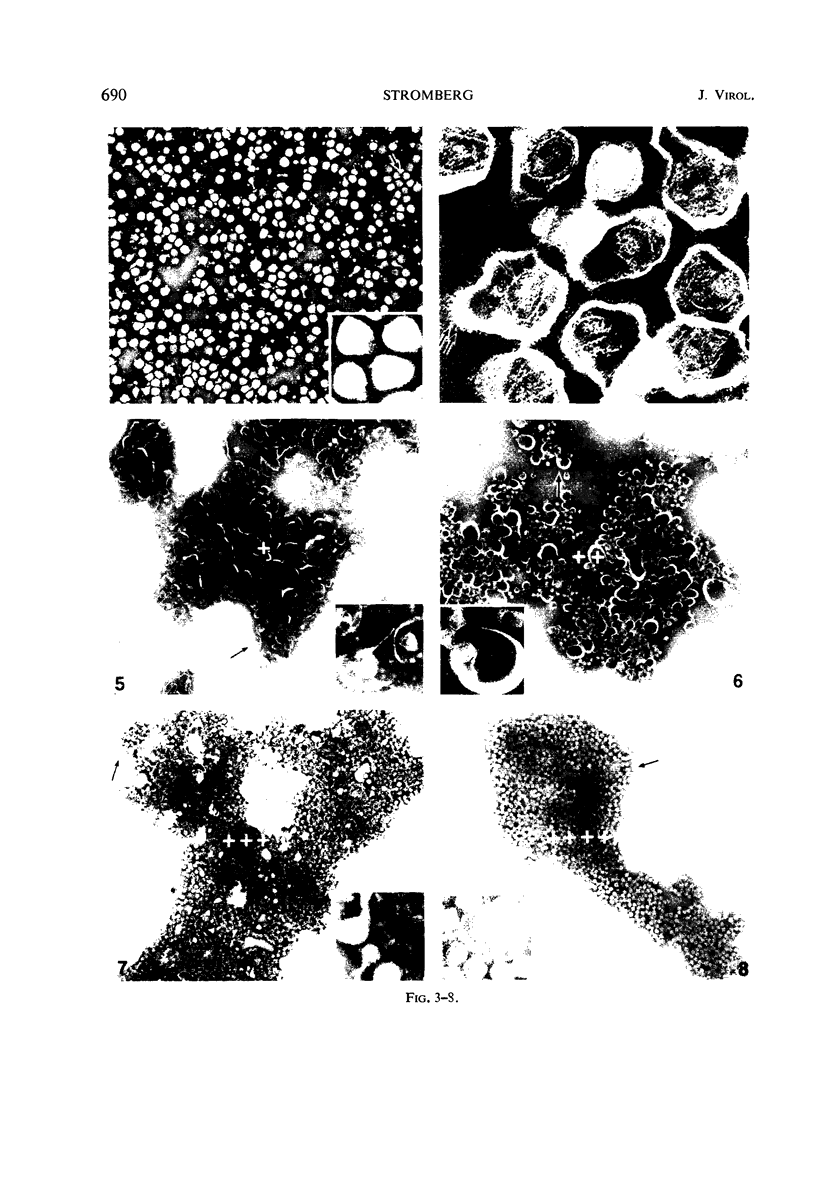
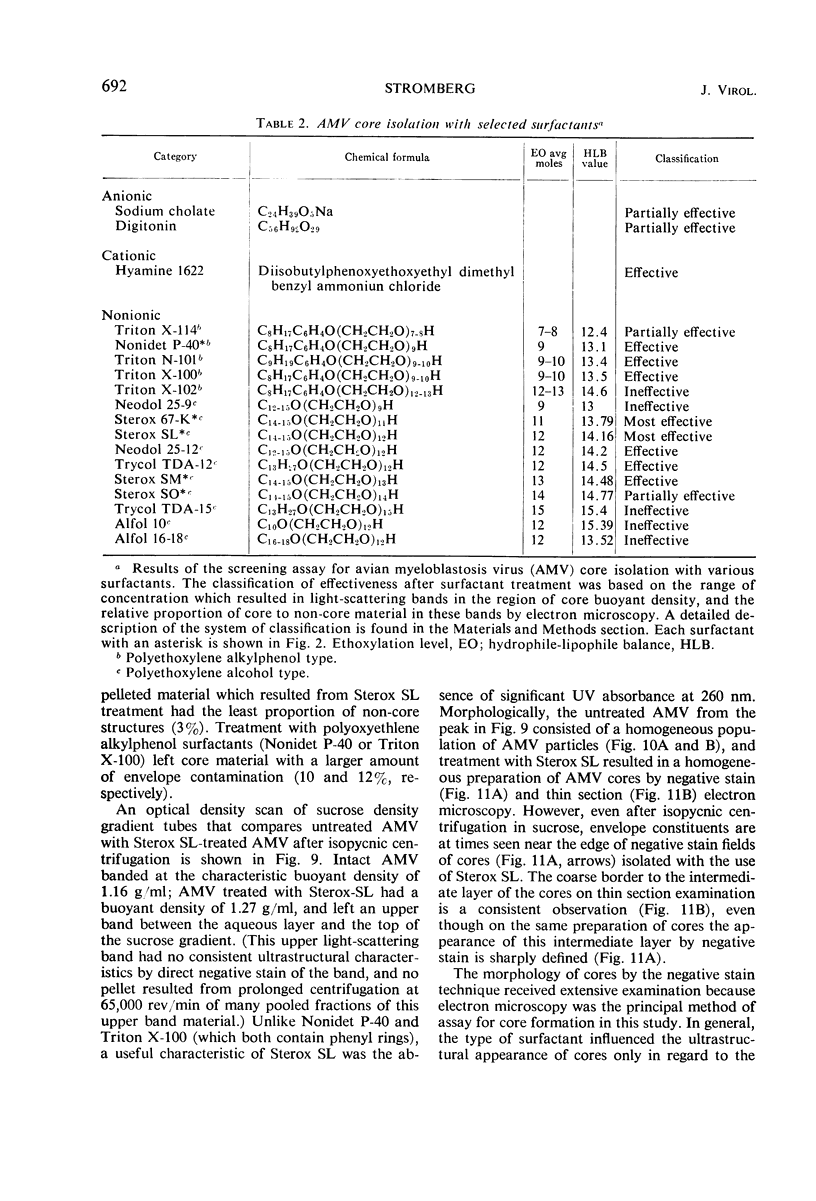
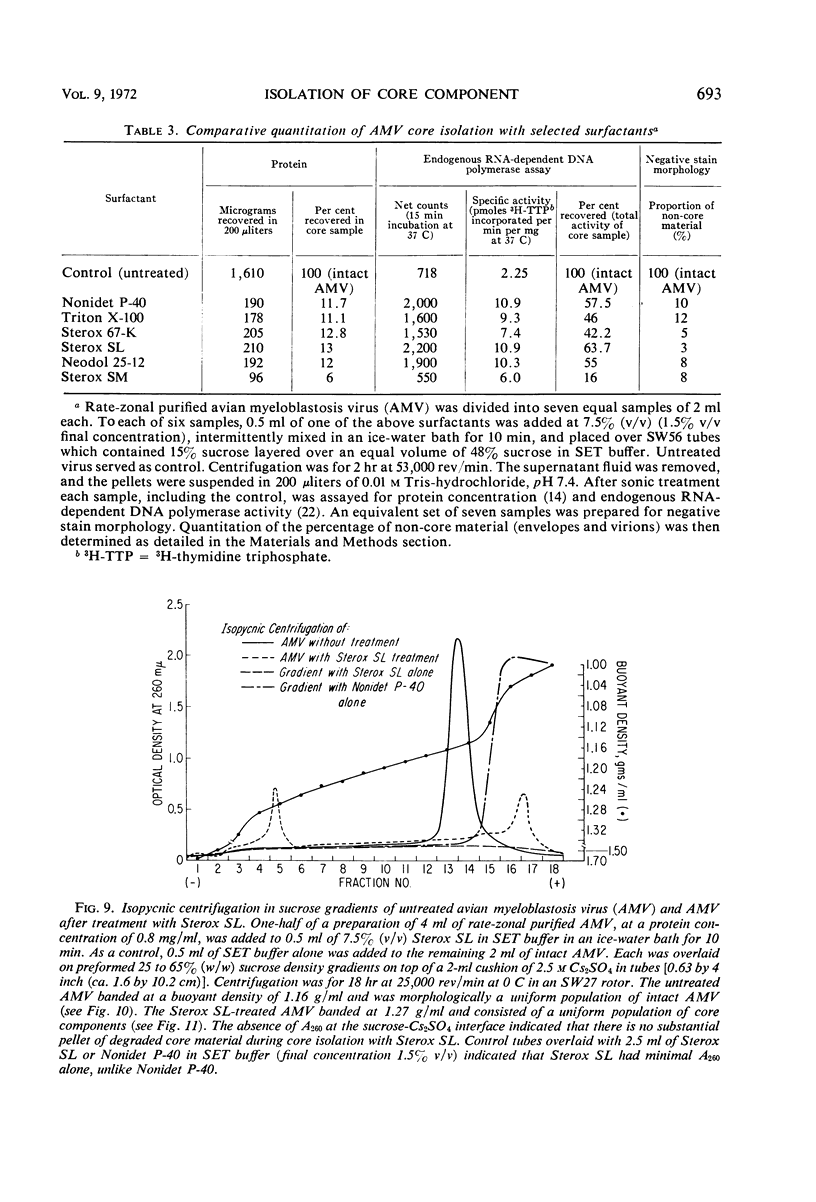
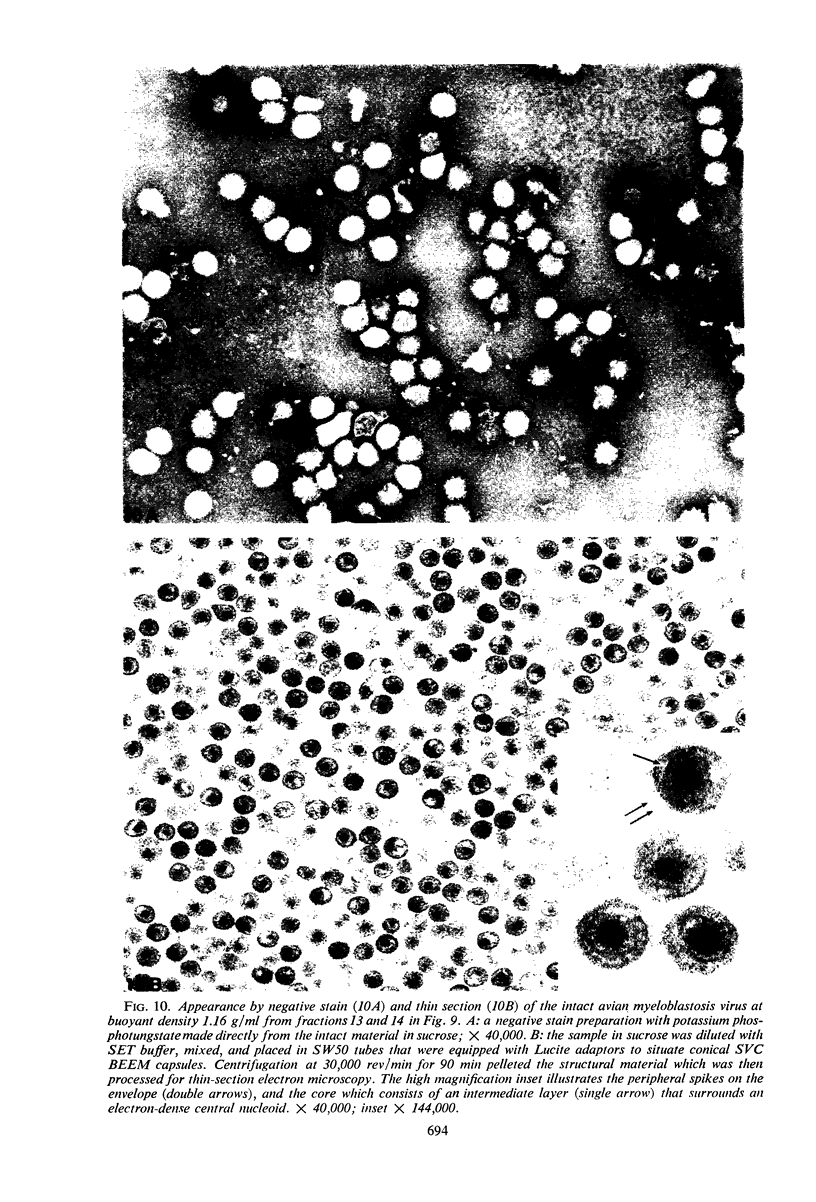
Abstract
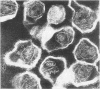
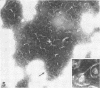
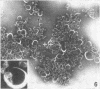
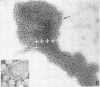
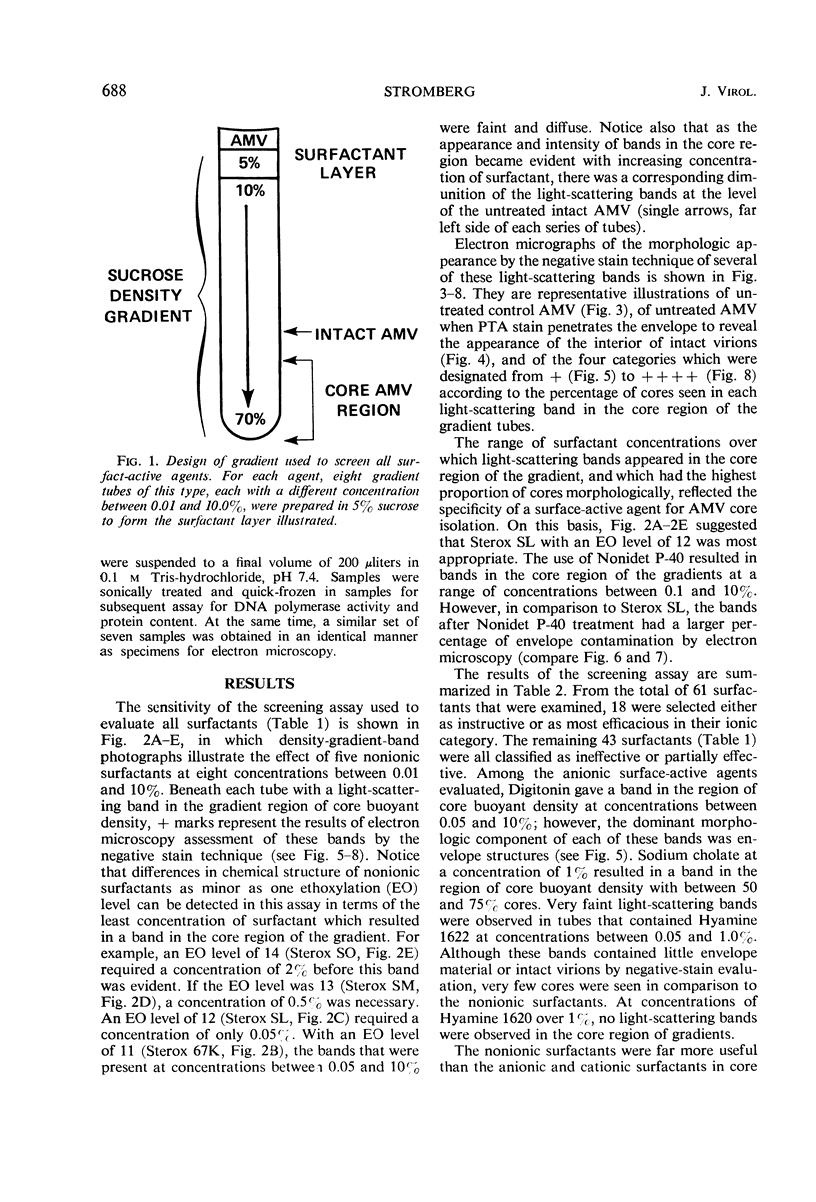
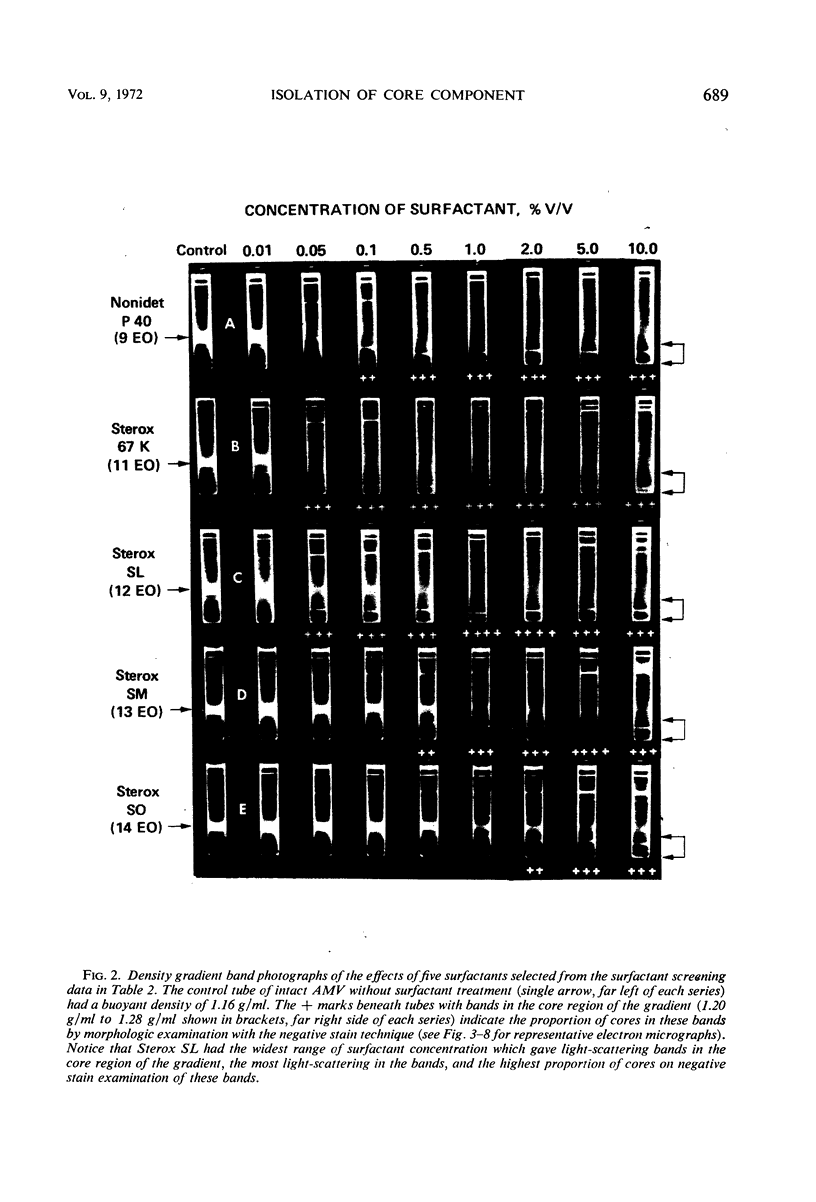
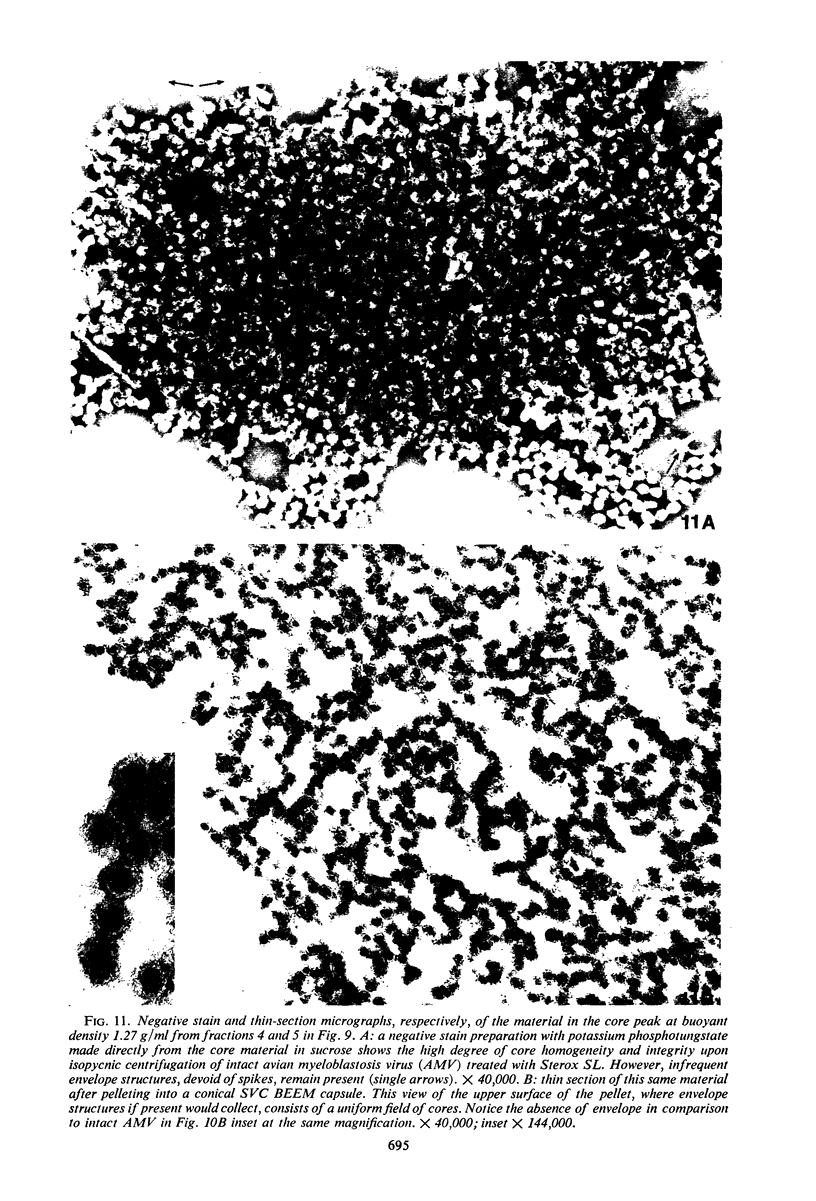
Sixty-one surface-active agents were evaluated in a procedure designed to assess their ability to remove the envelope from the core component of avian myeloblastosis virus (AMV). The procedure consisted of centrifugation of intact AMV through a series of sucrose gradients each containing an upper layer of agent at one of eight concentrations between 0.01 and 10%. The effectiveness of an agent in producing AMV cores was indicated by (i) the appearance of light-scattering bands in the region of core buoyant density in gradient tubes; (ii) the range of surfactant concentration over which these bands appeared; and (iii) an electron microscopy assessment by the negative-staining technique of the relative proportion of core to non-core material in each of these bands. Six nonionic surfactants were selected by this screening method for comparison in regard to recovery of core protein and endogenous ribonucleic acid (RNA)-dependent deoxyribonucleic acid (DNA) polymerase activity, as well as further morphologic evaluation by electron microscopy. The nonionic surfactants of the polyoxyethylene alcohol class (particularly, Sterox SL) were most effective. Nonionic surfactants of the polyoxyethylene alkylphenol class (particularly, Nonidet P-40) were also effective. Sterox SL and Nonidet P-40 each gave a more than fivefold increase in specific activity of endogenous RNA-dependent DNA polymerase, and each gave a low recovery of core protein. Sterox SL did not interfere to the extent that Nonidet P-40 did in procedures which involved spectrophotometric assay at 260 nm. The use of Sterox SL resulted in the least envelope contamination of core preparations by electron microscopy examination, the most recovery of protein and endogenous RNA-dependent DNA polymerase activity, and a core buoyant density in sucrose of 1.27 g/ml.
Full text
PDF






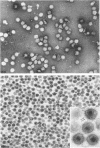
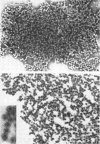
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAN H. S. Solubilisation by surface active agents. Pharm Acta Helv. 1960 Sep-Oct;35:512–525. [PubMed] [Google Scholar]
- BONAR R. A., BEAUDREAU G. S., SHARP D. G., BEARD D., BEARD J. W. Virus of avian erythroblastosis. V. Adenosinetriphosphatase activity of blood plasma from chickens with the disease. J Natl Cancer Inst. 1957 Nov;19(5):909–922. [PubMed] [Google Scholar]
- Bader J. P., Brown N. R., Bader A. V. Characteristics of cores of avian leuko-sarcoma viruses. Virology. 1970 Aug;41(4):718–728. doi: 10.1016/0042-6822(70)90436-8. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Comparison of Rous sarcoma virus-specific deoxyribonucleic acid polymerases in virions of Rous sarcoma virus and in Rous sarcoma virus-infected chicken cells. J Virol. 1971 May;7(5):625–634. doi: 10.1128/jvi.7.5.625-634.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Thé G., O'Connor T. E. Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology. 1966 Apr;28(4):713–728. doi: 10.1016/0042-6822(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Todaro G. J., Zeve V., Scolnick E. M., Aaronson S. A. Separation of RNA-dependent DNA polymerase activity from the murine leukaemia virion. Nature. 1970 Oct 31;228(5270):435–438. doi: 10.1038/228435a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- MOMMAERTS E. B., SHARP D. G., ECKERT E. A., BEARD D., BEARD J. W. Virus of avian erythromyeloblastic leukosis. I. Relation of specific plasma particles to the dephosphorylation of adenosine triphosphate. J Natl Cancer Inst. 1954 Apr;14(5):1011–1025. [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Enzymes and nucleotides in virions of Rous sarcoma virus. J Virol. 1971 Oct;8(4):409–416. doi: 10.1128/jvi.8.4.409-416.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley G. P., Carleton F. J., Wright B. S., Schidlovsky G., Monroe J. H., Mayyasi S. A. Comparison of the biologic and biophysical properties of the progeny of intact and ether-extracted Rauscher leukemia viruses. Cancer Res. 1969 Apr;29(4):905–911. [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D. Pelleting virus-infected cells for thin-section electron microscopy. Proc Soc Exp Biol Med. 1969 Apr;130(4):1117–1119. doi: 10.3181/00379727-130-33731. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. DNA-directed DNA polymerase activity in oncogenic RNA viruses. Nature. 1970 Sep 5;227(5262):1029–1031. doi: 10.1038/2271029a0. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]