayonnaise), and it was recommended that eggs be considered short shelf-life products. They should be refrigerated <8°C throughout the production chain and during retail, catering, and domestic storage, and consumed within 3 weeks of the date of lay [18]. In 1989 the government introduced a raft of legislation, including the Zoonoses Order, which required that all Salmonella isolates from live animals or birds, carcasses, or feedstuffs be reported. Movement restrictions were implemented along with compulsory slaughter, compensation, and disinfection procedures. The more draconian procedures were usually reserved for Salmonella Typhimurium and Salmonella Enteritidis [24]. The requirement for compulsory slaughter of poultry flocks was revoked following a recommendation from the Advisory Committee on the Microbiological Safety of Food in 1993 to review the policy in light of the fact that Salmonella Enteritidis in flocks had reduced substantially [18]. In 1989, >600 000 birds from 58 infected flocks were slaughtered. In 1992, <300 000 birds from 38 infected flocks were slaughtered [18]. Alongside legislation was a voluntary, industry-led vaccination scheme that began in broiler-breeder flocks in 1994 and in laying flocks in 1998 [16]. A “Lion Mark,” stamped on eggs, which had been introduced in 1957 but dropped by 1971, was revived in 1998 (http://www.lioneggs.co.uk/page/lionmark). The Lion Mark can only be used by subscribers to the British Egg Industry Council for eggs that have been produced in accordance with UK and EU law and the Lion Quality Code of Practice. The code of practice requires mandatory vaccination of all pullets destined to lay Lion eggs against Salmonella; independent auditing; full traceability of hens, eggs, and feed; and a “best-before” date stamped on the shell and pack, in addition to on-farm stamping of eggs and packing station hygiene controls.
When, in 1989, a Junior Health Minister stated in a British television interview that “Most of the egg production in this country, sadly, is now infected with Salmonella,” the sale of eggs collapsed by 60% almost overnight. Moreover, despite government efforts to improve the safety of eggs, sales continued to fall by around 8% per year over the next 10 years, which was a disaster for the industry. The British Egg Industry Service began a major consumer research program in 1997 and, in 1998, the majority of UK producers and packers made a voluntary investment of £8 million to assist the British Egg Industry Council to relaunch British eggs. A total of £4 million was spent on the stringent new Code of Practice described above, and £4 million supported a new promotional campaign to restore consumer confidence and increase consumption. The cost of the vaccination program (including Lion sampling and testing) is estimated to be around £52 million to date (Mark Williams, written personal communication, September 2012). However, between 1998 and 2009, the egg market grew from 9.8 billion to 11 billion eggs per year, and Lion eggs now account for around 85% of the total market. Within the retail sector the market share of Lion eggs share rose from approximately 60% in 1998 to 95% in 2010 (http://www.lioneggs.co.uk/files/lioneggs.co.uk/pdfs/marketing.pdf).
Alas, Salmonella was not the only “food scare” during the 1980s and 1990s. Scandals surrounding, for example, bovine spongiform encephalopathy in the United Kingdom, dioxins in Belgium, and Salmonella EU-wide prompted new legislation providing for a risk-based “farm to fork” approach to food safety policy, which was enacted in 2002 (European General Food Law [Regulation (EC) No. 178/2002]) [24]. EU Zoonoses Regulation (EC) No. 2160/2003 required member states to take effective measures to detect and control Salmonella species of public health significance in specified animal species at all relevant stages of production [24]. Each EU member state was obliged to undertake a standardized baseline survey to determine the prevalence of Salmonella within their industry sectors. EC Regulation (EC) 1168/2006 laid down an annual reduction target for Salmonella Enteritidis and Salmonella Typhimurium for each member state.
NATIONAL CONTROL PROGRAMS FOR SALMONELLA IN THE POULTRY SECTOR
Four National Control Programmes (NCPs) for Salmonella have been implemented in the UK poultry sector between 2007 and 2010. These postdate the rapid decline in Salmonella Enteritidis in the United Kingdom but are designed to achieve and maintain low rates EU-wide. For the most part, the targets set by the EU have already been met or exceeded in the United Kingdom [24].
The NCP for breeding chickens (implemented in 2007): The target for this NCP was that no more than 1% of adult breeding flocks should be infected with 5 specific regulated serovars (Salmonella Enteritidis, Salmonella Typhimurium, Salmonella Hadar, Salmonella Infantis, and Salmonella Virchow) by the end of 2009. Results from UK holdings have been significantly below the EU target of 1% every year for the last 4 years [24].
The NCP for commercial laying chickens (implemented in 2008): An EU-wide baseline survey of commercial laying chicken flock holdings was undertaken in 2004–2005. In a survey of Salmonella species on 454 commercial layer flock holdings in the United Kingdom, 54 (11.7%) were Salmonella positive [35]. Salmonella Enteritidis was the serovar most commonly identified (prevalence = 5.8%) and PTs 4, 6, 7, and 35 comprised 70% of isolates. Salmonella Typhimurium was the second most commonly identified serovar (prevalence = 1.8%). The UK prevalence figures were among the lowest of the major egg-producing countries (7.9% of holdings positive compared with a 20.4% average across the EU) [36]. Across the EU, the incidence rate of salmonellosis in member states varies between 16 and 11 800 per 100 000 population and has been shown to be significantly correlated with the prevalence of Salmonella Enteritidis in laying hens [10], so controlling levels of Salmonella Enteritidis in laying flocks is important for improving public health.
The NCP for broilers (implemented in 2009): The target for this NCP was that no more than 1% of flocks should be infected with Salmonella Enteritidis and Salmonella Typhimurium by the end of 2011. In a baseline survey of broiler chickens in 2005–2006 in the United Kingdom, the prevalence of Salmonella Enteritidis and Salmonella Typhimurium was very low (0.2% [37] compared with an EU average of 11.0% [38]) and remains well below the EU target [24].
The NCP for turkeys (implemented in 2010): A baseline survey for Salmonella in turkey breeding and fattening flocks was carried out across the EU in 2006–2007. In the United Kingdom, the prevalence of Salmonella in breeding flock holdings was 20.1% and in fattening flocks the holdings prevalence was 37.7% [39]. The flock prevalence of Salmonella Typhimurium was very low on breeding holdings at 0.7% (EU weighted average = 1.8%) but higher on fattening holdings at 4.6% (EU weighted average = 3.7%) [24]. The target for Salmonella reduction is that only 1% of breeding flocks and 1% of fattening flocks should be positive by the end of 2012. Early indications are that this target will be met.
WHAT NEXT?
There is no room for complacency. During the 2000s, new Salmonella problems emerged. Notable among these were national outbreaks of Salmonella Enteritidis PT14b linked to raw shell eggs originating in Spain [40, 41]. Unbelievably, perhaps, hospital caterers in the United Kingdom were found serving raw shell eggs again to patients, with consequent outbreaks [42]. The first outbreak of Salmonella Typhimurium PT8 linked to consumption of duck eggs since 1949 occurred in the United Kingdom [43], and Salmonella outbreaks linked to fresh produce were increasingly recognized [44, 45], reflecting a pattern also seen in the United States [46].
CONCLUSIONS
The nature of public health interventions often means that evaluating their impact is complex as they are often implemented in combination and/or simultaneously. It is interesting to reflect on the fact that the various legislative measures in the United Kingdom in the late 1980s and early 1990s appear to have slowed down the increase in Salmonella Enteritidis PT4, whereas the decrease in laboratory-confirmed human cases coincides quite closely with the introduction of vaccination programs in broiler-breeder and laying flocks and prior to much of the EU legislation being implemented. It is probable that no single measure contributed to the decline in Salmonella Enteritidis PT4 and that the combination of measures was successful, but the temporal relationship between vaccination programs and the reduction in human disease is compelling and suggests that these programs have made a major contribution to improving public health.
There has also been a reduction in reported human salmonellosis cases across the EU (on average 12% per year between 2005 and 2009). The European Commission and European Food Safety Authority are attributing this, at least in part, to successful control of Salmonella in broiler, laying, and breeding hen flocks and eggs [24].
If success in public health is defined by illnesses averted, then the story of Salmonella Enteritidis PT4 in the United Kingdom, which has come down and stayed down, is good news. However, history teaches us that something else may come along to take its place. Robust surveillance, incorporating state-of-the-art microbiological, epidemiological, and biostatistical methods, and maintaining a prompt and comprehensive response to outbreaks is just as important now as it ever was.
Notes
Acknowledgments. I thank Mark Williams of the British Egg Industry Council for information on the cost of the vaccination programs.
Potential conflicts of interest. Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Majowicz SE, Musto J, Scallan E, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–9. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Adak GK, Meakins SM, Yip H, Lopman BA, O'Brien SJ. Disease risks from foods, England and Wales, 1996–2000. Emerg Infect Dis. 2005;11:365–72. doi: 10.3201/eid1103.040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domingues AR, Pires SM, Halasa T, Hald T. Source attribution of human salmonellosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012;140:959–69. doi: 10.1017/S0950268811002172. [DOI] [PubMed] [Google Scholar]
- 4.Wahlström H, Andersson Y, Plym-Forshell L, Pires SM. Source attribution of human Salmonella cases in Sweden. Epidemiol Infect. 2011;139:1246–53. doi: 10.1017/S0950268810002293. [DOI] [PubMed] [Google Scholar]
- 5.Pires SM, Vigre H, Makela P, Hald T. Using outbreak data for source attribution of human salmonellosis and campylobacteriosis in Europe. Foodborne Pathog Dis. 2010;7:1351–61. doi: 10.1089/fpd.2010.0564. [DOI] [PubMed] [Google Scholar]
- 6.Ravel A, Nesbitt A, Marshall B, Sittler N, Pollari F. Description and burden of travel-related cases caused by enteropathogens reported in a Canadian community. J Travel Med. 2011;18:8–19. doi: 10.1111/j.1708-8305.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 7.Guo C, Hoekstra RM, Schroeder CM, et al. Application of Bayesian techniques to model the burden of human salmonellosis attributable to U.S. food commodities at the point of processing: adaptation of a Danish model. Foodborne Pathog Dis. 2011;8:509–16. doi: 10.1089/fpd.2010.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton Behravesh C, Jones TF, Vugia DJ, et al. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996–2005. J Infect Dis. 2011;204:263–7. doi: 10.1093/infdis/jir263. [DOI] [PubMed] [Google Scholar]
- 10.Havelaar AH, Ivarsson S, Löfdahl M, Nauta MJ. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol Infect. 2012:1–10. doi: 10.1017/S0950268812000568. [Epub ahead of print] Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam CC, Rodrigues LC, Viviani L, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler JG, Sethi D, Cowden JM, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ. 1999;318:1046–50. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CC, O'Brien SJ, Tompkins DS, et al. Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis. 2012;54:1275–86. doi: 10.1093/cid/cis028. [DOI] [PubMed] [Google Scholar]
- 14.Barrow PA. Salmonella control—past, present and future. Avian Pathol. 1993;22:651–69. doi: 10.1080/03079459308418954. [DOI] [PubMed] [Google Scholar]
- 15.Rowe B, Hall ML, Ward LR, de Sa JD. Epidemic spread of Salmonella Hadar in England and Wales. BMJ. 1980;280:1065–6. doi: 10.1136/bmj.280.6221.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward LR, Threlfall J, Smith HR, O'Brien SJ. Salmonella Enteritidis epidemic. Science. 2000;287:1753–4. doi: 10.1126/science.287.5459.1753d. [DOI] [PubMed] [Google Scholar]
- 17.Ward LR, de Sa JD, Rowe B. A phage-typing scheme for Salmonella Enteritidis. Epidemiol Infect. 1987;99:291–4. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Advisory Committee on the Microbiological Safety of Food (ACMSF) London: HMSO; Report on Salmonella in eggs. 1993. Available at: http://www.food.gov.uk/multimedia/pdfs/acmsfsalmonellaineggs.pdf. Accessed 4 July 2012. [Google Scholar]
- 19.Baird-Parker AC. Foodborne salmonellosis. Lancet. 1990;336:1231–5. doi: 10.1016/0140-6736(90)92844-8. [DOI] [PubMed] [Google Scholar]
- 20.Cogan TA, Humphrey TJ. The rise and fall of Salmonella Enteritidis in the UK. J Appl Microbiol. 2003;94(Suppl):114S–9S. doi: 10.1046/j.1365-2672.94.s1.13.x. [DOI] [PubMed] [Google Scholar]
- 21.Hardy A. Salmonella: a continuing problem. Postgrad Med J. 2004;80:541–5. doi: 10.1136/pgmj.2003.016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigue DC, Tauxe RV, Rowe B. International increase in Salmonella Enteritidis: a new pandemic? Epidemiol Infect. 1990;105:21–7. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Health Protection Agency. Identification of Salmonella species. UK Standards for Microbiology Investigations; ID 24 Issue 2.2. 2011. Available at: http://www.hpa.org.uk/SMI/pdf. Accessed 24 September 2012. [Google Scholar]
- 24.Department for Environment, Food and Rural Affairs (Defra) Zoonoses Report UK 2010. Available at: http://www.defra.gov.uk/publications/files/pb13627-zoonoses-report2010.pdf. Accessed 4 July 2012. [Google Scholar]
- 25.Gormley FJ, Little CL, Rawal N, Gillespie IA, Lebaigue S, Adak GK. A 17-year review of foodborne outbreaks: describing the continuing decline in England and Wales (1992–2008) Epidemiol Infect. 2011;139:688–99. doi: 10.1017/S0950268810001858. [DOI] [PubMed] [Google Scholar]
- 26.Coyle EF, Palmer SR, Ribeiro CD, et al. Salmonella Enteritidis phage type 4 infection: association with hen's eggs. Lancet. 1988;2:1295–7. doi: 10.1016/s0140-6736(88)92902-9. [DOI] [PubMed] [Google Scholar]
- 27.Cowden JM, Lynch D, Joseph CA, et al. Case-control study of infections with Salmonella Enteritidis phage type 4 in England. BMJ. 1989;299:771–3. doi: 10.1136/bmj.299.6702.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowden JM, Chisholm D, O'Mahony M, et al. Two outbreaks of Salmonella Enteritidis phage type 4 infection associated with the consumption of fresh shell-egg products. Epidemiol Infect. 1989;103:47–52. doi: 10.1017/s095026880003034x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillespie IA, O'Brien SJ, Adak GK, Ward LR, Smith HR. Foodborne general outbreaks of Salmonella Enteritidis phage type 4 infection, England and Wales, 1992–2002: where are the risks? Epidemiol Infect. 2005;133:795–801. doi: 10.1017/S0950268805004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessel AS, Gillespie IA, O'Brien SJ, Adak GK, Humphrey TJ, Ward LR. General outbreaks of infectious intestinal disease linked with poultry, England and Wales, 1992–1999. Commun Dis Public Health. 2001;4:171–7. [PubMed] [Google Scholar]
- 31.Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. 2006;43:512–7. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- 32.Thomson NR, Clayton DJ, Windhorst D, et al. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008;18:1624–37. doi: 10.1101/gr.077404.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gast RK, Guraya R, Guard J, Holt PS. The relationship between the numbers of Salmonella Enteritidis, Salmonella Heidelberg, or Salmonella Hadar colonizing reproductive tissues of experimentally infected laying hens and deposition inside eggs. Avian Dis. 2011;55:243–7. doi: 10.1637/9540-092810-Reg.1. [DOI] [PubMed] [Google Scholar]
- 34.Humphrey TJ, Whitehead A, Gawler AH, Henley A, Rowe B. Numbers of Salmonella Enteritidis in the contents of naturally contaminated hens’ eggs. Epidemiol Infect. 1991;106:489–96. doi: 10.1017/s0950268800067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow LC, Davies RH, Christiansen KH, et al. Survey of the prevalence of Salmonella species on commercial laying farms in the United Kingdom. Vet Rec. 2007;161:471–6. doi: 10.1136/vr.161.14.471. [DOI] [PubMed] [Google Scholar]
- 36.European Food Safety Authority. Preliminary report on analysis of the baseline study on the prevalence of Salmonella in laying hen flocks of Gallus gallus. Available at: http://www.efsa.europa.eu/en/efsajournal/pub/81r.htm. Accessed 4 July 2012.
- 37.Snow LC, Davies RH, Christiansen KH, et al. Survey of the prevalence of Salmonella on commercial broiler farms in the United Kingdom, 2005/06. Vet Rec. 2008;163:649–54. doi: 10.1136/vr.163.22.649. [DOI] [PubMed] [Google Scholar]
- 38.Report of the Task Force on Zoonoses Data Collection on the analysis of the baseline survey on the prevalence of Salmonella in broiler flocks of Gallus gallus in the EU, 2005–2006—Part A: Salmonella prevalence estimates. Available at: http://www.efsa.europa.eu/en/efsajournal/pub/98r.htm . Accessed 4 July 2012.
- 39.Snow LC, Davies RH, Christiansen KH, et al. Survey of Salmonella prevalence on commercial turkey breeding and fattening farms in the UK in 2006 to 2007. Vet Rec. 2011;169:493. doi: 10.1136/vr.d4408. [DOI] [PubMed] [Google Scholar]
- 40.Little CL, Surman-Lee S, Greenwood M, et al. Public health investigations of Salmonella Enteritidis in catering raw shell eggs, 2002–2004. Lett Appl Microbiol. 2007;44:595–601. doi: 10.1111/j.1472-765X.2007.02131.x. [DOI] [PubMed] [Google Scholar]
- 41.Janmohamed K, Zenner D, Little C, et al. National outbreak of Salmonella Enteritidis phage type 14b in England, September to December 2009: case-control study. Euro Surveill. 2011;16 pii:19840. [PubMed] [Google Scholar]
- 42.Lund BM, O'Brien SJ. Microbiological safety of food in hospitals and other healthcare settings. J Hosp Infect. 2009;73:109–20. doi: 10.1016/j.jhin.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Noble DJ, Lane C, Little CL, et al. Revival of an old problem: an increase in Salmonella enterica serovar Typhimurium definitive phage type 8 infections in 2010 in England and Northern Ireland linked to duck eggs. Epidemiol Infect. 2012;140:146–9. doi: 10.1017/S0950268811000586. [DOI] [PubMed] [Google Scholar]
- 44.Horby PW, O'Brien SJ, Adak GK, et al. A national outbreak of multi-resistant Salmonella enterica serovar Typhimurium definitive phage type (DT) 104 associated with consumption of lettuce. Epidemiol Infect. 2003;130:169–78. doi: 10.1017/s0950268802008063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gajraj R, Pooransingh S, Hawker JI, Olowokure B. Multiple outbreaks of Salmonella Braenderup associated with consumption of iceberg lettuce. Int J Environ Health Res. 2012;22:150–5. doi: 10.1080/09603123.2011.613114. [DOI] [PubMed] [Google Scholar]
- 46.Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect. 2009;137:307–15. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]