Abstract
The development and improvement of cardiopulmonary bypass technology is an ongoing process. During the past decade, a number of publications on improvements and best practices have appeared, especially in the areas of biocompatibility, materials sciences, instrumentation, monitoring of physiological parameters and knowledge base (education and evidence-based medicine). Biocompatibility may be defined not only as an inherent property of a particular composition of matter, but also as a set of properties concerning shape, finish, fabrication techniques and choice of application. Materials in use for cardiopulmonary bypass have changed and coated components have been used frequently. Improvements in the area of instrumentation were achieved by adaptation of conventional cardiopulmonary bypass circuits. Miniaturization and re-design of cardiopulmonary bypass circuits (so-called minimized perfusion circuits or minimal extracorporeal circulation circuits) have made cardiopulmonary bypass technology less traumatic. A team approach, including the cardiac surgeon, the anesthesiologist and the cardiovascular perfusionist, was deemed beneficial in order to achieve further improvements. Next to choice of technology and material for a given operation, adjunct measures such as pharmaceutical treatment and blood conservation strategies need to be taken into consideration. Monitoring of variables during cardiopulmonary bypass has made some progress, while the knowledge base has expanded due to studies on best practices. For the immediate future, sound scientific knowledge and intelligent monitoring tools will allow cardiopulmonary bypass to be tailored to individual patients’ needs.
Keywords: cardiopulmonary bypass, extracorporeal circulation, biocompatibility, minimized perfusion circuit, systemic inflammatory response
INTRODUCTION
The development of cardiopulmonary bypass (CPB) has been an ongoing process since its first clinical use. Equipment and techniques have undergone significant refinements [1].
Today, this technology is used in more than one million cases annually worldwide [2].
The optimal technical characteristics of a CPB system for any given patient as well as the optimal operative strategy are still under debate. Recently, an array of articles on best practices and guidelines for the conduct of CPB has been published.
Biocompatibility
According to a widely used definition, the term biocompatibility refers to “... the ability of a material to perform with an appropriate host response in a specific application” [3].
The extent of body reactions to the exposure to non-physiologic materials is a function of the characteristics of foreign materials and the nature, location and length of time in use [4]. However, questions in conjunction with biomaterials are whether it is possible to synthesize biomaterial with reliable predictability of its properties (appropriate host response), and whether this material has any effect on increased patient safety during CPB [5]. A more complex definition of the term reads as follows: “Biocompatibility refers to the ability of a biomaterial to perform its desired function with respect to a medical therapy, without eliciting any undesirable local or systemic effects in the recipient or beneficiary of that therapy, but generating the most appropriate beneficial cellular or tissue response in that specific situation, and optimising the clinically relevant performance of that therapy” [6].
Physiologically, several mechanisms prevent the organism from blood loss due to injured blood vessels: the coagulation system, endothelium and regulatory proteins, platelets and fibrinolysis. This hemostatic system of a patient is activated during cardiac surgery with and without CPB [7].
Furthermore, the so-called systemic inflammatory response syndrome (SIRS) is triggered. Surgical trauma, blood contact with CPB surfaces, endotoxemia and ischemia trigger mediators, transcription factors and adhesion molecules, leading to leukocyte extravasation, lipid peroxidation, edema and eventually cell death [8]. Clinically, SIRS can lead to coagulopathy, arrhythmias, endothelial dysfunction, neurological manifestations and end organ failure [1].
In order to improve the above scenario, several strategies have been developed. First of all, use of the heart-lung machine could be avoided, whenever feasible. However, activation of the hemostatic system is still detectable in off-pump cardiac surgery [9]. A second strategy would be to use more advanced perfusion circuits, such as minimized perfusion circuits (MPCs), for those operations where conventional circuits are not necessary. The biomarker profile measured during the use of MPCs is comparable to the profile measured when conventional circuits are in use [10].
Minimized perfusion circuits
Minimized perfusion circuits are usually comprised of venous and arterial tubings, a centrifugal pump, a membrane oxygenator (optionally with integrated arterial line filter) and cannulae. A venous reservoir and suction devices (vent and field suction) are usually not incorporated [1]. All components of the circuits are either heparin coated or treated with alternative coating agents.
The use of centrifugal arterial pumps is advocated. Further, low priming volume of MPCs in contrast to conventional circuits, the use of cell salvage devices instead of intraoperative retransfusion of untreated suction blood as well as venous line air handling devices are characteristics of these miniaturized systems [11].
Initial concerns about a lack of safety in air-handling or cases of major blood loss could be refuted by the results of studies focusing on that matter. Kutschka et al. even demonstrated superiority in the handling of air in an MPC compared to conventional bypass. Modular concepts of MPCs allow the quick integration of additional suckers and reservoirs if major bleeding occurs [12].
A variety of studies was undertaken in order to determine differences in patient outcomes when conventional CPB circuits were compared to minimized perfusion circuits. These early studies, however, included small patient groups with low risk profiles. Subsequently, only limited evidence was available in favour of these circuits [13,14,15,16].
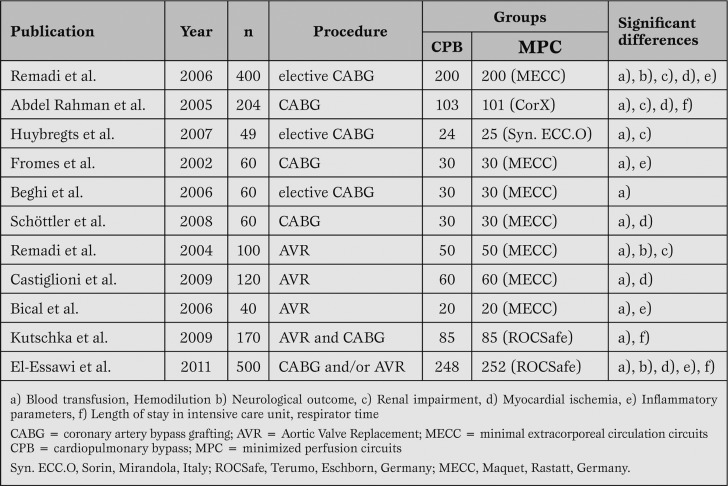
To date, a number of studies with prospective randomized design and a cohort of more than 40 patients have been published [17,18,19,20,21,22] (Table 1).
Table 1.
Clinical experience and findings with MPCs.
Vohra et al. describe the effect of minimized circuits on inflammatory markers and end-organ effects.
Although a reduction in the amount of circulating inflammatory markers can be measured, the authors state that survival rates of patients operated upon with conventional CPB do not differ from those of patients operated on with MPC [1].
In contrast, Anastasiadis et al. in their metaanalysis found that so-called minimal extracorporeal circulation improved short-term patient outcome by reducing the mortality and morbidity associated with conventional systems [23].
The requirement for blood transfusion is today regarded as a risk factor for adverse long-term outcome in cardiac surgery [24]. Avoiding transfusion by reducing hemodilution, caused by excessive priming volume of conventional CPB circuits, is recommended. The use of mini-circuits is advocated especially in patients with high risks for adverse effects of hemodilution [25].
MPCs are associated with significantly reduced hemodilution and higher hematocrit at the end of the extracorporeal circulation, as compared with conventional CPB [23].
A retrospective study on transfusion requirements in 285 coronary artery bypass grafting (CABG) patients compared off-pump procedures, conventional circuits (with cold hydroxyethyl starch cardioplegia) and MPCs (with warm blood cardioplegia).
The authors stated that significantly fewer blood transfusions were needed in the MPC group than in the off-pump and conventional CPB groups. For the conventional CPB group, the use of thrombocyte concentrates was higher on the day of operation than in the MPC group. However, the results of this study may be questionable, since the type of cardioplegia differed between the on-pump groups [26].
A metaanalysis of randomized controlled studies, which included pooled data from 1051 patients, found that MPCs decreased the risk of red blood cell transfusion and the amount of red blood cells transfused per patient when compared with conventional CPB in CABG patients [27].
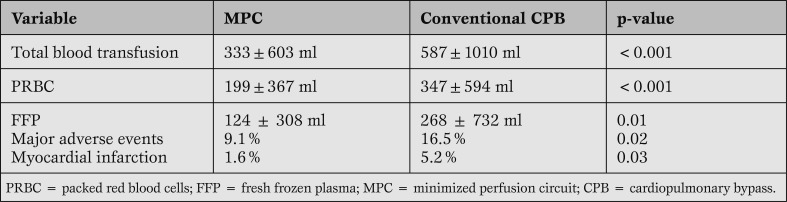
In a prospective randomized multicenter study (6 hospitals), an MPC device was compared to conventional CPB. Five hundred patients were included; 252 patients were assigned to the same type of MPC, 248 patients were assigned to the standard open CPB system of the respective hospital.
In this study, favorable results were noted for the MPC procedures regarding transfusion requirements, incidence of atrial fibrillation and the incidence of major adverse events (death, myocardial infarction, major cerebrovascular accidents, re-operation).
Furthermore, an optimal outcome, defined as freedom from blood transfusion or any adverse event, was clearly in favour of the MPC group (52% vs. 41%; p = 0.02). The findings are summarized in Table 2.
Table 2.
Benefits of MPCs.
This study also showed significant differences regarding biochemical parameters in favour of the MPC group. Beside platelets, red and white blood cells, granulocytes and lymphocytes, plasma free hemoglobin, creatinine and LDH were measured [22].
Miniaturized CPB in pediatric surgery
In analogy to the developments in adult cardiac surgery, interest in reducing hemodilution and the subsequent necessity for transfusion of homologous blood components in pediatric cardiac surgery has increased recently. Alongside the potential for transmission of infection, the use of fresh whole blood for priming heart lung machines for children and the use of blood components are triggers for altered immunologic function. For this reason, avoiding blood components for priming of the CPB circuit may have beneficial effects [28].
Miniaturization of conventional CPB has been achieved in experimental surgery and in clinical practice. The asanguineous priming fluid in the animal model described by Hickey et al. was found to improve postoperative right ventricular function, pulmonary compliance, alveolar gas exchange, recovery of cerebral perfusion and the inflammatory cytokine load [28].
Clinically, the use of an asanguineous prime is feasible as well. Initial experiences with blood-free priming of a conventional CPB circuit for a neonatal cardiac operation [29] showed that this approach was possible. Subsequently, neonates in several series were operated on without the use of blood prime [30, 31]. In a retrospective analysis on 288 children from the same institution, children weighing from 1.7 to 15.9 kg were divided into the three categories no transfusion, postoperative transfusion only, and intraoperative as well as postoperative transfusion.
Of these children, 24.7% did not require any form of blood transfusion during their hospital stay, 23.6% received transfusions postoperatively, and 51.7% received intra- and postoperative transfusion. It was noted that this achievement was only possible because of the concerted efforts of the surgeon, anaesthesiologist and perfusionist in addition to the availability of the appropriate equipment [32].
Conduct and monitoring of CPB
While the conduct of CPB was referred to as “experience based” in contrast to based on evidence only a short while ago [33], a number of publications have subsequently dealt with this issue.
Shann et al. gathered available evidence on the practice of CPB in adults, mainly focusing on neurologic injury, glycemic control, hemodilution and the inflammatory response. One of the recommendations of this paper was to avoid direct retransfusion of unprocessed blood exposed to pericardial and mediastinal surfaces. Also, hemodilution should be minimized to avoid subsequent allogeneic blood transfusion [34].
In a following paper, Murphy et al. focused on management of physiologic parameters during CPB, namely on determinants of tissue oxygen supply and demand, such as mean arterial pressure, systemic bypass flow rates, hematocrit values, oxygen delivery, systemic temperatures, pulsatility and acid-base management. The authors addressed these topics extensively, but also concluded that since there is limited high quality data on perfusion-related issues, evidence-based guidelines are of uncertain reliability [35].
The term “goal-directed perfusion management” was created by the working group of Ranucci et al. One of the key findings of a recent publication is the statement that oxygen delivery should be preserved by reducing hemodilution and maintaining high pump flows, since a nadir oxygen delivery of 262ml/min/m² during CPB is associated with acute kidney failure [36].
In the future, more patient-targeted pharmaceutical strategies, including genetic risk profiles for hemostatic activation, will make it possible to select the appropriate CPB technology for a given patient [7].
CONCLUSION
The combined strategies of avoiding excessive hemodiluion, decreasing CPB circuit size, avoiding blood transfusion, limiting the use of cardiotomy suction, and the use of re-engineered and optimized perfusion circuits may make cardiopulmonary bypass more patient-friendly.
The knowledge base for cardiopulmonary bypass related technologies has expanded, and evidence-based guidelines have been established in some areas. Well-controlled studies on the effect of interventions are warranted to help in choosing the right technology for the right patient.
Acknowledgments
We thank Anne Gale, ELS (Editor in the Life Sciences), for editorial assistance.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Presented at the 3rd Expert forum of the Roland Hetzer International Cardiothoracic and Vascular Surgery Society on the occasion of the 6th Oriental Congress of Cardiology, Shanghai, May 25, 2012
Cite as: Merkle F, Haupt B, El-Essawi A, Hetzer R. State of the Art in Cardiovascular Perfusion - Now and in the next decade. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012; 4(4): 211-216
References
- Vohra H A, Whistance R, Modi A. et al. The inflammatory response to miniaturised cxtracorporeal circulation: a review of the literature. Mediators Inflamm. 2009; 2009: 707042. 2009;2009 doi: 10.1155/2009/707042. [707042] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M A, Antman E M. 2003. Perioperative evalutaion for cardiac surgery. pp. 235–248. [Cohn LH, Edmungs LH (eds): Cardiac Surgery in the Adult, New York, McGraw-Hill, ] [Google Scholar]
- Williams D F. Definitions in biomaterials. [Elsevier, Amsterdam, 1987] [Google Scholar]
- Glasmacher B. Materials Sciences - Biocompatibility. 2006:109–126. [In: Feindt P, Harig F, Weyand M (eds): Darmstadt, Steinkopff ] [Google Scholar]
- Rubens F D. Cardiopulmonary bypass technology transfer: musings of a cardiac surgeon. J Biomater Sci Polymer Edn. 2002;13:485–499. doi: 10.1163/156856202320253974. [DOI] [PubMed] [Google Scholar]
- Williams D F. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Scniecinski R M, Chandler W L. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;13:1319–1333. doi: 10.1213/ANE.0b013e3182354b7e. [DOI] [PubMed] [Google Scholar]
- Paparella D, Yau T M, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–244. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- Levy J H, Tanaka K A. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:715–720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- Castellheim A, Hoel T, Videm V. et al. Biomarker profile in off-pump and on-pump coronary artery bypass grafting surgery in low-risk patients. Ann Thorac Surg. 2008;85:1994–2002. doi: 10.1016/j.athoracsur.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Harling L, Punjabi P, Athanasiou T. Miniaturized extracorporeal circulation vs. off-pump coronary artery bypass grafting: What the evidence shows? Perfusion. 2011;26:40–47. doi: 10.1177/0267659110396578. [DOI] [PubMed] [Google Scholar]
- Kutschka I, Schönrock U, El Essawi A. et al. A new minimized perfusion circuit provides highly effective ultrasound controlled deairing. Artif Organs. 2007;31:215–220. doi: 10.1111/j.1525-1594.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Fromes Y, Gaillard D, Ponzio O. et al. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg. 2002;22:527–533. doi: 10.1016/s1010-7940(02)00372-x. [DOI] [PubMed] [Google Scholar]
- Agostinelli A, Beghi C, Nicolini F. et al. Mini-cardiopulmonary bypass system: Results of a prospective randomized study. Ann Thorac Surg. 2006;81:1396–1400. doi: 10.1016/j.athoracsur.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Huybregts R A, Morariu A M, Rakhorst G. et al. Attenuated renal and intestinal injury after use of a mini-cardiopulmonary bypass system. Ann Thorac Surg. 2007;83:1760–1766. doi: 10.1016/j.athoracsur.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Schöttler J, Lutter G, Böning A. et al. Is there really a clinical benefit of using minimized extracorporeal circulation for coronary artery bypass grafting? Thorac Cardiovasc Surgeon. 2008;56:65–70. doi: 10.1055/s-2007-989336. [DOI] [PubMed] [Google Scholar]
- Remadi J P, Marticho P, Butoi I. et al. Clinical experience with the mini-extracorporeal circulation system: an evolution or a revolution? Ann Thorac Surg. 2004;77:2172–2176. doi: 10.1016/S0003-4975(03)00977-9. [DOI] [PubMed] [Google Scholar]
- Remadi J P, Rakotoarivelo Z, Marticho P, Benamar A. Prospective randomized study comparing coronary artery bypass grafting with the new mini-extrcorporeal circulating Jostra System or with a standard cardiopulmonary bypass. Am Heart J 2006; 151: 198. 2006;151:198. doi: 10.1016/j.ahj.2005.03.067. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman U, Keller F, Özaslan S. et al. Erfahrungen mit einem geschlossenen minimierten EKZ-System (CORx). Kardiotechnik. 2005;4:113–118. [Google Scholar]
- Castiglioni A, Verzini A, Colangelo N. et al. Comparison of minimally invasive closed circuit versus standard extracorporeal circulation for aortic valve replacement: a randomized study. Interact Cardiovasc Thorac Surg. 2009;9:37–41. doi: 10.1510/icvts.2008.192559. [DOI] [PubMed] [Google Scholar]
- Kutschka I, Skorpil J, El Essawi A. et al. Beneficial effects of modern perfusion concepts in aortic valve and aortic root surgery. Perfusion 2009; 24: 37-44. 2009;24:37–44. doi: 10.1177/0267659109106727. [DOI] [PubMed] [Google Scholar]
- El-Essawi A, Hajek T, Skorpil J. et al. Are minimized perfusion circuits the better heart lung machines? Final results of a prospective randomized multicentre study. Perfusion. 2011;26:470–478. doi: 10.1177/0267659111419035. [DOI] [PubMed] [Google Scholar]
- Anastiasidis K, Antonitsis P, Haidich A B. et al. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Engoren M C, Habib R H, Zacharias A. et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–1186. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- Ferraris V A, Brown J R, Despotis G J. et al. 2011 Update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Gerritsen W B, van Boven W J, Wesselink R M. et al. Significant reduction in blood loss in patients undergoing minimal extracorporeal circulation. Transfusion Medicine, 2006;16:329–334. doi: 10.1111/j.1365-3148.2006.00676.x. [DOI] [PubMed] [Google Scholar]
- Benedetto U, Angeloni E, Refice S. et al. Is minimized extracorporeal circulation effective to reduce the need for red blood cell transfusion in coronary artery bypass grafting? Meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg 2009; 138: 14-3. 2009;138:1450–1453. doi: 10.1016/j.jtcvs.2009.03.042. [DOI] [PubMed] [Google Scholar]
- You J, Hickey E, Karamlou T. et al. Effects of circuit miniaturization in reducing inflammatory response to infant cardiopulmonary bypass by elimination of allogeneic blood products. Ann Thorac Surg. 2006;81:2367–2372. doi: 10.1016/j.athoracsur.2006.02.071. [DOI] [PubMed] [Google Scholar]
- Boettcher W, Merkle F, Koster A. Safe minimization of cardiopulmonary bypass circuit volume for complex cardiac surgery in a 3.7 kg neonate. Perfusion. 2003;18:377–379. doi: 10.1191/0267659103pf686oa. [DOI] [PubMed] [Google Scholar]
- Huebler M, Koster A, Boettcher W. et al. A new miniaturized cardiopulmonary bypass system reduces transfusion requirements during neonatal cardiac surgery: Initial experiences in13 consecutive patients. J Thorac Cardiovasc Surg. 2009;137:1565–1568. doi: 10.1016/j.jtcvs.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Redlin M, Huebler M, Boettcher W. et al. Minimizing intraoperative hemodilution by use of a very low priming volume cardiopulmonary bypass neonates with transposition of the great arteries. J Thorac Cardiovasc Surg. 2011;142:875–881. doi: 10.1016/j.jtcvs.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Redlin M, Habazettl H, Boettcher W. et al. Effects of a comprehensive blood-sparing approach using body weight-adjusted miniaturized cardiopulmonary bypass circuits on transfusion requirements in pediatric cardiac surgery. J Cardiovasc Surg. 2012;144:493–499. doi: 10.1016/j.jtcvs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Bartels C, Gerdes A, Babin-Ebell J. et al. Cardiopulmonary bypass: Evidence or experience based? J Thorac Cardiovasc Surg. 2002;124:20–27. doi: 10.1067/mtc.2002.121506. [DOI] [PubMed] [Google Scholar]
- Shann K G, Likosky D S, Murkin J M. et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–290. doi: 10.1016/j.jtcvs.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Murphy G S, Hessel E A, Groom R C. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg. 2009;108:1394–1417. doi: 10.1213/ane.0b013e3181875e2e. [DOI] [PubMed] [Google Scholar]
- De Somer F, Mulholland J, Bryan M. et al. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: time for a goal-directed perfusion management? Critical Care. 2011;15:192. doi: 10.1186/cc10349. [DOI] [PMC free article] [PubMed] [Google Scholar]