Abstract
Acute thoracic aortic aneurysm is one of the most life-threatening vascular disorders recognized to date. The majority of these aortic ruptures rapidly end in mortality, with 50% of patients suffering death before reaching the hospital. Thus, acute management through surgical intervention is often indicated, especially in cases of ascending aortic rupture. Physical examination is critical in making the diagnosis, as clinical signs and symptoms often vary depending on the location of the dissection. Clinicians should have a low threshold for including thoracic aortic dissection in their differential diagnosis, especially when a patient presents with acute onset chest or back pain. In this report, we discuss the different categories of aortic dissections and the current treatment modalities for each. These include endovascular aortic repair, which has become a viable treatment modality in certain cases of type B dissection. Offering a less invasive approach, the technique known as thoracic endovascular repair currently affords a treatment option to a patient population who would have otherwise been deemed non-surgical candidates. Hybrid thoracic endovascular aortic repair has also become a pertinent surgical technique, and successful outcomes have been demonstrated when it is employed to repair ascending aortic aneurysms. We also describe our Acute Aortic Treatment Center, a rapid multicentric triage system for the management of acute aortic pathologies, which has resulted in significant improvements in patient outcomes.
Keywords: aortic aneurysm, aortic dissection, DeBakey classification system, Stanford classification system, Acute Aortic Treatment Center, Acute Aortic Treatment Center
INTRODUCTION
Acute thoracic aortic aneurysm (TAA) is the most life-threatening vascular disorder recognized to date. Aortic ruptures have an 80% mortality rate, with 50% of patients suffering death before reaching the hospital [1]. The risk of death from untreated type A dissections is 25%, 50%, 75%, and 90% at 24 hours, 2 days, 1 week and 1 month, respectively [2]. Due to their severity, all acute ascending aortic dissections require emergency surgery in order to prevent aortic rupture and death.
HISTORY
Death of King George II of Great Britain
On the morning of October 25, 1760, King George II started his usual 6:00 a.m. routine, drank a cup of hot chocolate and went to his closet stool. After a few minutes, his valet heard a loud crash and entered the room to find the king on the floor. The king was lifted on to his bed and Princess Amelia, his daughter, was sent but, when she reached him, he was dead. The post-mortem examination of King George II of Great Britain ( 1683-1760 ) revealed an aortic aneurysm and rupture of the right ventricle. This examination was carried out by the king’s physician, Frank Nicholls ( 1699-1778 ), who was the first person to document a case of aortic dissection [3].
The man on the table devised the surgery
In 1955, Dr. Michael E. DeBakey and colleagues reported the first series of patients with aortic dissections successfully treated with primary surgical repair [4] employing techniques that are still widely used today [5, 6]. Fifty years later, on the late afternoon of December 31, 2005, Dr. DeBakey, then 97 years old, was alone at home preparing a lecture when a sharp pain ripped through his upper chest, between his shoulder blades, and then moved into his neck. “It never occurred to me to call 911 or my physician,” Dr. DeBakey said, adding: “As foolish as it may appear, you are, in a sense, a prisoner of the pain, which was intolerable. You’re thinking, what I could do to relieve myself of it. If it becomes intense enough, you’re perfectly willing to accept cardiac arrest as a possible way of getting rid of the pain” [7]. But when his heart kept beating, Dr. DeBakey suspected that he was not having a heart attack. It was these symptoms, and his years of experience, that lead him to suspect he was having dissecting aortic aneurysm. Two months later, he would undergo aortic aneurysm surgical intervention, and became the oldest patient ever to benefit from such a repair.
Classification
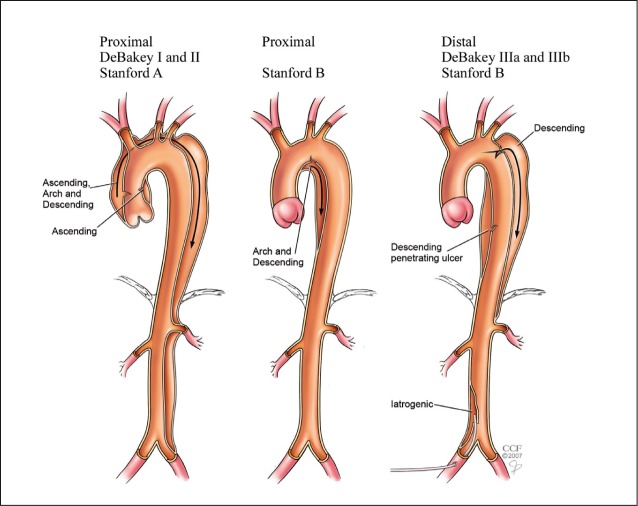
There are two gold-standard classification systems for aortic dissections: the DeBakey system and the Stanford system. They differ in that the former is based on the anatomy, and the latter on management of the patient (Figure 1).
Figure 1.
The DeBakey and Stanford classification systems for aortic dissection. Reprinted with permission from the Cleveland Clinic Foundation.
In general, Stanford type A dissections affect the ascending root, while type B affects the descending aorta. Type A is a surgical emergency, in contrast to type B, which is less lethal and can be treated conservatively with medical therapy and concomitant endovascular intervention. As described in a publication based on the International Registry of Acute Aortic Dissection, the less invasive endovascular treatment has been shown to provide better in-hospital survival in patients with acute type B dissection [8]. However, findings such as leaking, rupture or end organ compromise are indications for surgical intervention.
Clinical presentation
Sudden onset of severe chest pain is the typical presenting feature of aortic dissections. This pain may be excruciating, sharp, ripping, or tearing in nature. Nearly 90% of patients report acute chest pain. Anterior chest pain is typically seen in type A dissections, while type B dissections present back and abdominal pain. Acute aortic dissections can be misdiagnosed as acute myocardial infarction when the dissection extends into the coronary ostia. Clinical signs resembling those of acute myocardial ischemia (i.e. Electrocardiography changes and elevated cardiac biomarkers) are often discovered on initial workup.
Less common symptoms that may be seen in acute aortic dissection include congestive heart failure (7%), syncope, cerebrovascular accident, ischemic peripheral neuropathy, paraplegia, cardiac arrest and sudden death [9]. Acute aortic dissection into the pericardium resulting in tamponade is the second most common cause of death in acute aortic dissection [10]. Complications of aortic insufficiency occur in one-half to two-thirds of ascending aortic dissections and may lead to congestive heart failure or cardiogenic shock. The most common cause of death in type B dissection is mesenteric ischemia [11]. Respiratory complications that can also be observed include tachypnea, dyspnea and orthopnea due to mass effects on the tracheobronchial tree. Cerebral ischemia and stroke syndromes are the most common central nervous system effects of proximal acute aortic dissection, occurring 5-15% of the time [12].
Risk factors
The most common medical risk factor for aortic dissection is hypertension (70-90%), especially if uncontrolled [2]. The highest incidence of aortic dissection occurs in individuals who are 50 to 70 years of age. Two-thirds of patients with acute aortic dissections are male. Patients with type B dissections tend to be older than type A patients (mean age of 66 vs. 61 years, respectively) [13, 14]. Additionally, of the dissections occurring in females younger than age 40, about half occur during pregnancy (typically in the third trimester or early postpartum period) [15].
It has been shown that 50% of patients younger than 40 years of age who suffer an aortic dissection have a Marfan-like phenotype [11]. Having a congenital bicuspid aortic valve increases the risk of aortic dissection by 10 times compared to the risk of the general population and is a factor in 14% of all cases [11]. Additionally, individuals who have undergone aortic valve replacement for insufficiency are at particularly high risk. Interestingly, 18% of individuals who present with an acute aortic dissection have a history of cardiac surgery and/or catheterization [11].
Diagnostic imaging
Plain chest X-ray is abnormal in most patients suspected of having an acute aortic dissection and is abnormal (i.e. shows mediastinal widening) in about 90% of cases [16]. Double aortic knob sign, irregularity of the aortic contour, tracheal displacement, and pleural effusion are all non-specific findings that should prompt more precise investigation. Helical computed tomography (CT) is the most commonly utilized study for making the diagnosis of acute aortic dissection because of its relatively quick turnover, broad availability, and high sensitivity/specificity for this complication. Diagnostic CT provides detailed information about the location and extent of anatomic anomalies, and can be further enhanced with contrast employment.
The benefit of magnetic resonance angiography (MRA) is that it provides enhanced details of the aorta, arch vessels and aortic valve insufficiency without the use of ionizing radiation or iodinated contrast material. However, MRA is best used for monitoring patients with chronic aortic dissection and in postoperative follow-up. Transthoracic/transesophageal echocardiography (TTE/TEE) is a relatively non-invasive test that may provide a safer method for confirming or excluding aortic dissection. TTE/TEE also gauges the severity of certain valve problems and contributes valuable information by detecting involvement of the coronary arteries, communications between true/false lumens, and aortic regurgitation.
Acute aortic treatment center
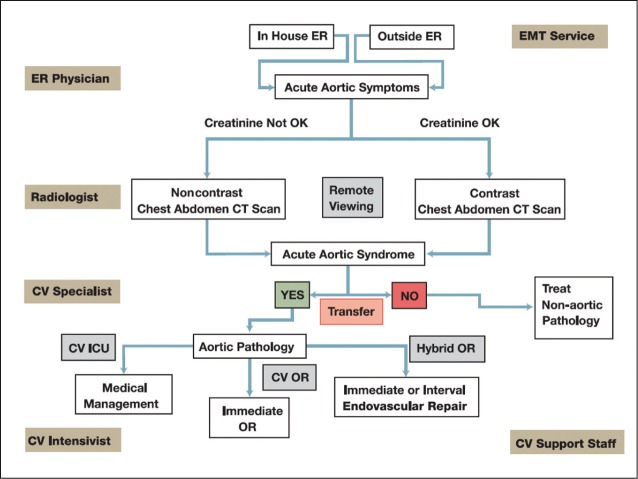
Key elements that have significantly reduced mortality from acute aortic syndromes include rapid patient transportation, employment of a dedicated multi-disciplinary team and subsequent prompt diagnosis and intervention (Figure 2) [17].
Figure 2.
Acute Aortic Treatment Center Passway. Image reproduced with permission from Davies MG, Lumsden AB. Acute Aortic Treatment Center. Methodist DeBakey Cardiovasc J. 2011; 7: 8-10.
OR = operating room; ER = emergency room; EMT = emergency medical technician; CV = cardiovascular; ICU = intensive care unit; CT = computed tomography.
As pioneers in the treatment of acute aortic manifestations, the Methodist DeBakey Heart & Vascular Center has developed the Acute Aortic Treatment Center (AATC) to rapidly triage and treat acute aortic disease. The AATC has resulted in a 30% increase in volume, a 64% reduction in time to definitive treatment and a reduction in intensive care unit time, while maintaining clinical efficacy despite significantly more acute admissions [18]. Our experience with the AATC has demonstrated its clinical relevance and we will continue to develop this system to optimize outcomes in the future [18].
Surgical repair
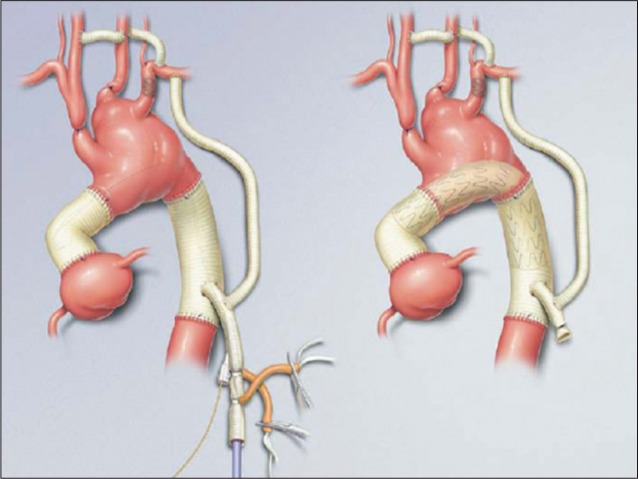
Aortic dissection involving the ascending aorta or proximal aortic arch (DeBakey type I and II or Stanford type A) requires immediate surgical repair. A Dacron graft may be used to replace portions of the ascending aorta or total aortic arch (Figure 3).
Figure 3.
Dacron graft repair of ascending and descending aortic dissection. Reprinted from Journal of Vascular Surgery, Volume 44 Issue 4, Wei Zhou, Michael Reardon, Eric K. Peden, Peter H. Lin, Alan B. Lumsden, pages 688-693, Copyright 2006, with permission from Elsevier.
If the patient’s aortic valve is competent, David’s valve-sparing aortic root replacement has shown clinical efficacy and is currently the most favorable technique. The major advantage is the avoidance of anticoagulation. The modified Bentall procedure has promising short- and long-term results for patients with severely dilated aortic root and valves [19].
Patients with DeBakey type III and Stanford type B dissection receive surgical intervention only in certain conditions. The majority of these patients can be treated conservatively with blood pressure control. Surgery or endovascular therapies are performed when there are complications from the aortic dissection, such as rupture, organ or limb perfusion dysfunction, a large size aneurysm (>5 cm), or rapid expansion in aneurysm diameter during a 1-year period.
Open surgical repair of distal thoracic aortic dissection is often associated with unacceptably high risks of paralysis (4.2%), fatal bleeding (2%), stroke (2.5%), and renal failure (3.5%) [14]. While spinal cord ischemia and renal failure warrant the most consideration, the most important methods are: cerebrospinal fluid drainage, left heart bypass, perfusion of renal arteries with 4°C crystalloid solution, and reattachment of segmental arteries, especially between T8 and L1 [20].
Endovascular repair
Although medical therapy is still the first line treatment in patients with type B dissection and aneurysm, thoracic endovascular aortic repair (TEVAR) has emerged as an acceptable treatment. TEVAR offers a minimally invasive approach to aneurysm stabilization and repair, while avoiding the increased prothrombotic state associated with thoracotomy and clamping of the proximal aorta [21].
The procedure involves the insertion of a presized endograft that is secured under real time fluoroscopic imaging. Successful endograft repair depends on accuracy of deployment and landing zone, and that the aneurysm is absent of any residual leaks. Clinical follow up should confirm complete retraction and thrombosis of the aneurysm sac, and the patient should be free of any signs and symptoms [22]. Current data supports a paradigm shift, with TEVAR (introduced in 1991) emerging as the preferred therapy for all patients presenting with descending aortic rupture [23, 24].
A large meta-analysis study of centers across the United States has demonstrated improved shorter perioperative outcomes when compared to open aortic repair (OAR) procedures, despite a targeted older patient population. The same study also showed that TEVAR is associated with a shorter total length of stay and less complications. However, TEVAR did have significantly greater hospital charges when compared to OAR [25].
Hybrid thoracic endovascular aortic repair
Aneurysms involving the ascending aorta and arch have traditionally been treated with open surgery involving cardiopulmonary bypass with or without deep hypothermic circulatory arrest. In some cases, a staged elephant trunk procedure is required. TEVAR can be limited by inadequate proximal and distal landing zones. A novel technique for repair of these aneurysms has emerged using debranching or hybrid TEVAR. Unlike TEVAR, which is performed by a vascular surgeon, hybrid TEVAR is performed by a multidisciplinary team of vascular and cardiac surgeons. For this technique, aortic arch debranching is required before the placement of endografts [26, 27]. Debranching is performed for two fundamental reasons:
to provide an appropriate landing zone for thoracic stent grafts;
to ensure ongoing perfusion of the supra-aortic vessels (Figure 4).
Figure 4.
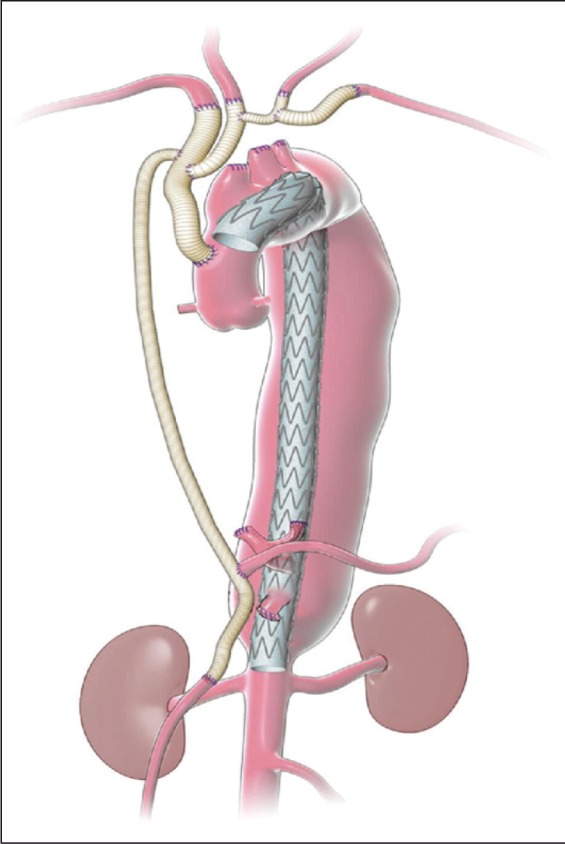
Schematic representation showing arch and visceral debranching inflow from the ascending aorta. Reprinted with permission from the Department of Cardiovascular Surgery, Methodist DeBakey Heart & Vascular Center.
The technical advantages of the hybrid procedure is that it again eliminates the need for aortic clamping, while allowing direct visualization and preparation of the proximal landing zone for subsequent TEVAR. The debranching can be completed via right anterior minithoracotomy, which avoids the morbidity and complications associated with sternotomy [28]. Despite preliminary success with the procedure, there are no objective studies that have established superiority over conventional open approach and further studies are merited [29].
CONCLUSION
Improvements in diagnostic protocol, surgical technique and guideline-driven management have changed the way acute and chronic aortic manifestations are approached and treated. Crucial to patient management has been the employment of advanced and timely diagnostic imaging procedures, such as helical CT, MRA, and TEE. Prompt surgical intervention for Stanford type A aortic dissection and proximal thoracic aortic aneurysm has demonstrated excellent survival with acceptable morbidity and is currently the gold-standard treatment.
However, hybrid procedures employing branched/fenestrated endografts, as well as percutaneous aortic valves, have emerged as relevant alternatives to traditional surgical intervention. They have demonstrated significant improvement in mortality and morbidity.
Moreover, these procedures have been employed as a bridge to subsequent TEVAR and should be considered in high-risk older patients. In closing, logistical and technological advances have significantly improved patient outcomes in patients suffering acute abdominal aneurysms. We look forward to further investigating the benefits of employing a designated care team and center, as our experience with the AATC has shown higher success rates afforded by rapid diagnosis and management.
Acknowledgments
The authors thank Amanda Hodgson, PhD, for critical reading of the manuscript.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Presented at the 3rd Expert forum of the Roland Hetzer International Cardiothoracic and Vascular Surgery Society on the occasion of the 6th Oriental Congress of Cardiology, Shanghai, May 25, 2012
Cite as: Loebe M, Ren D, Rodriguez L, La Francesca S, Bismuth J, Lumsden A. Acute and chronic thoracic aortic disease: surgical considerations. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012; 4 (4): 243-250
References
- Isselbacher E M, Eagle K A, Zipes D P, 1997. Diseases of the aorta. In: Braunwald E, ed. Heart disease: a textbook of cardiovascular medicine. pp. 1546–1581. [5th ed. Philadelphia, PA: WB Saunders. ] [Google Scholar]
- Chen K, Varon J, Wenker O C. et al. Acute thoracic aortic dissection: the basics. J Emerg Med. 1997;15:859–867. doi: 10.1016/s0736-4679(97)00196-0. [DOI] [PubMed] [Google Scholar]
- Nicholls F. Observation concerning the body of his late majesty. J Philos Trans Lond. 1761;52:265–275. [Google Scholar]
- Debakey M E, Cooley D A, Creech O. et al. Surgical consideration of dissecting aneurysm of the aorta. Ann Surg. 1955;142:586–610. doi: 10.1097/00000658-195510000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley D A, DeBakey M E, Morris GC Jr. Controlled extracorporeal circulation in surgical treatment of aortic aneurysm. Ann Surg. 1957;146:473–485. doi: 10.1097/00000658-195709000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBakey M E, Henly WS, Cooley D A. et al. Surgical management of dissection aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–149. [PubMed] [Google Scholar]
- Altman L K. The man on the table devised the surgery. [The New York Times. December 25, 2006. Accessed August 28, 2012] [Google Scholar]
- Fattori R, Tasi T T, Myrmel T. et al. Complicated acute type B dissection: is surgery still the best option?: a report from the international registry of acute aortic dissection. JACC Cardiovasc Interv. 2008;1:395–402. doi: 10.1016/j.jcin.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kamalakannan D, Rosman H S, Eagle K A. Acute aortic dissection. Crit Care Clin. 2007:779–800. doi: 10.1016/j.ccc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Rogers R L, McCormack R. Aortic disasters. Emerg Med Clin North Am. 2004;22:887–908. doi: 10.1016/j.emc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Hiratazka L F, Bakris G L, Beckman J A. et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patient with thoracic aortic disease. Circulation. 2010;12:266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- Knaut A L, Cleveland JC Jr. Aortic emergencies. Emerg Med Clin North Am. 2003;21:817–845. doi: 10.1016/s0733-8627(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Meszaros I, Morocz J, Szlavi J. et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–1278. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- Hagan P G, Nienaber C A, Isslbacher EM. et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- Immer F F, Bansi A G, Immer-Bansi A S. et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;76:309–314. doi: 10.1016/s0003-4975(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002;287:2262–2272. doi: 10.1001/jama.287.17.2262. [DOI] [PubMed] [Google Scholar]
- Lumsden A B, Crawford D J, Peden E K. et al. Establishing an acute aortic treatment center. Endovascular Today. 2007:28–31. [Supplement:] [Google Scholar]
- Davies M G, Younes H K, Harris P W. et al. Outcomes before and after initiation of an acute aortic treatment center. J Vasc Surg. 2010;52:1478–1485. doi: 10.1016/j.jvs.2010.06.157. [DOI] [PubMed] [Google Scholar]
- Etz C D, Homann T M, Silovitz D. et al. Long-term survival after the Bentall procedure in 206 patients with bicuspid aortic valve. Ann Thorac Surg. 2007;84:1186–1194. doi: 10.1016/j.athoracsur.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Coselli J S, LeMaire S A. 2004. Thoracic aortic aneurysms and aortic dissection. pp. 691–691. [In: Schwartz’s Principles of Surgery. 8th ed. New York, NY: McGraw-Hill] [Google Scholar]
- Conrad M F, Cambria R P. Contemporary management of descending thoracic and thoracoabdominal aortic aneurysms: endovascular versus open. Circulation. 2008;117:841–852. doi: 10.1161/CIRCULATIONAHA.107.690958. [DOI] [PubMed] [Google Scholar]
- Grabenwöger M, Alfonso F, Bachet J. et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2012;33:1558–1563. doi: 10.1093/eurheartj/ehs074. [DOI] [PubMed] [Google Scholar]
- Coselli J S, Conklin L D, LeMaire S A. Thoracoabdominal aortic aneurysm repair: Review and update of current strategies. Ann Thorac Surg. 2002;74:1881–1884. doi: 10.1016/s0003-4975(02)04139-5. [DOI] [PubMed] [Google Scholar]
- Jonker F H, Trimarchi S, Verhagen H J. et al. Meta-analysis of open versus endovascular repair for ruptured descending thoracic aortic aneuryam. J Vasc Surg. 2010;51:1026–1032. doi: 10.1016/j.jvs.2009.10.103. [DOI] [PubMed] [Google Scholar]
- Gopaldas R R, Huh J, Dao T K. et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg. 2010;140:1001–1010. doi: 10.1016/j.jtcvs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Patel H J, Williams D M, Upchurch GR Jr. et al. A comparative analysis of open and endovascular repair for the ruptured descending thoracic aorta. J Vasc Surg. 2009;50:1265–1270. doi: 10.1016/j.jvs.2009.07.091. [DOI] [PubMed] [Google Scholar]
- Zhou W, Reardon M, Peden E K. et al. Hybrid approach to complex thoracic aortic aneurysms in high-risk patients: surgical challenges and clinical outcomes. J Vasc Surg. 2006;44:688–693. doi: 10.1016/j.jvs.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Anaya-Ayala J E, Cheema Z F, Davies M G. et al. Hybrid thoracic endovascular aortic repair via right anterior minithoracotomy. J Thorac Cardiovasc Surg. 2011;142:314–318. doi: 10.1016/j.jtcvs.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Benedetto U, Melina G, Angeloni E. et al. Current results of open total arch replacement versus hybrid thoracic endovascular aortic repair for aortic arch aneurysm: A meta-analysis of comparative studies. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.09.011. [Epub ahead of print. PMID: 23040321.] [DOI] [PubMed] [Google Scholar]